Abstract
Objective
To investigate the role of the vascular endothelial growth factor receptor 2 (VEGFR2) in the proliferation, migration, invasion, and radiation-induced apoptosis of the non-small cell lung cancer (NSCLC) cell line Calu-1.
Methods
VEGFR2 gene was silenced by RNA interference in Calu-1 cells, and the expression of VEGFR2 was measured by qRT-PCR and Western blot analysis. The cells were divided into control, VEGF-treated, VEGFR2 knockdown, and VEGFR2 knockdown and VEGF-treated groups. A CCK8 assay and Transwell assay were performed to assess cell proliferation, migration, and invasion, respectively, after VEGFR2 knockdown. Western blot assays were used to detect signaling proteins downstream of VEGFR2. Cells in the groups listed above were also subjected to radiation treatment, followed by apoptosis analysis.
Results
(1) RNA interference of VEGFR2 in Calu-1 cells reduced VEGFR2 mRNA (P < 0.01) and protein levels (P < 0.01). (2) VEGFR2 knockdown inhibited proliferation (P < 0.05), migration (P < 0.05), and invasion (P < 0.05) in Calu-1 cells. (3) VEGFR2 knockdown blocked the phosphorylation of protein kinase B (Akt, also known as PKB), extracellular regulated kinase (ERK) 1/2, and p38 mitogen-activated protein kinase (p38 MAPK) to various extent (P < 0.05), but did not change their total protein expression. (4) Knockdown of VEGFR2 suppressed HIF-1α protein synthesis (P < 0.05), and exacerbated apoptosis induced by radiation (P < 0.05).
Conclusion
VEGFR2 gene knockdown significantly suppressed a number of cellular activities in Calu-1 cells and increased radiation-induced cell death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the world, lung cancer has been the highest mortality of any malignancy, and its incidence is still on the rise [1]. Non-small cell lung cancer (NSCLC) accounts for 80–89 % of the all cases of lung cancer. One of the standard treatments for NSCLC is radiotherapy [2]. However, radiotherapy alone has yielded limited effectiveness clinically, and the patients receiving radiotherapy alone have a 5 year survival rate of only 5–10 % [3]. The reasons for therapy failure are usually tumor recurrence and distant metastasis [4]. There is a pressing need to identify a novel therapeutic target that could improve the efficacy of radiotherapy.
Vascular endothelial growth factor receptor 2 (VEGFR2), also known as kinase insert domain receptor (KDR) [5]. When activated, it stimulates proliferation and chemotaxis in endothelial cells and increases vascular permeability [6]. Proliferation of tumor cell and endothelial cell will consume a large amount of oxygen, and even the endothelial cell will consume more, so both can increase the hypoxia in tumor [7, 8]. However, hypoxia is right one of the main causes of radioresistance [9]. Here, fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC) assays were used to determine VEGFR2 protein levels and KDR gene copy number gains (CNG) in human NSCLC tissues, respectively. VEGFR2 was here found to be expressed in large amounts in the cytoplasm and on the cell membrane of tumor cells. VEGFR2 expression and KDR gene amplification were found to be correlated with the degree of tumor tissue differentiation, and the patients with tumors that expressed high levels of VEGFR2 had shorter PFS (Progression-Free Survival) and OS (Overall Survival) than those with tumors that expressed lower levels [10]. This shows that VEGFR2 may be one of the main causes of radioresistance in NSCLC. To further understand the mechanisms of radioresistance induced by VEGFR2 expression, RNA interference (RNAi) was used to silence VEGFR2 in a lung cancer cell line expressing high levels of VEGFR2, Calu-1. Then the role of VEGFR2 in the proliferation, migration, invasion, and radioresistance of Calu-1 cells was investigated.
Materials and methods
Cell line
Calu-1, a human lung squamous cell carcinoma cell line, was purchased from the Chinese Academy of Sciences Shanghai Institutes for Biological Sciences.
