Abstract
Background
The application of newer signaling pathway-targeted agents has become an important addition to chemotherapy in the treatment of advanced non-small cell lung cancer (NSCLC). In this study, we evaluated the efficacy and toxicities of PKC inhibitors combined with chemotherapy versus chemotherapy alone for patients with advanced NSCLC systematically.
Patients and materials
Literature retrieval, trials selection and assessment, data collection, and statistic analysis were performed according to the Cochrane Handbook 5.1.0. The outcome measures were tumor response rate, disease control rate, progression-free survival (PFS), overall survival (OS), and adverse effects.
Results
Five randomized controlled trials, comprising totally 1,005 patients, were included in this study. Meta-analysis showed significantly decreased response rate (RR 0.79; 95 % CI 0.64–0.99) and disease control rate (RR 0.90; 95 % CI 0.82–0.99) in PKC inhibitors-chemotherapy groups versus chemotherapy groups. There was no significant difference between the two treatment groups regarding progression-free survival (PFS, HR 1.05; 95 % CI 0.91–1.22) and overall survival (OS, HR 1.00; 95 % CI 0.86–1.16). The risk of grade 3/4 neutropenia, leucopenia, and thrombosis/embolism increased significantly in PKC inhibitors combination groups as compared with chemotherapy alone groups.
Conclusion
The use of PKC inhibitors in addition to chemotherapy was not a valid alternative for patients with advanced NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide [1]. Approximately 85 % of patients with lung cancer have non-small cell lung cancer (NSCLC), three-quarters of who present with locally advanced or metastatic disease at the time of diagnosis [2]. Treatment approaches to advanced and metastatic NSCLC vary depending on patients’ life expectancy, overall status, tumor characteristics, and patients’ preference. Platinum-based doublet is the backbone of the first-line treatment for advanced NSCLC, but the response rate is only less than 40 %, and a plateau in efficacy has been reached [3]. The addition of newer signaling pathway-targeted agents to current therapies may improve the clinical efficacy of existing treatments.
Protein kinases C (PKC) are a family of serine/threonine kinases, which are involved in cell proliferation, differentiation, and apoptosis [4]. PKC overexpression and increased activity have been reported to be linked to many cancers including NSCLC [4]. They affect tumor formation and progression by virtue of their function as tumor promoters that enhance the signaling cascade which influences the fate of tumor cells [5]. Thus, inhibition of specific isoenzymes of PKC may be a reasonable approach to achieve therapeutic benefit. Within the last decade, several small molecular weight inhibitors and antisense molecules have been developed, targeting the calcium-dependent classical isoforms of PKC, for the treatment of NSCLC, such as bryostatin I [6], UCN-01 [7, 8], PKC412 [9], LY90003 [10], LY317615 [11], aurothiomalate (ATM) [12], and et al.
Preclinical experiments showed that PKC inhibitors could potentiate the antitumor effects of many cytotoxic agents [13]. Enzastaurin (LY317615), an oral PKC-β inhibitor, combined with pemetrexed demonstrated synergistic inhibition of NSCLC cell growth [14]. In clinical studies, the combination of PKC inhibitors and platinum-based regimens was well tolerated and showed promising antitumor activities in the treatment of NSCLC [15, 16]. A phase I/II trial of aprinocarsen (LY90003, a PKC-α antisense oligonucleotides) in combination with gemcitabine and cisplatin demonstrated a response rate of 33 % and stable disease of 53 % in patients with advanced NSCLC [16]. To date, several randomized controlled trials have been published regarding the clinical efficacy and toxicities of PKC inhibitors combined with various kinds of chemotherapeutic regimens versus chemotherapy alone for advanced NSCLC, but they have not reached a final conclusion systematically. Therefore, we performed a meta-analysis to determine the outcomes of synthetic clinical effect of PKC inhibitors combined with cytotoxic drugs in the treatment of advanced NSCLC.
