Abstract
Objective
To examine the expression of cortactin in epithelial ovarian cancer, and discuss the relationship between the expression of cortactin and the clinical pathology characteristics in epithelial ovarian cancer, as well as clinical significance.
Methods
The expression of cortactin was detected using real-time fluorescence quantitative PCR and immunohistochemical SP method in epithelial ovarian cancer.
Results
(1) The relative content of cortactin mRNA in epithelial ovarian cancer tissue was higher than that in benign control tissue, and expression was related to histological classification and FIGO stage. (2) Cortactin protein was localized in the cytoplasm and membrane of tumor cells. The positive rate of cortactin was 73.3 % in epithelial ovarian cancer, and the rate of cortactin expression was related to histological classification. (3) The average survival period of epithelial ovarian cancer patients with positive expression of cortactin was 19.5 ± 1.2 months (95 % CI 14.6–21.4 months), compared with 34.5 ± 4.3 months in the negative expression group (95 % CI 22.1–25.9 months). Univariate survival analysis showed that: negative expression of cortactin had a significant survival advantage (χ 2 = 5.739, P = 0.017). A cox regression model for multivariate analysis revealed that cortactin was an independent prognostic factor for epithelial ovarian cancer (P = 0.001; RR = 6.452, 95 % CI 2.289–7.112).
Conclusions
Negative expression of cortactin was an independent prognostic factor and had a survival advantage. This suggested that cells with poor differentiation showed increasing motility. Cortactin is closely related to poor prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithelial ovarian cancer has the highest mortality of all gynecological tumors [1], with metastasis being the main cause of mortality. The process of metastasis has been viewed as a series of steps, including direct invasion, lymphatic metastasis, hematogenous dissemination, overflow, and implantation metastasis. From a genetic perspective, the instability of cancer cells allows them to adapt to their new microenvironment, which includes fiber cells, immune cells, endothelial cells, and extracellular matrix changes [2]. Tumor cells released from organizational structures show increased motility [3], and this motility and the consequent invasion and metastasis depend on the properties of the cells themselves [4], which in turn depend on the structural characteristics of the cytoskeleton and the conduction of signals from the nucleus. The cortactin gene is located on chromosome 11q13 and cortactin protein is located on the cell surface or at the poles. Cortactin recruits Arp2/3 complex proteins to actin, causing actin nucleation and regulating actin cytoskeleton assembly and adhesion. It thus plays an important role in podocyte and lamellipodia formation, membrane transport, intracellular phagocytosis, axon guidance, neuronal polarization, and tumor formation [5]. Cortactin is a p80/85 protein; the 85-kDa subtype is a modification of the 80-kDa protein, which is involved in tumorigenesis.
Materials and methods
Patients and samples
Seventy-five patients diagnosed with epithelial ovarian cancer underwent cytoreductive surgery at Liaocheng People’s Hospital between April 2009 and October 2013. The patients were grouped according to age, cell differentiation, FIGO stage, lymph node metastasis, and ascites. Patient ranged from 30 to 66 years. There were 22 highly differentiated and 53 moderate–poorly differentiated tumors. Twenty-two were FIGO stage I–II and 53 were stage III–IV. Thirty patients had lymph node metastasis and 18 had ascites. None of the patients had any prior history of chemoradiation, radiation, or hormonal therapy. None of the patients had any contact with radiation, or any other risk factors, including smoking. None of the patients had histories of infectious hepatitis, autoimmune diseases, or other complications. The postoperative follow-up period continued until the date of death or December 2013 (2–54 months). During the same period, we also selected 40 patients with benign ovarian epithelial tumors who underwent surgery at Liaocheng People’s Hospital, and 20 perimenopausal control patients who underwent ovariectomy because of other diseases (uterine fibroids and other benign diseases). Fresh tissues were frozen rapidly in liquid nitrogen and stored at −80 °C. Conventional specimens were fixed in 10 % formalin and embedded in paraffin.
