Abstract
Purpose
To report interim results from a single-institution study conducted to assess accelerated hypofractionated radiotherapy (AHRT) delivered with 3D conformal radiotherapy in two groups of patients with non-small cell lung cancer: (1) patients with early stage disease unable to tolerate surgery and ineligible for stereotactic body radiation therapy, and (2) patients with locally advanced disease unsuitable for concurrent chemoradiotherapy.
Methods/patients
A total of 83 patients (51 stage I–II, 32 stage III) were included. Radiotherapy targets included the primary tumor and positive mediastinal areas identified on the pre-treatment PET–CT. Mean age was 77.8 ± 7.8 years. ECOG performance status (PS) was ≥2 in 50.6 % of cases. Radiotherapy was delivered in daily fractions of 2.75 Gy to a total dose of 66 Gy (BED10 84 Gy). Acute and late toxicities were evaluated according to NCI CTC criteria.
Results
At a median follow-up of 42 months, median overall survival (OS) and cause-specific survival (CSS) were 23 and 36 months, respectively. On the multivariate analysis, PS [HR 4.14, p = 0.0001)], stage [HR 2.51, p = 0.005)], and maximum standardized uptake values (SUVmax) [HR 1.04, p = 0.04)] were independent risk factors for OS. PS [HR 5.2, p = 0.0001)] and stage [HR 6.3, p = 0.0001)] were also associated with CSS. No cases of severe acute or late treatment-related toxicities were observed.
Conclusions
OS and CSS rates in patients treated with AHRT for stage I–II and stage III NSCLC were good. Treatment was well tolerated with no grade three or higher treatment-related toxicity. PS, stage, and SUV max were predictive for OS and CSS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related death world-wide [1]. Non-small cell lung cancer (NSCLC) accounts for 80 % of all lung cancers and prognosis is poor, even in patients eligible for curative-intent treatment. Surgical resection is the treatment of choice in early stage (I–II) disease, although radiotherapy offers an efficacious alternative in the 20–25 % of patients considered unfit for surgery due to advanced age, poor lung function, or comorbidities, such as cardiopathy, vascular disease, diabetes mellitus, or other comorbid conditions [2] or in patients who refuse surgery. Concomitant chemoradiotherapy is the treatment of choice in locally advanced (stage III) NSCLC, offering improved overall survival compared with sequential treatment schemes. However, concomitant treatment is associated with more treatment-related side effects, primarily in the esophagus, with higher rates (from 4 to 18 % higher) of ≥grade 3 acute esophagitis compared with sequential treatment [3, 4].
The number of elderly patients diagnosed with lung cancer is expected to increase in coming decades due to population demographics. Consequently, the percentage of patients with early stage NSCLC considered ineligible for surgery is also expected to increase [5, 6]. Various studies have demonstrated the importance of accurately assessing the patient’s general condition, performance status (PS), and comorbidities, because these factors have been shown to be independent predictors of overall survival [7, 8].
The conventional radiotherapy for NSCLC consists of a total dose ≥60 Gy delivered in daily fractions of 2 Gy/day for 6–6.5 weeks. [9] In recent years, various alternative treatment schemes have been evaluated in an effort to improve treatment outcomes. One such strategy—hypofractionated radiotherapy—involves increasing the dose per fraction while reducing the number of sessions, an approach that is believed to limit tumor repopulation [10]. Hypofractionated radiotherapy may be particularly advantageous in patients with poor PS, because fewer treatment sessions are needed. This is an important advantage given that lower survival rates have been reported in elderly patients versus younger patients, possibly due to reduced access to treatment as a consequence of poor PS [8].