Major reagents and equipment
McCoy’s 5A medium was purchased from GIBCO (U.S,), and fetal bovine serum was purchased from Hangzhou Sijiqing (Zhejiang, China). Primary antibodies for Western blot analysis were obtained from CST (US), and secondary antibodies were purchased from ZSGB Bio (Beijing, China). The RNA extraction reagent Trizol and SYBR® Green Kit were purchased from Invitrogen (US). The reverse transcription kit was obtained from Fermentas (US). The nucleic acid and protein analyzer were purchased from Eppendorf (Germany), the protein electrophoresis system was purchased from Bio-Rad (US), and the real-time fluorescence PCR system (ABI7500) was purchased from Applied Biosystems (US). The cell apoptosis detection kit was purchased from Nanjing KeyGEN Biotech (Jiangsu, China). The flow cytometer was purchased from Beckman-Coulter (US), and the linear accelerator was purchased from Siemens (German). The VEGFR2 siRNA was purchased from GenePharma (Shanghai, China) and the sequences were as follows: sense strand: 5′-CCGGGAUAUUUAUAAAGAUTT-3′, antisense strand: 5′-AUCUUUAUAAAUAUCCCGGTT-3′.
Cell culture and transfection
Calu-1 cells were grown in McCoy’s 5A medium supplemented with 10 % FBS in a humidified incubator at 37 °C with 5 % CO2. Cells were transfected with siRNA when they reached 80 % confluence. siRNA was diluted in serum-free MEM medium, let stand for 5 min, and then gently mixed with Lipofectamine 2000. This transfection mixture was placed at room temperature for 20 min before it was added to the cells. Transfected cells were placed back into the incubator for 8 h, after which the medium was replaced to McCoy’s 5A medium containing 10 % FBS but no antibiotics. The final concentration of siRNA (labeled with a fluorescent) was 50 nmol/mL. The transfection efficiency was monitored by fluorescence between 8 and 24 h after transfection.
Cell proliferation and viability analysis
Twenty-four hours after transfection, control cells(transfected with negative control siRNA) and transfected cells were trypsinized and resuspended in McCoy’s 5A medium containing 10 % FBS to 1 × 105 cells/ml. Cells were seeded into 96-well plates at 5000 cells per well in 100 μl medium. Blank wells contained an equal volume of medium but no cells. Cells were put into the following groups: control, VEGF stimulation, VEGFR2 gene knockdown, VEGFR2 gene knockdown plus VEGF stimulation. The concentration of VEGF was 20 ng/mL. Each group contained six replicate wells. At designated points in time, plates were removed from the incubator and added with 10 μl CCK-8 solution per well. The plates were placed back into the incubator for 2 h, and then the reaction signals were detected with a microplate reader. The tumor cell growth inhibition rate (IR) was calculated as follows: IR = (1—absorbance of treated group/absorbance of control group) × 100 %. This experiment was performed three times.
Cell migration assay
Twenty-four hours after transfection, 1 × 105 cells in 100 μl of medium containing 5 % FBS were seeded into the upper chambers of Transwells placed in 24-well plates. Then 600 μl medium containing 15 % FBS, with or without VEGF, was added to the lower chambers. The concentration of VEGF was 20 ng/mL. Cell grouping was the same as above. After 24 h of culturing, unmigrated cells left in the upper chambers were removed, and the Transwells were washed twice with PBS. Migrated cells were fixed with methanol, stained with Giemsa, and photographed under a microscope. Cells in the photographs were counted using Image J software. This experiment was performed three times.
Invasion assay
Twenty-four hours after transfection, 2 × 104 cells in 100 μl of medium containing 5 % FBS were seeded into the upper chambers of Matrigel-coated Transwells placed in 24-well plates. Then 600 μl medium containing 20 % FBS, with or without VEGF, was added to the lower chambers. The concentration of VEGF was 20 ng/mL. Cells grouping was the same as above. After 24 h of culturing, unmigrated cells left in the upper chambers were removed, and the Transwells were washed twice with PBS. Migrated cells were fixed with methanol, stained with Giemsa, and photographed under a microscope. Cells in the photographs were counted using Image J software. This experiment was performed three times.
Real-time quantitative RT-PCR (qRT-PCR)
Total RNA was extracted from cells in accordance with the manufacturer′s instructions and reverse-transcribed into cDNA. Primers used for PCR were: VEGFR2: forward 5′-GGACTCTCTCTGCCTACCTCAC-3′, reverse 5′-GGCTCTTTCGCTTACTGTTCTG-3′; GAPDH: forward 5′-AGAAGGCTGGGGCTCATTTG-3′, reverse 5′-AGGGGCCATCCACAGTCTTC-3′. The real-time PCR program was: 95 °C 30 s, 40 cycles of 95 °C 5 s, 58 °C 10 s, and 72 °C 30 s, followed by melting curve analysis. The relative levels of VEGFR2 mRNA were calculated using the 2−ΔΔCt method using GAPDH as an internal reference gene. This experiment was performed three times.