Methods
RNA extraction
One hundred micrograms of tissue was homogenized with 1 ml Trizol reagent (Invitrogen, USA) in an ice bath and then transferred to a fresh tube. Chloroform (200 µl) was added followed by shaking vigorously by hand for 30 s. The samples were then incubated on ice for 5 min and centrifuged at 4 °C at 12,000 rpm for 10 min. The cleared homogenate was transferred to a fresh tube and insoluble material and the upper fat layer were discarded. Isopropyl alcohol (500 µl) was added followed by mixing and incubation at room temperature for 10 min and centrifuging at 4 °C at 12,000 rpm for 10 min. One milliliter of 75 % ethanol was added to the samples, which were then mixed. The precipitate was washed, centrifuged at 4 °C at 7500 rpm for 5 min, the supernatant was discarded, and the RNA pellet was air-dried for 5 min before adding 20 µl RNase-free water and mixing by gentle pipetting. The purity and concentration were determined using a NanoDrop2000 trace ultraviolet spectrophotometer (Thermo Electron Corporation, USA) and the RNA was stored at −80 °C until required.
Real-time fluorescent quantitative polymerase chain reaction to detect Smad4 and cortactin
Samples were incubated at 37 °C for 60 min and the purity and concentration of the cDNA reverse transcription product were tested using a NanoDrop2000 trace ultraviolet spectrophotometer. cDNA was stored at −80 °C until required. Primers were designed according to the gene sequences of cortactin: upstream, 5′-GGATGGATAAGAATGCGTCAAC-3′; downstream, 5′-GTTACTTGTTTTGCTGGTCACAG-3′. Primers used for GAPDH as an internal control were 5′-AGAAGGCTGGGGCTCATTTG-3′, and 5′-AGGGGCCATCCACAGTCTTC-3′ (Shanghai Sangon Biotech Co. Ltd, China). The cortactin real-time fluorescent quantitative polymerase chain reaction (PCR) reaction conditions were as follows: 95 °C predegeneration 15 min, 95 °C transgender 10 s, 54 °C annealing 20 s, 72 °C extension 32 s, for 40 cycles. Dissolution curves were automatically generated to determine the specificities of both amplification products. The CT value of the cDNA for each group sample was determined and the formula RQ = 2−ΔΔCt was used to compare differences in gene expression between the cancer and control groups.
Immunohistochemical staining of cortactin proteins
Paraffin-embedded specimens were cut into 3- to 4-μm serial sections, incubated in a water bath at 37 °C, allowed to flatten, and incubated at 75 °C for 30 min. The sections were then dewaxed in three xylene solutions for 10 min each and processed through 100 % ethanol for 2 min, followed by 95, 80, and 75 % ethanols for 1 min each. The sections were heated in a microwave for antigen repair in 0.01 mmol/l citrate salt buffer and cooled to room temperature. Peroxidase blocking solution was added followed by incubation at room temperature for 10 min, washing in phosphate-buffered saline (PBS), incubation at room temperature for 10 min in non-immune animal serum, and removal of the serum. Primary antibodies to Smad4 and cortactin, respectively, were added at 4 °C overnight, followed by washing with PBS. Secondary biotin-labeled antibodies were added and incubated at room temperature for 10 min and then washed in PBS. Streptomycin resistance-biotin peroxidase solution was added and incubated at room temperature for 10 min, followed by washing in PBS, addition of 3,3′-diaminobenzidine, counterstaining with hematoxylin for 3 min, differentiation in hydrochloric acid solution, washing with PBS to reveal the blue color, and dehydration through graded alcohols. The slides were mounted and examined under a light microscope after drying. PBS solution was used in place of primary antibodies as a negative control.
Positive staining was indicated by brown–yellow staining around the cell membrane at high-power magnification. “+” indicates >10 % of tumor cells with weak staining on the cell membrane, “++” indicates >10 % of tumor cells with moderate staining on the cell membrane, and “+++” indicates >10 % of tumor cells with strong staining on the cell membrane. The results were judged by pathologists blinded to the slides’ origins.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS, USA). Data were described as mean ± standard deviation (SD). Differences between groups were compared by single factor analysis of variance and t tests, and numerical data were compared using χ2 tests. Correlation analysis was performed using Spearman’s correlation and rank correlation analyses. The degree of correlation was judged based on the correlation coefficient as follows: R < 0.1 no correlation; 0.1–0.39 low correlation; 0.4–0.69 moderate correlation; 0.7–0.99 high correlation. The Kaplan–Meier method was used to calculate survival curves, and differences were evaluated using the log-rank test. Cox proportional hazards regression analysis was used to estimate the impact of clinicopathologic factors and the expression of each marker on overall survival. Values of P < 0.05 were considered significant.