In early stage NSCLC, stereotactic body radiotherapy (SBRT) has proven superior to the conventional radiotherapy in inoperable patients [11–13]. However, SBRT must be administered according to a very strict protocol to assure treatment accuracy and safety. In addition, certain patient characteristics—including tumor size and/or location and PS—could complicate the use of SBRT; moreover, SBRT is not available in all centers. For these reasons, accelerated hypofractionated radiotherapy (AHRT) delivered with three-dimensional conformal radiotherapy (3D-CRT) could offer an acceptable alternative to SBRT. Several studies (using slightly different doses and fractionation schemes) have reported promising results for AHRT compared with the conventional radiotherapy [14, 15]. Sakaguchi et al. [16] retrospectively assessed 29 patients, finding that the only factor that was significantly associated with local control was a biological equivalent dose (BED) ≥80 Gy. However, data on AHRT remain limited and all but two of the studies published to date have been retrospective.
Given this context, we conducted the present prospective study to investigate the efficacy of AHRT delivered with 3D-CRT as an alternative to the conventional fractionation in two groups of patients with NSCLC, both of which underwent AHRT. The first group consisted of patients with early stage disease who were ineligible for surgery and unable to undergo SBRT (lack of SBRT technique; comorbidities; elevated PS; refusal to travel to another center; etc.). The second group comprised patients with stage III disease considered ineligible for concomitant chemoradiotherapy and, therefore, treated with sequential chemotherapy and AHRT.
Materials and patients
Patient selection
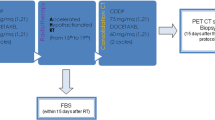
Inclusion criteria for the first group included a histological diagnosis of stage I–II NSCLC and ineligibility for surgical resection or SBRT. For the second group, inclusion criteria were a histological diagnosis of stage III NSCLC and eligibility for sequential chemoradiotherapy. Exclusion criteria included any of the following: diagnosis of small cell lung cancer; eligibility for surgery or SBRT; history of prior chest irradiation; and eligibility for concomitant chemotherapy treatment. Patients eligible for inclusion were treated between March 2009 and June 2015.
All patients were staged with contrast-enhanced thoraco-abdominal computed tomography (CT), 18F-fluorodeoxyglucose positron-emission-computed tomography (FDG–PET–CT), brain magnetic resonance imaging (MRI), and respiratory function tests. Histologic assessment was performed by endobronchial ultrasound (EBUS) or mediastinoscopy depending on the results of the imaging tests. In general, histological evaluation was performed if the mediastinal nodes had a diameter >1 cm and/or nodal uptake was observed on the PET–CT. The treatment decision was made by a multidisciplinary tumor board. All patients signed the informed consent form prior to treatment. The Research Ethics Board of Parc de Salut Mar gave ethical approval for this project.
Radiotherapy technique
Treatment planning was based on the PET–CT images. The patient was placed in the treatment position (supine decubitus with arms raised above the head) using an immobilization device. No endovenous contrast was used [17]. PET–CT images were interpreted jointly with the nuclear medicine specialist. The gross tumor volume (GTV) was defined as the primary tumor plus the involved lymph nodes (>1 cm on the CT and/or hypermetabolic on the PET–CT). Under our institutional protocol, the GTV and CTV are the same. To obtain the PTV, we applied an automatic 1.5-cm margin to the GTV in the antero-posterior and lateral directions, and a 2-cm margin in the cranial-caudal direction. No elective nodal irradiation was performed.
The following organs at risk (OARs) were contoured: esophagus, heart, spinal cord, and lung. The brachial plexus was contoured when necessary (according to the location of the primary tumor). The lung volume considered as the OAR was defined as the total lung volume minus the GTV (i.e., total lung-GTV).
Treatment planning and dosimetric calculations were performed with the Oncentra Master Plan (Nucletron B.V., Veenendaal, The Netherlands). Radiotherapy was administered in 24 daily sessions of 2.75 Gy/session to a total dose of 66 Gy (BED10 84 Gy). Plan acceptance required that 95 % of the PTV receive 95 % of the prescribed dose and that 50 % of the PTV receive 100 % of the prescribed dose.