Western blot analysis
Total protein was extracted from cells with RIPA buffer (supplemented with PMSF protease inhibitor and phosphatase inhibitors). Denatured proteins were separated using electrophoresis and then transferred onto PVDF membranes. Concentrations of the gels were selected according to the molecular weight of the protein to be detected. The membranes were blocked with 5 % BSA for 1 h and then incubated with the primary antibodies (1:1000) to VEGFR2, P-VEGFR2, HIF-1α, Akt, P-Akt, ERK1/2, P-ERK1/2, p38, or P-p38 at 4 °C overnight. The membranes were then washed three times with PBST (10 min each), incubated with secondary antibodies (1:1000) for 1 h at room temperature, and washed again with PBST. The secondary antibodies were conjugated with alkaline phosphatase and appropriate substrate was used for color development. This experiment was performed three times.
Flow cytometry analysis of cell apoptosis following radiation
Forty-eight hours after transfection, cells were irradiated in a Siemens accelerator at a dose of 2 GY (gantry angle 180°, 1 cm thick dosage compensation pads were placed underneath the culture plates). The cells were fed with fresh medium with or without VEGF and further cultured in a 37 °C incubator for 24 h. The concentration of VEGF was 20 ng/mL. For apoptosis analysis, cells were washed with cold PBS twice and dissociated with an EDTA-free trypsin solution. Cells were collected and washed twice with cold PBS (cells were centrifuged at 1500 rpm for 5 min after each wash). Cells were resuspended with 100 μl Annexin V/FITC binding buffer, and then 5 μl Annexin V-FITC and PI were added to stain the cells at 4 °C in the dark for 30 min, after which 400 μl of binding buffer was added and cells were analyzed by flow cytometry. This experiment was performed three times.
Statistical analysis
Statistical analysis was performed with SPSS 16.0. All data are presented as mean ± standard deviation (x ± s). Comparison of the means across multiple samples was performed with single factor analysis of variance; comparison between two groups was conducted with the two sample t test; correlation analysis was conducted with two-factor correlation analysis. P < 0.05 was designated as statistically significant.
Results
RNA interference reduced VEGFR2 mRNA and protein levels in Calu-1 cells
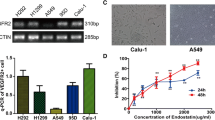
Eight hours after siRNA transfection, examination with a fluorescence microscope showed that the transfection rate reached about 80 % (Fig. 1a, b). VEGFR2 mRNA and protein levels in Calu-1 cells were measured by qRT-PCR and Western blot assays, respectively. Twenty-four hours after siRNA transfection, VEGFR2 mRNA level decreased significantly (F = 103.429, P < 0.01) (Fig. 1c, d). By 48 h after siRNA transfection, VEGFR2 protein level was also significantly decreased (F = 39.095, P < 0.01) (Fig. 1e, f).
RNA interference of VEGFR2 reduced VEGFR2 gene and protein levels in Calu-1 cells. a Phase contrast and b fluorescence images of cells 8 h after transfection. c, d qRT-PCR analysis showed that VEGFR2 mRNA levels were significantly lower (P < 0.01) 24 h after transfection. e, f VEGFR2 protein levels were significantly lower (P < 0.01) 48 h after transfection. *P < 0.05; **P < 0.01, n = 3
Knockdown of VEGFR2 reduced the proliferation, migration, and invasion of Calu-1 cells
The CCK8 assay was used to determine the number of viable cells and monitor cell proliferation. There was significantly less Calu-1 cell proliferation at 24 and 48 h after VEGFR2 siRNA transfection, with a slightly greater effect at 48 h. VEGF induced the proliferation of Calu-1 cells (F = 88.720, P < 0.05), but this effect was diminished by VEGFR2 knockdown (Fig. 2a).
VEGFR2 knockdown inhibited Calu-1 cell proliferation, migration, and invasion. The CCK8 assay, and Transwell assay were conducted to assess (a) cell proliferation, (b, c) migration, and (d, e) invasion. The concentration of VEGF was 20 ng/mL. a 24 h and 48 h after transfection, cells in the VEGFR2 knockdown group showed significantly less growth than cells in the control group (P < 0.05 and P < 0.05 for 24 and 48 h, respectively). (b, c) There was less cell migration in VEGFR2 knockdown cells than in control cells (P < 0.05). (d, e) There was less cell invasion in VEGFR2 knockdown cells than in the control cells (P < 0.05). *P < 0.05; **P < 0.01, n = 3
The Transwell assay was performed to determine cell migration. VEGFR2 knockdown inhibited cell migration significantly. VEGF induced the migration of cells with VEGFR2 knockdown slightly, but this effect was not statistically significant. However, VEGF stimulated migration in control cells significantly (F = 23.749, P < 0.05) (Fig. 2b, c).