Results
Cortactin mRNA expression in ovarian tumor tissue
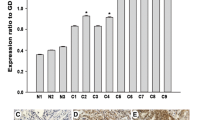
Cortactin mRNA levels were higher in epithelial ovarian cancer tissues compared with benign control tissues (Table 1; Fig. 1). The formula RQ = 2−ΔΔCt was used to calculate relative cortactin mRNA levels. Cortactin mRNA levels were 2.31 times higher in ovarian epithelial cancer tissue than in benign ovarian tumor tissue.
The relationship of cortactin mRNA expression in ovarian epithelial carcinoma tissues with clinical pathological features
We next investigated the correlation between cortactin mRNA expression and the clinicopathological characteristics of the ovarian epithelial carcinoma, including age, histologic classification, FIGO staging, lymph node metastasis, and ascites. As shown in Table 2, we found that cortactin mRNA expression was significantly different in patients with different histological classification and FIGO stage (high differentiation vs low differentiation 1.5245 ± 0.4596 vs 0.7396 ± 0.5996, respectively, p < 0.001; I–II period vs III–IV period 1.2878 ± 0.7869 vs 0.8378 ± 0.5643, respectively, p < 0.05). There was no significant association between the cortactin mRNA expression and other clinicopathological characteristics (p > 0.05).
Cortactin expression in ovarian tumor tissue
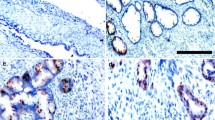
Cortactin was localized in the cytoplasm and membrane of tumor cells. The positivity rate for cortactin was significantly higher (73.30 %) in epithelial ovarian cancer compared with benign ovarian tumor tissue (<25 %) (P < 0.05) (Table 3; Fig. 2a, b).
The expression of cortactin and the correlation with clinical pathological features of epithelial ovarian cancer
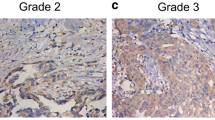
As shown in Table 4, we found that the expression of cortactin was significantly different in patients with high differentiation (20/22, 90.91 %) and low differentiation (35/53, 66.04, χ 2 = 4.918, p < 0.05), but not age, FIGO stage, ascites, or lymph node metastasis (p > 0.05).
The analysis of the relationship between the expression level of cortactin in ovarian epithelial carcinoma with clinical pathological characteristics and survival prognosis
Among 75 cases of ovarian epithelial cancer, 55 cases were positive for cortactin protein expression, and had an average survival period of 19.5 ± 1.2 months (95 % CI 14.6–21.4 months), and 20 cases were cortactin-negative, with an average survival of 34.5 ± 4.3 months (95 % CI 22.1–25.9 months) (P = 0.017) (Fig. 3), cortactin showed the opposite effect (χ2 = 5.739, P = 5.739). Single factor survival analysis showed that patients with high differentiation, early stage, and no lymph node metastasis or ascites had a survival advantage (Table 5).
Cox regression analysis to investigate the independent prognostic factors for ovarian cancer patients
We analyzed the factors correlated with patient survival using Cox’s proportional hazards regression model. The following factors were identified as independent prognostic factors in ovarian epithelial cancer: FIGO grade (P = 0.036, RR = 1.460, 95 % CI 1.030–2.160), histological grade (P = 0.019, RR = 0.581, 95 % CI 0.481–0.932), lymph node metastasis (P = 0.010, RR = 2.436, 95 % CI 1.218–4.500), cortactin expression (P = 0.001, RR = 6.452, 95 % CI 2.289–7.112) (Table 5).
Discussion
Cell movement is a universal phenomenon involving organelle movement and cell membrane movement. The mechanochemical cytoskeletal system is the source of the active movement force. Many cytoskeletal proteins are involved in cell movement, and the newly discovered actin-bundling protein fascin, and the actin-filament-binding protein cortactin play important roles in the regulation of cell active movement.