Dose restrictions to the OARs were set according to standard limits [18, 19]. Dose limits in the lung were: V20 ≤30 %, and mean lung dose (MLD) <20 Gy. In the spinal cord, the maximum dose was set at <45 Gy. For the heart, the V30 limit was <46 % with a total mean dose <26 Gy. In the brachial plexus, the limit was <66 Gy. Finally, the recommended mean dose to the esophagus was <34 Gy, with V30 <50 %, V50 <40 %, and V70 <20 % [20, 21].
Statistical analysis
The statistical analysis was performed with SPSS version 22 (IBM SPSS, Chicago, IL). A descriptive analysis was performed using means and standard deviations. The survival analysis was performed with the Kaplan–Meier method. Overall survival (OS) and cause-specific survival (CSS) were calculated from radiotherapy initiation to death or final follow-up evaluation. The multivariate analysis was performed with a Cox regression.
Follow-up
During the course of radiotherapy, patients were examined at least once per week (more often if necessary). All patients were scheduled for a follow-up consultation at the following time points: at 3 weeks post-treatment, every 3 months for the first 2 years, and every 6 months thereafter. All follow-up evaluations consisted of a full history and physical examination, including assessment of adverse events. Acute and late radiation effects were evaluated and graded according to Common Terminology Criteria for Adverse Events (http://evs.nci.nih.gov/ftp1/CTCAE). Chest CT was used to assess tumor response at all follow-up consultations except for the first post-treatment follow-up (week 3).
Results
A total of 83 patients with a histologically confirmed diagnosis of NSCLC (62 % stage I–II, 38 % stage III) were included in the study. Patient characteristics are shown in Table 1. Most patients (50/83) had no mediastinal node involvement; of the other 33 patients, 9 were classified as N1, 19 as N2, and 5 as N3. All patients were staged by PET–CT, with a maximum standardized uptake value (SUVmax) of 10.0 ± 6.4. Most (90.6 %) of the stage III patients received sequential chemotherapy, which consisted of platinum-based treatment combined with vinorelbine in most cases.
PS ranged from 2 to 3 in 50.6 % of the patients. Respiratory function tests showed the following mean values: forced expiratory volume in 1 s (FEV1), 57.1 ± 23.7 %; diffusing capacity for carbon monoxide (DLCO), 48.2 ± 28.6 %; and carbon monoxide transfer coefficient (KCO), 58.9 ± 36.6 %.
All patients were treated with 6-MV photon 3D-CRT delivered in 24 sessions of 2.75 Gy/day. The final OAR values were as follows: lung: V5 37.8 ± 15.5 %; V20 18.9 ± 6.4 %; mean dose 11.6 ± 3.8 Gy; esophagus: V30 16.5 ± 20.1 %; V50 11.4 ± 16.6 %; mean dose 14.3 ± 11.9 Gy; maximum dose 41.4 ± 24.5 Gy; heart: V30 11.5 ± 14.5 %; mean dose 12.4 ± 10.6 Gy; and maximum dose 53.9 ± 20.6 Gy. In the brachial plexus and spinal cord, the maximum doses in all cases were, respectively, <66 Gy and <45 Gy (Table 1).
At a median follow-up of 42 months, median OS and CSS were, respectively, 23 and 36 months (Fig. 1). By stage, 2-year OS and CSS rates were, respectively, 51.1 and 82.1 % (stage I patients), 50.6 and 70.8 % (stage II), and 37.5 and 41.5 % (stage III).
Thirty-seven patients developed a recurrence, as follows: local (21.6 %), 5 stages I–II and 3 stage III); distant (18.9 %), 3 stages I–II and 4 stage III; or both local and distant (59.5 %), 8 stages I–II, and 14 stage III.
We assessed the impact of the following variables on survival: age, PS, stage, tumor size, SUV max on the PET–CT, FEV1, DLCO, and KCO. As shown in Table 2, the only variables found to be independent prognostic factors for OS and CSS were PS (0–1 vs 2–3), early vs advanced stage disease, and SUV max.