In the cell invasion assay, cells with VEGFR2 knockdown displayed significantly less invasiveness, even in the presence of VEGF. However, VEGF was able to stimulate the invasion of the control cells (F = 21.917, P < 0.05) (Fig. 2d, e).
VEGFR2 knockdown inhibited the activity of VEGFR2 and its downstream signaling proteins in Calu-1 cells
Western blot assays were conducted to detect VEGFR2 and its downstream signaling proteins. VEGFR2 knockdown decreased the levels of VEGFR2 and p-VEGFR2 in Calu-1 cells significantly (F = 39.095 and 73.875; P < 0.01 and 0.01 for VEGFR2 and p-VEGFR2, respectively). The levels of total AKT, extracellular regulated protein kinase (ERK) 1/2, and p38 mitogen-activated protein kinase (MAPK) proteins were not changed by VEGFR2 knockdown, but the phosphorylation of all these proteins was significantly lower, albeit to various extent (F = 46.603, 48.701, and 5.739; P < 0.05, 0.01, and 0.05 for p-AKT, p-ERK1/2, and p-p38, respectively). VEGF stimulation induced VEGFR2 expression and the phosphorylation of AKT, ERK1/2, and p38 in control cells but did not change their total levels. In VEGFR2 knockdown cells, VEGF only slightly increased the phosphorylation of AKT, ERK1/2, and p38, but the effect did not reach statistical significance (Fig. 3). In addition, after VEGFR2 knockdown, hypoxia-inducible factor 1 α (HIF-1α) protein level was significantly lower (F = 25.501, P < 0.05). The level of HIF-1α protein positively correlated with VEGFR2 protein level (r = 0.955, P < 0.01) (Fig. 4b, c).
Phosphorylation of VEGFR2 and its downstream signaling proteins was reduced by VEGFR2 knockdown in Calu-1 cells. 48 h after transfection, western blot assays were performed to detect VEGFR2 and its downstream proteins. The concentration of VEGF was 20 ng/mL. In VEGFR2 knockdown cells, the levels of VEGFR2 and P-VEGFR2 were significantly decreased (P < 0.01, 0.01 for VEGFR2 and P-VEGFR2, respectively). The corresponding phosphoproteins protein levels of AKT, ERK1/2, and p38 were reduced to various extents (P < 0.05, 0.01, 0.05 for P-AKT, P-ERK1/2, and P-P38, respectively). *P < 0.05; **P < 0.01, n = 3
VEGFR2 knockdown exacerbated radiation-induced apoptosis in Calu-1 cells. 48 h after transfection, cells were irradiated in a Siemens accelerator at a dose of 2 GY. The cells were fed with fresh medium with or without VEGF and further cultured in a 37 °C incubator for 24 h. The concentration of VEGF was 20 ng/mL. a, b Cells were analyzed by flow cytometry to detect apoptotic cells. VEGFR2 knockdown cells had a higher rate of apoptosis rate than control cells (P < 0.01); VEGF stimulation did not alter the apoptosis rate in VEGFR2 knockdown cells but reduced apoptosis in control cells (P < 0.05). c, d There was significantly less HIF-1α protein in VEGFR2 knockdown cells (P < 0.01). *P < 0.05; **P < 0.01, n = 3
VEGFR2 gene knockdown exacerbated radiation-induced apoptosis in Calu-1 cells
Flow cytometry was performed to determine the percentage of apoptotic cells after radiation. VEGFR2 knockdown cells showed more apoptosis after radiation than control cells, even in the presence of VEGF (t = 32.83, P < 0.05). VEGF treatment prevented radiation-induced apoptosis in control cells (t = 18.55, P < 0.01) (Fig. 4a, b).
Discussion
In this study, blocking the expression of VEGFR2 inhibited proliferation, migration, and invasion in Calu-1 cells. Similar findings have been reported [11]. Adham et al. reported that VEGFR2 gene knockdown in ovarian cancer cells led to accelerated cell proliferation due to elevated expression of NRP-1 and VEGF [12]. The discrepancy between their study and the current study suggests that VEGFR2 plays cell type-specific roles.