Animal cells possess a 0.2- to 0.5-μm-thick transparent layer under the cell membrane known as the cell cortex, which is a three-dimensional network structure composed mainly of cortactin, connected closely with the plasma membrane. The gaps in the cortactin network are only about 0.1 nm, preventing the passage of some organelles and macromolecules. The plasma membrane constitutes the most active structure in the cell. Cell movement and many other important physiological activities, such as changes in cell shape, cell adhesion, endocytosis, cytokinesis, transmembrane protein composition, and cell surface tension involve the cortactin cytoskeleton.
Cortactin regulates the actin cytoskeleton through cell movement, adhesion, polarization, and shrinkage. It is a substrate of Src tyrosine kinase [6], and plays key roles in the cytoskeleton and in reconstruction of signal pathways. A biochemical study showed that cortactin promoted migration via phosphorylation, to regulate cell movement [7]. The cytoskeleton and its regulatory proteins are critical for the migration of tumor cells, and changing the cytoskeleton causes migration. The invasion and metastatic abilities of the tumor cells are closely related to cell movement. The structural characteristics of the cytoskeleton are responsible for caused the different capacities of cells to move, and this in turn reflects specific genetic difference between tumor and normal cells.
The study found that some epithelial cells lost their epithelial morphology and characteristics, and acquired the morphology and characteristics of mesenchymal cells, along with motility. This transformation is known as epithelial–mesenchymal transition (EMT).
Epithelial cells lost polarity and the epithelial phenotype connecting with basement membrane through EMT, and obtained the mesenchymal phenotype which had high ability of migration and invasion, anti-apoptosis and extracellular matrix degradation. EMT is an important biological process in the migration and invasion of malignant tumors of epithelial origin. Tumor cells are found in the extracellular matrix after EMT, which is hydrolyzed by proteolytic enzymes such as MMP. Tumor cells are released into the systemic circulation, spread to distant organs, and form secondary tumors. EMT is regarded as the key step in tumor invasion.
EMT is important for the invasion and metastasis of epithelial tumor cells, which acquire the characteristics of stem cells. Cortactin may be a key gene in the processes of tumor invasion and metastasis. Tumor cell lines overexpressing cortactin demonstrate strong transfer ability. The results of the current study confirmed that cortactin was localized in the cytoplasm and cell membrane, and that cortactin expression levels were significantly higher in ovarian epithelial cancer compared with benign ovarian tumors. Cortactin expression was positively related to histological classification. These results suggest that the migratory ability of tumor cells is responsible for their invasive and metastatic characteristics.
The most obvious characteristics of malignant tumors are proliferation and metastasis. Tumor initiation and progression are complicated processes, in which tumor cell migration is a basic condition. Each stage of tumor metastasis requires tumor cell movement, and tumor cells rely mainly on active movement to achieve invasion and metastasis via a variety of signaling pathway.
Cancer cell movement is an essential feature of cancer cell migration, including pseudopodial extension of the cell membrane, membrane fluidity, and cell-polarity changes [8, 9]. One of the most important conditions for cancer cell invasion is the formation of invadopodia [10]. Artym [11] proposed a four-stage model of invadopodia formation. Cortactin initially aggregates and adheres to the membrane matrix; MT1-MMP then accumulates where the cortactin has gathered; the extracellular matrix is degraded; and cortactin regulates the secretion of MMPs (mainly MMP-2, MMP-9 and MT1-MMP) [12]. Cortactin may become phosphorylated by Erk1/Erk2, Src family kinases and PAK [5]. Cortactin regulates cell invasion and migration into the extracellular matrix through invadopodia in vivo. Invadopodia formation requires the polymerization G-actin to form F-actin [13]. Cortactin promotes the formation of Arp2/3 complexes and the nucleation of branched actin. Overexpression of cortactin thus induces cell motility and metastasis, inhibits intercellular adhesion, and accelerates the spread of tumors [14, 15].
Increased expression of cortactin may play a role in the metastasis of HCC by affecting cell locomotor activity. Cortactin might thus be a sensitive marker of intrahepatic metastasis in HCC [16]. About 44 % of head and neck squamous cell carcinomas overexpress cortactin, and its expression was shown to be positively related TNM stage and histological differentiation. Increased cortactin expression correlated with increased local recurrence rate and reduced 5-year survival [17].