Figure 2 shows the effect of PS on survival in both patient groups. In patients with stage I–II disease, 2-year OS was 20.5 % in the subset of patients with a poor PS (2–3) vs 61 % in patients with good PS (0–1) (p = 0.002). For 2-year CSS, the corresponding survival values were 26 vs 68.5 %, respectively (p = 0.01).
The treatment was well tolerated and no cases of toxicity >grade 2 were observed. In terms of acute side effects, grade 1 and grade 2 dermatitis, respectively, were observed in 41 and 16.8 % of patients; no dermatitis was observed in the remaining 42.2 % of patients. Most patients (65.1 %) did not develop any esophageal toxicity; however, 25.3 and 9.6 %, respectively, presented grade 1 or grade 2 esophagitis. Most patients (72 %) presented no acute pulmonary toxicity. Acute grade 1 pneumonitis was reported in the remaining 28 % of patients. Chronic grade 1 pneumonitis was observed in 50.6 % of the patients, with one case of grade 2 lung toxicity. No treatment-related deaths were observed.
Discussion
The aim of this study was to assess the role of AHRT as an alternative to the conventional fractionation in NSCLC. We evaluated two groups of patients, both treated with AHRT delivered by 3D-CRT. The first group consisted of patients with early stage NSCLC ineligible for surgery and unable to receive SBRT; this group received AHRT alone. The second group consisted of stage III patients unfit for concomitant chemoradiotherapy and, consequently, treated with sequential chemotherapy and AHRT. Median OS and CSS were good (23 and 36 months, respectively) and consistent with other reports. Moreover, treatment was well tolerated without any severe (≥grade 3) treatment-related toxicity. Taken together, these results support the use of AHRT in patients ineligible for SBRT or concomitant chemoradiotherapy.
Table 3 summarizes the most relevant studies published to date on AHRT for NSCLC. As that table shows, most studies conducted to date have been retrospective, with highly heterogenous doses schemes among those studies. Similarly, treatment planning (2D, 3D, or even 4D in the most recent studies) is also heterogeneous. As a result of this variability, it is difficult to reliably compare the available studies [14–16, 22–28, 30].
Soliman et al. [24] used a hypofractionated treatment regimen (4 Gy/day; total dose 48–60 Gy) to treat 118 patients with stage T1–3 N0M0 NSCLC, reporting favorable local control and survival rates. They observed 45 recurrences, of which 13 were exclusively local. They did report, however, one death due to radiation pneumonitis, five cases of pneumonitis requiring corticosteroid therapy, and four rib fractures. Yung et al. [25] evaluated 60 patients with T1–2 N0 disease, most of which (70 %) were treated with 20 fractions of 3 Gy/day. Survival rates were similar to those reported by Soliman et al., but with a better tolerance (no cases of ≥grade 3 toxicity). Two other studies used a daily fractionation schedule similar to ours [14, 15]. Din et al. [15] retrospectively assessed a series of 609 patients (20 fractions of 2.75 Gy; total dose: 55 Gy), reporting a 2-year OS of 72 % (stage IA), 51 % (stage IB), and 40 % (stage III), without any grade ≥3 toxicity. Importantly, less than 20 % of patients developed grade 1 or 2 pneumonitis. Lester et al. [14] evaluated 135 patients, most (72 %) of which received 20 fractions of 2.75 Gy (total dose 50 Gy). At a mean follow-up of 48 months, 2-year OS and CSS were 48.2 and 51.6 %, respectively, in stage I–II patients and 26.1 and 28.6 % in stage III patients. No severe acute or late toxicity was reported. Notably, although the OS and CSS rates reported by those authors showed a little variation, we observed a much greater disparity in our outcomes: 2-year OS and CSS were 51.1 and 82.1 %, respectively, in our stage I patients, 50.6 and 70.8 % in stage II patients, and 37.5 and 41.5 % in stage III patients. This disparity between our results and those reported by Lester et al. could be attributable to differences between the studies in terms of mean PS value: nearly three-quarters (72 %) of patients in this study had a PS of 0–1, whereas 49.4 % of patients in our study had a PS 0–1.