Methods
Literature retrieval
Electronic database PubMed, EMBASE, and the Cochrane Library were searched using the following search strategy: substance name term ‘7-hydroxystaurosporine’ or keywords ‘UCN-01, Staurosporine, PKC inhibitor’; OR substance name term ‘PKC412’ or keywords ‘Staurosporine, PKC inhibitor’; OR substance name term ‘aprinocarsen’ or keywords ‘PKC alpha inhibitor, phosphorothioate oligonucleotides, oligonucleotides antisense’; OR substance name term ‘enzastaurin’ or keywords ‘LY317615.HCl, PKC beta inhibitor’; OR substance name term ‘lestaurtinib’ or keywords ‘CEP-701, KT-5555, PKC inhibitor; OR substance name term ‘ruboxistaurin’ or keywords ‘LY-333531, arrxant, PKC beta inhibitor’; OR substance name term ‘gold sodium thiomalate’ or keywords ‘sodium aurothiomalate, gold thiomalic acid, aurothiomalate, PKC iota inhibitor’; AND MeSH term ‘lung neoplasms’ or keywords ‘lung tumor*, lung neoplasm*, lung cancer*’. Conference abstracts presented at major meetings including ASCO, ESMO, European Cancer Organization (ECCO), and World Congress on Lung Cancer (WCLC) were also searched. Elementary results were limited by clinical trials in human beings published in English.
Inclusion and exclusion criteria
Studies meeting the following criteria were considered for inclusion: randomized clinical trials (RCTs) published as articles that compared PKC inhibitors plus chemotherapy with chemotherapy alone for patients with recurrent or metastatic NSCLC. When trials were published reduplicatively, only the complete or the most recent ones were included.
Data extraction
The following information was extracted in order to understand the baseline of each included study: regimens, number of patients, age, histology, region, performance status of patients, stage of disease, line of treatment, and previous treatments as well. The following items were regarded as clinical endpoints in our analysis: survival endpoints in terms of tumor response rate, disease control rate, progression-free survival (PFS), and overall survival (OS); toxicity endpoints in terms of grade 3/4 hematologic laboratory abnormalities and grade 3/4 general non-hematologic toxicities. The grade of toxicities was assessed by Common Toxicity Criteria v2.0 of National Cancer Institute.
Quality assessment
The risk of bias in each trial was assessed according to Cochrane methodology, considering randomization, allocation concealment, blinding, loss of follow-up, dropout, and other biases. Overall quality of RCTs was graded as A, B, or C as follows: A, Minimization of bias in all categories above; B, each of the criteria in A was partially met; and C, one or more of the criteria in A was not met. Once high risk of bias was established, individual study characteristics were examined or subgroup analyses were performed to explain potential causes.
Statistical methods
Statistical analysis was performed, and forest plots were generated using version 5.3 of Review Manager. Risk ratios (RR) and their 95 % confidence intervals (CIs) were calculated for tumor response rate, disease control rate, and side effects endpoints as dichotomous outcomes. Hazard ratios (HR) were summarized, and their corresponding standard errors were computed to analyze the time-to-event data as generic inverse variance outcomes. Statistical heterogeneity between studies was assessed by means of Cochrane’s χ 2 statistic and the extent of inconsistency with I 2 statistic. We considered I 2 < 50 % as low-level heterogeneity, while I 2 > 50 % as significant heterogeneity. A fixed-effect model was used for calculations of the summary estimates unless significant (I 2 > 50 %) heterogeneity existed, in which case a random-effect model was used after possible reasons of the heterogeneity were explored.
Results
Study selection
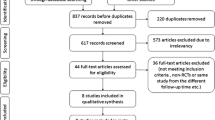
The literature search yielded 33 potentially assessable publications. Of these, 27 were excluded for the following reasons: phase I studies, single-arm studies, retrospective studies, pilot studies, and reviews. After reviewing the remaining six trials, we excluded one trial because of maintenance treatment with PKC inhibitor. Finally, five relevant RCTs, comprising a total of 1,005 patients, were included [10, 11, 17–19]. The five RCTs contained the following two PKC inhibitors: enzastaurin (an inhibitor of PKC-β) and aprinocarsen (a PKC-α antisense oligonucleotide). The characteristics and qualities of included trials are shown in Tables 1 and 2.
Response rate and disease control rate
The response rates ranged from 3.9 to 28.9 % in PKC inhibitors-chemotherapy groups versus 2.6 to 34.9 % in chemotherapy groups. The fixed-effect model evaluation (χ 2 = 1.77; P = 0.78; I 2 = 0 %), including totally 917 patients, showed a decreased response rate in PKC inhibitors-chemotherapy groups versus chemotherapy groups (RR 0.79; 95 % CI 0.64–0.99) (Fig. 1a).