To further understand the mechanism underlying VEGFR2's function, we analyzed the expression and activity of downstream signaling proteins. After VEGFR2 knockdown, the levels of total AKT, ERK1/2, and P38 were not changed, but their levels of phosphorylation were significantly lower. This was consistent with the role of VEGFR2 as a tyrosine kinase. ERK1/2 (also known as p44/42 MAPK) and p38 MAPK are both important members of the MAPK family. They become activated upon phosphorylation. ERK1/2 is involved in the regulation of cell proliferation [13], and p38 MAPK regulates cell migration and invasion [14]. Similar results have been reported in neuroblastoma cells [15] and in liver cancer cells [11].
Cell apoptosis analysis showed that Calu-1 cells with VEGFR2 knockdown underwent more cell death after radiation. Similar findings reported by Shi, miR-200c increases the radiosensitivity of NSCLC cell line A549 by targeting VEGFR2 [16]. In addition, Hypoxia-inducible factor 1α(HIF-1α), unlike other VEGFR2 downstream proteins, showed significantly reduced protein levels.
HIF-1α is induced throughout the body during hypoxia. It promotes tumor invasion and resistance to radiotherapy and chemotherapy [17]. However, recent studies have reported that HIF-1α was stably expressed in tumor cells under normoxic conditions, and its expression could lead to increased radioresistance [18]. But silencing or blocking HIF-1α could increase radiosensitivity in A549 cells [19] and esophageal cancer cells [20]. Nilsson et al. reported that a number of tyrosine kinase receptors (VEGFR2 not evaluated) can affect the expression of HIF-1α in neuroblastoma cells [21]. It is therefore possible that VEGFR2 controls radioresistance of Calu-1 cells by regulating the expression of HIF-1α.
Alvarez et al. found that through growth factor-mediated activation of the phosphatidylinositol 3-kinase (PI3 K) pathway, tumor cells could express HIF-1α at high levels even in normoxia [22]. These growth factors activated their respective tyrosine kinase receptors (RTKs, such as VEGFR2) by bound to them, which in turn activated PI3 K and mammalian target of rapamycin (mTOR). mTOR then upregulated HIF-1α protein levels in cells under normoxic conditions. Hofer et al. reported that MAPK prevented the phosphorylation of serine residues in HIF-1α, thereby inducing the binding of HIF-1α to the transcription cofactor CBP/P300 and enhanced their transcriptional activity [23]. Befani et al. showed that the PI3 K/AKT pathway regulated HIF-1α expression at the transcriptional level and MAPK (ERK1/2) regulated HIF-1α expression at the protein level [24]. It is possible that VEGFR2 also regulates HIF-1α expression in Calu-1 cells through the PI3 K/AKT and MAPK pathways.
According to the above results, the research suggests that gene silencing VEGFR2 expression not only can inhibit the neovascularization of tumor, but also can inhibit the proliferation, migration and invasion ability of calu-1 cells, reduce the expression of HIF-1α, thereby possibly improving hypoxia and enhancing the radiosensitivity in tumor.
Because a transient siRNA transfection method was used to knock down VEGFR2, the level of VEGFR2 in Calu-1 cells could not remain low for a prolonged period of time. For this reason, it was not possible to obtain cell survival curves following radiation. Future experiments should involve lentivirus-packaged siRNA to knock down VEGFR2 and create an animal model of VEGFR2 knockdown. This would facilitate further study of the function of VEGFR2 in tumor cells. These studies may provide new ideas for treating NSCLC.
Abbreviations
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
- HIF-1α:
-
Hypoxia-inducible factor-1α
- VEGF:
-
Vascular endothelial growth factor
References
Ahmed K, Emran AA, Jesmin T, Mukti RF, Rahman MZ, Ahmed F. Early detection of lung cancer risk using data mining. Asian Pac J Cancer Prev. 2013;14(1):595–8.
de Jong WK, Schaapveld M, Blaauwgeers JL, Groen HJ. Pulmonary tumours in the Netherlands: focus on temporal trends in histology and stage and on rare tumours. Thorax. 2008;63(12):1096–102.
Gressen EL, Curran WJ. Hyperfractionated radiotherapy for lung cancer. Curr Oncol Rep. 2000;2(1):71–5.
Chi A, Liao Z, Nguyen NP. Xu j, Stea B, Komaki R. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol. 2010;94(1):1–11.
Terman BI, Carrion ME, Kovacs E, Rasmussen BA, Eddy RL, Shows TB. Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene. 1991;6(9):1677–83.
Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull. 2011;34(12):1785–8.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62.
Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, Angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–63.