Two prospective studies have been conducted to evaluate hypofractionated 3D-CRT. The CALGB 39904 trial [26] reported favorable results with a total dose of 70 Gy (2.41–4.11 Gy/fraction). Mean survival was 38.5 months with a local failure rate of only 10 %. The NCIC-CTG BR 25 [27] phase II trial assessed 80 patients with peripheral lung tumors (≤5 cm) without nodal involvement. Treatment consisted of 60 Gy of hypofractionated radiotherapy delivered in 15 fractions. At a mean follow-up of 49 months, 2-year local control and OS were 87.4 and 68.7 %, respectively.
More recently, several authors have compared SBRT with AHRT. Lucas et al. [28] analyzed 160 stages I–II NSCLC patients treated with a mean dose of 54 Gy in three fractions (RTOG 0236 regimen) and 70.2 Gy in 26 fractions (CALGB 39904 regimen), respectively. At 3 years of follow-up, there were no significant differences between the groups in terms of local control (87.7 vs 71.7 %) or OS (63.4 vs 56.7 %); however, it is important to note that the groups were not well balanced in terms of PS, tumor size, and tumor localization. Chiang et al. [29], retrospectively, reviewed outcomes in 114 patients diagnosed with stage T1–T4 N0M0 NSCLC. Patients were equally divided into two treatment groups (57 patients per group): SBRT (49.7 ± 1.9 Gy; BED10 100–119.6 Gy) vs AHRT (49.8 ± 2.8 Gy; BED10 67.2–84 Gy). The results of the AHRT group had previously been reported by Soliman et al. [24]. In that group, treatment planning was 3D (3D-CRT), with weekly control via portal imaging. In the SBRT group, 4D treatment planning was utilized and the immobilization systems were more rigid. Daily cone beam CT was used for image guidance. The findings showed that patients treated with SBRT had better OS and local control, although no differences between the groups were observed in terms of progression-free survival and distant failure. Importantly, a higher proportion (p < 0.001) of patients in the SBRT group underwent complete staging (i.e., both PET–CT and CNS imaging). On the multivariate analysis, tumor size [HR (per 1 cm increase): 1.29, p = 0.009] and complete staging with PET–CT [HR 0.34, p = 0.004)] were the only factors significantly associated with OS. Moreover, no differences in OS were observed among the treatment groups. By contrast, in our study, all patients were staged with PET–CT; CNS imaging was performed only in stage III patients and in patients with adenocarcinomas ≥stage IIA. We found that tumor stage and SUV max values were independent prognostic factors for OS. Based on these data and the results of other related studies, we agree with the conclusions of the aforementioned comparative studies: SBRT seems to be superior to AHRT in the treatment of patients with inoperable early stage NSCLC. However, in institutions that lack the equipment necessary to perform SBRT, or in patients who cannot follow the strict protocols necessary for SBRT, AHRT appears to be a reasonable option.
In general, AHRT is well tolerated. Although we did observe some toxicity in our study, it was limited, without any grade 2 or greater acute or chronic side effects. These findings are consistent with the published results of other AHRT studies. Notably, the good toxicity outcomes in our study (≤grade 2) were achieved even in patients with mediastinal involvement (19 patients with N2 disease, and 5 with N3). However, the use of higher dose fractions appears to increase side effects, primarily dyspnea and esophagitis [24], although it should be noted that Westover et al. [30] found no significant association between these side effects and dose levels.