The current results confirmed that cortactin mRNA levels were significantly higher in epithelial ovarian cancer tissue compared with benign control tissue, and expression levels were related to histological classification and FIGO stage, while cortactin protein was expressed in 73.3 % of epithelial ovarian cancer tissues and was related to histological classification. Univariate survival analysis showed that negative cortactin expression conferred a survival advantage, while multivariate analysis identified negative cortactin expression as an independent prognostic factor. These results suggest that the malignant phenotype was associated with cortactin.
TGFβ1 is a multifunctional cytokine able to induce the occurrence of EMT and apoptosis in some epithelial cells. TGF-β1 can reduce the intracellular storage protein ferritin heavy chain, release iron stored in ferritin heavy chain, and increase the intracellular labile iron pool.
Expression of TGF-β1 is high in most tumor tissues and plasma [18]. Expression of TGF-β1 is increased in esophageal squamous cell carcinoma, which is negatively correlated with E-cadherin, but positively with vimentin. This indicates that TGF-β1 promotes EMT. When the TGF-β1 signaling pathway is inhibited in esophageal squamous carcinoma cell line EC9706, EMT is blocked.
Smad4 is a cellular signal transduction protein that is a key component of TGF-β1 signal transduction pathways. Abnormal expression and phosphorylation of Smad4 leads to disturbance of TGF-β1. The loss or mutation of Smad4 expression is closely related to tumor occurrence and development. The TGF-β1 signaling pathways might regulate tumor cell movement through cytoskeletal proteins controlled by Smad4, and affect tumor cell invasion and metastasis.
Silencing of Smad4 in pancreatic cancer by RNA interference (RNAi) affected TGF-β downstream genomic activity and multiple cell functions, such as movement, cytoskeleton regulation, cell cycle regulation, nuclear transport, and oxidation damage. Stimulation of Smad4-silenced cells with TGF-β1 upregulated five motility genes. These results indicate that inhibition of Smad4 expression might influence tumor cell movement. In addition to changes in tumor cell shape, cytoskeletal proteins are also upregulated during the process of metastasis [19, 20].
Transfection of hepatoma SMMC-7721 cells with RNAi-Smad lentivirus vector alters cell migration by the method of cell scratch and three-dimensional culture detection of cells. The results indicate that scratch wound healing time is faster in RNAi-Smad4-2 and RNAi-Smad4-12 transfected cells, and fascin and cortactin protein expression is higher. This shows that decreased expression of Smad4 upregulates the level of fascin and cortactin, and promotes the migration of hepatoma SMMC-7721 cells.
Cortactin is an F-actin-binding protein that regulates cell movement and the adhesive junction assembly. However, the function of cortactin in EMT remains elusive. Here, we found that during TGF-β1-induced EMT, cortactin underwent tyrosine dephosphorylation. Inhibition of the dephosphorylation of cortactin blocked TGF-β1-induced EMT. Knockdown of cortactin by RNAi decreased expression of intercellular junction proteins E-cadherin and ZO-1 and induced expression of fibronectin. Additionally, knockdown of cortactin further promoted TGF-β1-induced EMT, as shown by EMT markers and cell morphological changes. Moreover, a migration assay showed that cortactin knockdown promoted the migration and enhanced TGF-β1-induced migration [21].
The TGF-β1 signal transduction pathway might regulate cytoskeletal protein by Smad4. Cortactin might be a downstream target gene in the Smad4 pathway. Tumor cell movement is regulated via the Smad4–cortactin pathway, which promotes tumor cell proliferation. In TGF-β pathway, Smad protein and cortactin are part of a tumor suppressor pathway. Any abnormal signal transduction can lead to disruption of the whole pathway. Tumor cells can escape the inhibitory effect of TGF-β1, and accelerate the proliferation, infiltration and metastasis of malignant tumor cells. The Smad4–cortactin motor pathway might have potential therapeutic value.