Moeller BJ, Dewhirst MW. HIF-1 and tumour radiosensitivity. Br J Cancer. 2006;95(1):1–5.
Jiang XD, Ding MH, Qiao Y, Liu Y, Liu L. Study on lung cancer cells expressing VEGFR2 and the impact on the effect of RHES combined with radiotherapy in the treatment of brain metastases. Clin Lung Cancer. 2014;15(2):e23–9.
Xiang QF, Chen WQ, Ren M, Wang JN, Zhang HW, Deng David YB, et al. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin Cancer Res. 2014;20(11):2959–70.
Adham SA, Sher I, Coomber BL. Molecular blockade of VEGFR2 in human epithelial ovarian carcinoma cells. Lab Invest. 2010;90(5):709–23.
Sun Chris K, Man K, Ng Kevin T, Ho Joanna W, Lim Zophia X, Cheng Q. Proline-rich tyrosine kinase 2 (Pyk2) promotes proliferation and invasiveness of hepatocellular carcinoma cells through c-Src/ERK activation. Carcinogenesis. 2008; 29(11): 2096–105.
Ghosh S, Kumar A, Tripathi RP, Chandna S. Connexin-43 regulates p38-mediated cell migration and invasion induced selectively in tumour cells by low doses of γ-radiation in an ERK-1/2-independent manner. Carcinogenesis. 2014;35(2):383–95.
Gomes E, Rockwell P. p38 MAPK as a negative regulator of VEGF/VEGFR2 signaling pathway in serum deprived human SK-N-SH neuroblastoma cells. Neurosci Lett. 2008;431(2):95–100.
Shi L, Zhang S, Wu H, Zhang L, Dai X, Hu J, Xue J, Liu T, Liang Y, Wu G. MiR-200c increases the radiosensitivity of non-small-cell lung cancer cell line A549 by targeting VEGF-VEGFR2 pathway. PLoS One. 2013;8(10):e78344.
Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58(7):1408–16.
Shachar I, Cohen S, Marom A, Becker HS. Regulation of CLL survival by hypoxia-inducible factor and its target genes. FEBS Lett. 2012;586(18):2906–10.
Li J, Song X, YU J. Analysis of radiosensitivity of A549 lung cancer cells with HIF-1 silencing by RNAi. Chinese Journal of Radiological Medicine and Protection. 2008, 28(2):120-3.
Yang X, Yang B, Cai J, Zhang C, Zhang Q, Xu L, et al. Berberine enhances radiosensitivity of esophageal squamous cancer by targeting HIF-1α in vitro and in vivo. Cancer Biol Ther. 2013;14(11):1068–73.
Nilsson MB, Zage PE, Zeng L, Xu L, Cascone T, Wu HK, et al. Multiple receptor tyrosine kinases regulate HIF-1alpha and HIF-2alpha in normoxia and hypoxia in neuroblastoma: implications for antiangiogenic mechanisms of multikinase inhibitors. Oncogene. 2010;29(20):2938–49.
Alvarez TM, Alfranca A, Aragonés J, Vara A, Landázuri MO, del PL. Lack of evidence for the involvement of the phosphoinositide 3-kinase/Akt pathway in the activation of hypoxia-inducible factors by low oxygen tension. J Biol Chem. 2002;277(16):13508–17.
Hofer T, Desbaillets I, Höpfl G, Gassmann M, Wenger RH. Dissecting hypoxia-dependent and hypoxia-independent steps in the HIF-1alpha activation cascade: implications for HIF-1alpha gene therapy. FASEB J. 2001;15(14):2715–7.
Befani CD, Vlachostergios PJ, Hatzidaki E, Patrikidou A, Bonanou S, Somos G. Bortezomib represses HIF-1α protein expression and nuclear accumulation by inhibiting both PI3 K/AKT/TOR and MAPK pathways in prostate cancer cells. J Mol Med (Berl). 2012;90(1):45–54.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NO. 81472792), Research Fund from Ministry of Health (W201210), and the National Natural Science Foundation of Jiangsu Province (BK2012661).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Our study did not refer to clinical trial and animal trial, so there was no ethics statement.
Conflict of interest
None.
Additional information
Y. Liu, Y. Qiao and C. Hu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, Y., Qiao, Y., Hu, C. et al. VEGFR2 inhibition by RNA interference affects cell proliferation, migration, invasion, and response to radiation in Calu-1 cells. Clin Transl Oncol 18, 212–219 (2016). https://doi.org/10.1007/s12094-015-1358-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1358-z