Forest plots and statistics for the efficacy endpoints. a Forest plot of risk ratio of total response rate (fixed-effect model); b forest plot of risk ratio of total disease control rate (fixed-effect model); c forest plot of hazard ratio of total median PFS (fixed-effect model); and d forest plot of hazard ratio of total median OS (fixed-effect model)
The disease control rate ranged from 49.4 to 85.0 % in PKC inhibitors-chemotherapy groups versus 48.7–100 % in chemotherapy groups. The fixed-effect model evaluation (χ 2 = 2.66; P = 0.62; I 2 = 0 %), including totally 919 patients, demonstrated a decreased disease control rate in PKC inhibitors combination groups as compared with chemotherapy alone groups (RR 0.90; 95 % CI 0.82–0.99) (Fig. 1b).
These results indicated that the combination of PKC inhibitor and chemotherapy was associated with a statistically significant decreased response rate and disease control rate as compared with chemotherapy alone.
PFS
The median PFS time ranged between 3.0 and 5.5 months in PKC inhibitors-chemotherapy groups versus 2.3 and 5.8 months in chemotherapy groups. The comparison showed a similar risk of disease progression (HR 1.05; 95 % CI 0.91–1.22) between the two treatment groups in the fixed-effect model (χ 2 = 0.16; P = 0.93; I 2 = 0 %) meta-analysis (Fig. 1c).
This result indicated that the combination of PKC inhibitors and chemotherapy could not improve the median PFS as compared with chemotherapy alone groups.
OS
The median OS ranged between 4.3 and 10.0 months in PKC inhibitor-based groups versus 5.1 and 10.4 months in placebo groups. The fixed-effect model (χ 2 = 2.33; P = 0.51; I 2 = 0 %) analysis demonstrated that there was no significant difference regarding OS between the two treatment groups (HR 1.00; 95 % CI 0.86–1.16) (Fig. 1d).
This result indicated that there was no significant difference regarding OS between the PKC inhibitors-chemotherapy and chemotherapy groups.
Adverse effects
The following grade 3/4 adverse events were more frequent in PKC inhibitor-chemotherapy groups than chemotherapy alone groups by meta-analysis: neutropenia, leucopenia, and thrombosis/embolism. No significant difference could be found between the two treatment groups with regard to febrile neutropenia, thrombocytopenia, anemia, nausea and vomiting, arorexia, and fatigue. Table 3 lists the results of the meta-analysis of grade 3/4 adverse events. In addition, limited evidence showed that the risk of grade 3/4 epistaxis was higher in PKC inhibitor-chemotherapy group (5.9 %) than chemotherapy alone group (1.2 %) (P = 0.022) [19]. Incidence of renal failure, hypokalemia, weight loss, hypertension, and diarrhea was similar between PKC inhibitors-chemotherapy groups (0.6, 2.8, 1.9, 12.5, 4.2 %) and chemotherapy groups (1.2, 0.6, 0.6, 22.2, 1.4 %) [11, 18, 19].
Discussion
Combining existing lung cancer therapies with novel agents that interfere with major signaling pathways is a promising approach in the treatment of metastatic NSCLC. There is an increasing evidence that targeting specific isoenzymes of PKC may enhance the effect of cytotoxic drugs in preclinical models of NSCLC. However, clinical benefits of PKC inhibitors combined with current therapies in the treatment of NSCLC are controversial. In the prospective randomized trials presented here, we found significantly decreased response rate (RR 0.79; 95 % CI 0.64–0.99) and disease control rate (RR 0.90; 95 % CI 0.82–0.99) in PKC inhibitors-chemotherapy groups, as compared with chemotherapy alone groups. The efficacy endpoints PFS (HR 1.05; 95 % CI 0.91–1.22) and OS (HR 1.00; 95 % CI 0.86–1.16) were similar between the two therapeutic groups after comparison. There are several potential explanations for the inefficacy of PKC inhibitors here. First, it is possible that the addition of PKC inhibitors can improve the therapeutic efficacy in a subset of NSCLC patients bearing specific biomarkers. Unfortunately, the predictive biomarkers for the sensitivity of PKC inhibitors have not been screened and identified in all the included trials. It has been postulated that the elevated levels of PKC-α and PKC-β and the high phosphorylation state of PKC-β downstream molecules may be associated with the efficacy of PKC inhibitors [20, 21]. In addition, more reports indicated that the expression levels of u-PAR or cyclin D1 might also be predictive of the response to PKC-β inhibitor enzastaurin [22, 23]. Moreover, overexpression of PKCι has been identified in lung squamous carcinoma. PKCι signals to downstream pathways, such as Mek-Erk and Hh, which crosstalk with each other, regulating tumor initiation. ATM is a highly selective inhibitor of PKCι that exhibits potent antitumor activity of lung cancer. Expression levels of PKCι and its downstream molecules, such as Par6 and Hh acyltransferase, will be useful in identifying lung cancer patients most likely to respond to ATM therapy [24, 25]. However, all the promising candidate biomarkers mentioned still need to be validated through clinical studies. Second, the decreased response rate and disease control rate in the meta-analysis indicated that the interaction between cytotoxic drugs and PKC inhibitors may also exist.