In addition to treatment-related variables, such as dose and toxicity, several studies have demonstrated that assessment of patient-related variables (age, PS, functional status) is very important. Moreover, in most patients, comorbidities associated with tobacco use are also present [5–8, 31]. In our sample, mean patient age was nearly 80 years, and slightly more than half had a PS ≥2 (an independent predictor of poor outcomes). These findings are consistent with those reported by Lester et al. [14], who also found an association between good PS and higher OS. As discussed previously, our results showed a large disparity between OS and CSS—particularly in early stage disease—a finding that indicates that the cause of death in most of our patients was not directly related to the cancer.
Conclusions
AHRT is safe and well tolerated by patients with stage I–II NSCLC who cannot receive SBRT and in stage III patients considered unsuitable for concomitant treatment. Importantly, AHRT does not require any special technology, which means that it can be performed at any radiation oncology department with 3D planning. Poor PS, tumor stage, and SUV max on the PET–CT are all predictors of survival in these patients.
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96.
Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer. Chest. 2013;143(Suppl 5):e278S–313S.
Auperin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–90.
Koning CC, Wouterse SJ, Daams JG, Uitterhoeve LL, Van den Heuvel MM, Belderbos JS. Toxicity of concurrent radiochemotherapy for locally advanced non-small-cell-lung cancer: a systematic review of the literature. Clin Lung Cancer. 2013;14:481–7.
Asmis TR, Ding K, Seymour L, Sheperd FA, Leighl NB, Winton TL, et al. Age and comorbidity as independent prognostic factors in the treatment of non-small-cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group trials. J Clin Oncol. 2008;26:54–9.
Haasbeek CJ, Lagerwaard FJ, Antonisse ME, Slotman BJ, Senan S. Stage I non-small cell lung cancer in patients aged > or = 75 years; outcomes after stereotactic radiotherapy. Cancer. 2010;116:406–14.
Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer. A comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010;5:620–30.
Firat S, Bousamra M, Gore E, Byhardt RW. Comorbidity and KPS independent prognostic factors in stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;52:1047–57.
Perez CA, Pajak TF, Rubin P, Simpson JR, Mohiuddin M, Brady LW, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the radiation therapy oncology group. Cancer. 1987;59:1874–81.
Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumour clonogen repopulation during radiotherapy. Acta Oncol. 1988;27:131–46.
Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol. 2012;13:802–9.
Zheng X, Schipper M, Kidwell K, Lin J, Reddy R, Ren Y, et al. Survival outcomes after stereotactic body radiation therapy and surgery for stage I non-small-cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys. 2014;90:603–11.
Zimmermann F, Wulf J, Lax I, Nagata Y, Timmerman RD, Stojkovski I, et al. Stereotactic body radiation therapy for early non-small cell lung cancer. Front Radiat Ther Oncol. 2010;42:94–114.
Lester JF, Macbeth FR, Brewster AE, Court JB, Igbal N. CT-planned accelerated hypofractionated radiotherapy in the radical treatment of non-small-cell lung cancer. Lung Cancer. 2004;45:237–42.
Din OS, Harden SV, Hudson E, Mohammed N, Pemberton LS, Lester JF, et al. Accelerated hypo-fractionated radiotherapy for non-small cell lung cancer: results from 4 UK centers. Radiother Oncol. 2013;109:8–12.
Sakaguchi M, Maebayashi T, Aizawa T, Ishibashi N, Fukushima S, Abe O, et al. Patient outcomes of monotherapy with hypofractionated three-dimensional conformal radiation therapy for stage T2 or T3 non-small cell lung cancer: a retrospective study. Radiat Oncol. 2016;11:3.
Rodríguez de Dios N, Sanz X, Trampal C, Foro P, Reig A, Lacruz M, et al. 18F-FDG PET definition of gross tumor volume for radiotherapy of lung cancer: is the tumor uptake value-based approach appropriate for lymph nodes delineation? Int J Radiat Oncol Biol Phys. 2010;78:659–66.
Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(suppl 3):S10–9.