In conclusion, expression of cortactin in epithelial ovarian cancer was significantly higher than in the control group. High expression of cortactin occurred mainly in poorly differentiated and late-stage cancer. Higher levels of cortactin expression were related to poorer differentiation, greater tumor cell motility, and greater malignancy. Cortactin expression in epithelial ovarian carcinoma was closely related to malignant biological behavior. Because the migration rate was determined by the movement ability of the tumor cells, up-regulation of cortactin as an important cytoskeletal protein was closely related to poor prognosis in ovarian cancer.
References
Hensley ML. A step forward for two-step screening for ovarian cancer. J Clin Oncl. 2010;28:2128–30.
Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95.
Condeelis J, Segall JE. Intravital imaging of cell movement in tumors. Nat Rev Cancer. 2003;3:921–30.
Kedrin D, Van Rheenen J, Hernandez L, Condeelis J, Seqall JE. Cell motility and cytoskeletal regulation in invasion and metastasis. J Mammary Gland Biol Neoplasia. 2007;12:143–52.
Ayala I, Baldassarre M, Giacchetti G, Caldidri G, Tete S, Luini A, et al. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci. 2008;121:369–78.
Schuuring E, Verhoeven E, Litvinov S, Michalides RJ. The product of the EMS1 gene, amplified and overexpressed in human carcinomas, is homologous to a v-src substrate and is located in cell-substratum contact sites. Mol Cell Biol. 1993;13:2891–8.
Minta JO, Yun JJ, Kabiawu O, Jones J. mRNA differential display identification of vascular smooth muscle early response genes regulated by PDGF. Mol Cell Biochem. 2006;281:63–75.
Wu H, Reynolds AB, Kanner SB, Vines RR, Parsons JT. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol Cell Biol. 1991;11:5113–24.
Tang ZY. Small hepatocellular carcinoma: current status and prospects. Hepatobiliary Pancreat Dis Int. 2002;1:349–53.
Buccione R, Caldieri G, Ayala I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009;28:137–49.
Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;15:3034–43.
Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–35.
Saitou T, Rouzimaimaiti M, Koshikawa N, Seiki M, Ichikawa K, Suzuki T. Mathematical modeling of invadopodia formation. J Theor Biol. 2012;298:138–46.
Luo ML, Shen XM, Zhang Y, Wei F, Xu X, Cai Y, et al. Amplification and overexpression of CTTN (EMS1) contribute to the metastasis of esophageal squamous cell carcinoma by promoting cell migration and anoikis resistance. Cancer Res. 2006;66:11690–9.
Rothschild BL, Shim AH, Ammer AG, Kelley LC, Irby KB, Head JA, et al. Cortactin overexpression regulates actin-related protein 2/3 complex activity, motility, and invasion in carcinomas with chromosome 11q13 amplification. Cancer Res. 2006;66:8017–25.
Chuma M, Sakamoto M, Yasuda J, Fujii G, Nakanishi K, Tsuchiya A, et al. Overexpression of Cortactin is involved in motility and metastasis of hepatocellular carcinoma. J Hepatol. 2004;41:629–36.
Hofman P, Butori C, Havet K, Hofman V, Selva E, Guevara N, et al. Prognostic significance of cortactin levels in head and neck squamous cell carcinoma: comparison with epidermal growth factor receptor status. Br J Cancer. 2008;98:956–64.
Antony ML, Nair R, Sebastian P, Karunagaran D. Changes in expression, and/or mutations in TGF-beta receptors (TGF-β RI and TGF-β RII) and Smad 4 in human ovarian tumors. J Cancer Res Clin Oncol. 2010;136:351–61.
Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin Exp Metastasis. 2009;26:273–87.
Jayo A, Parsons M. Fascin: a key regulator of cytoskeletal dynamics. Int J Biochem Cell Biol. 2010;42:1614–7.
Zhang K, Wang D, Song J. Cortactin is involved in transforming growth factor-beta1-induced epithelial-mesenchymal transition in AML-12 cells. Acta Biochim Biophys Sin Shanghai. 2009;41:839–45.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, A., Zhang, L., Zhang, X. et al. Expression and clinical significance of cortactin protein in ovarian neoplasms. Clin Transl Oncol 18, 220–227 (2016). https://doi.org/10.1007/s12094-015-1360-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1360-5