Kong FM, Ritter T, Quint DJ, Senan S, Gaspar LE, Komaki RU, et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs and brachial plexus. Int J Radiat Oncol Biol Phys. 2011;81:1142–457.
Rodríguez de Dios N, Algara M, Foro P, Lacruz M, Reig A, Membrive I, et al. Predictors of acute esophagitis in lung cancer patients treated with concurrent three-dimensional conformal radiotherapy (3D-CRT) and chemotherapy. Int J Radiat Oncol Biol Phys. 2009;73:810–7.
Palma D, Senan S, Oberije C, Belderbos J, Rodríguez de Dios N, Bradley JD, et al. Predicting esophagitis after chemoradiation therapy for non-small cell lung cancer: an individual patient data meta-analysis. Int Radiat Oncol Biol Phys. 2013;87:690–6.
Faria SL, Souhami L, Portelance L, Duclos M, Vuong T, Small D, et al. Absence of toxicity with hypofractionated 3-dimensional radiation therapy for inoperable, early stage non-small cell lung cancer. Radiat Oncol. 2006;1:42.
Cheung PCF, Mackillop WJ, Dixon P, Brundage MD, Youssef YM, Zhou S. Involved-field radiotherapy alone for early-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2000;48:703–10.
Soliman H, Cheung P, Yeung L, Poon I, Balogh J, Barbera L, et al. Accelerated hypofractionated radiotherapy for early-stage non-small cell lung cancer: long term results. Int J Radiat Oncol Biol Phys. 2011;79:459–65.
Yung T, Giuliani ME, Le LW, Sun A, Cho BC, Bezjak A, et al. Outcomes of accelerated hypofractionated radiotherapy in stage I non-small cell lung cancer. Curr Oncol. 2012;19:e264–9.
Bogart JA, Hodgson L, Seagren SL, Blackstock AW, Wang X, Lenox R, et al. Phase I study of accelerated conformal radiotherapy for stage I non-small cell lung cancer in patients with pulmonary dysfunction: CALGB 39904. J Clin Oncol. 2010;28:202–6.
Cheung P, Faria S, Ahmed S, Chabot P, Greenland J, Kurien E, et al. Phase II study of accelerated hypofractionated three-dimensional conformal radiotherapy for stage T1-3 N0M0 non-small cell lung cancer: NCI CTG BR. 25. J Natl Cancer Inst. 2014;106(8):dju164.
Lucas Jr JT, Kuremsky JG, Soike M, Hinson WW, Kearns WT, Hampton CJ, et al. Comparison of accelerated hypofractionation and stereotactic body radiotherapy for stage 1 and node negative stage 2 non-small cell lung cancer (NSCLC). Lung Cancer. 2014;85:59–65.
Chiang A, Thibault I, Warner A, Rodrigues G, Palma D, Soliman H, et al. A comparison between accelerated hypofractionation and stereotactic ablative radiotherapy (SABR) for early-stage non-small cell lung cancer (NSCLC): results of a propensity score-matched analysis. Radiother Oncol. 2016;118:478–84.
Westover KD, Loo BW, Gerber DE, Iyengar P, Choy H, Diehn M, et al. Precision hypofractionated radiation therapy in poor performing patients with non-small cell lung cancer: phase 1 dose escalation trial. Int J Radiat Oncol Biol Phys. 2015;93:72–81.
Kopek N, Paludan M, Petersen J, Hansen AT, Grau C, Hoyer M. Co-morbidity index predicts for mortality after stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer. Radiother Oncol. 2009;93:402–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors indicate no potential conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
All patients signed the informed consent form prior to treatment.
Rights and permissions
About this article
Cite this article
de Dios, N.R., Sanz, X., Foro, P. et al. Accelerated hypofractionated radiation therapy (AHRT) for non-small-cell lung cancer: can we leave standard fractionation?. Clin Transl Oncol 19, 440–447 (2017). https://doi.org/10.1007/s12094-016-1544-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-016-1544-7