Our analysis indicated that patients with NSCLC were not likely to benefit from PKC inhibitors in addition to platinum-based therapies. This result is consistent with a number of clinical studies demonstrating similar principle, namely combined therapy with targeted agents and chemotherapy results in negative response, with the exception of antiangiogenic agents, in the treatment of NSCLC. For example, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors, such as erlotinib and gefitinib, or EGFR antibody, such as cetuximab, combined with chemotherapy could not improve the efficacy of NSCLC even in patients with specific markers [26]. However, treatment of antiangiogenic agents, such as bevacizumab and cediranib, which target signaling pathways in tumor angiogenesis, in combination with chemotherapy, has been reported to be more efficacious than individual monotherapies for the treatment of NSCLC in several clinical trials [27].
Our toxicity analysis demonstrated that patients receiving PKC inhibitors experienced high risk of neutropenia, leucopenia, and thrombosis/embolism. Not surprisingly, the addition of PKC inhibitors could prominently increase the risk of thrombosis and embolism because PKC isoenzymes play important roles in the formation of thrombus [28]. Furthermore, inhibiting PKC-β, which is a component of the vascular endothelial growth factor (VEGF) signaling pathway, could reduce VEGF-induced endothelial regeneration, thus predisposing to thromboembolism [13, 28]. However, the incidence of neutropenia and leucopenia was also significant in PKC inhibitors-chemotherapy groups, which suggests that PKC inhibitors may enhance toxicities of the cytotoxic drugs in the chemotherapy regimens for NSCLC. Furthermore, the results of toxicities have to be clarified cautiously because it was only for thrombosis/embolism, febrile neutropenia, anemia, nausea and vomiting, and fatigue that the test for heterogeneity was I 2 = 0 %, demonstrating a small intertrial variation for these toxicities. For thrombocytopenia and arorexia, the test of heterogeneity was much higher with I 2 > 50 %, which indicates a large intertrial variability for the analysis.
Nevertheless, the results of our toxicity analysis were also partial influenced by the chemotherapeutic regimens that were used. Different chemotherapy regimens for advanced NSCLC may cause different distributions of toxicities. Gemcitabine/cisplatin regimen demonstrated more prominent thrombocytopenia, anemia, and renal toxicity, whereas pemetrexed-based regimen was more prone to inducing neutropenia, anemia, and fatigue [29, 30]. Toxicities introduced by chemotherapeutic regimens should be considered when analyzing the toxicities of the combined therapies. The pooled results of toxicities in our study demonstrated that the adverse events of combined PKC inhibitors with chemotherapy did not just resemble the safety profile of chemotherapeutic regimens with addition of typical adverse effects associated with PKC inhibitors; the interaction between PKC inhibitors and cytotoxic drugs may also augment or weaken the manifestations of some toxic effects.
The number of the included trials here is relatively small. The small number of trials, as well as low quality of most trials, might not allow for a reliable conclusion. Of the five included studies, two studies adopted the method of blinding [17, 18], whereas the other two did not and one was unclear. Without double blinding, high-performance bias and measuring bias may appear. All the included trials mentioned randomization and described their method of randomization. No trials reported adequate concealment of allocation of the outcome assessments, which might bring selective bias in these trials. Loss of follow-up during the study period, which may bring attrition bias, was not mentioned in all the included trials. Publication bias might also exist. In addition, most of the data included was from Europe and the USA; the efficiency and tolerability of PKC inhibitors and cytotoxic drugs for NSCLC may differ across the races and regions. More data from Asian, Africa, and Oceania are still needed to determine the outcomes in the future.
In conclusion, our findings demonstrated that the combination treatments of PKC inhibitors and chemotherapy were associated with significantly decreased response rate/disease control rate and similar PFS/OS in patients with advanced NSCLC. Thus, the use of PKC inhibitors in addition to chemotherapy is not a valid alternative for patients with advanced NSCLC at present. However, several early-phase clinical trials of newer inhibitors of PKC, such as PKCι inhibitor ATM, are ongoing for the treatment of NSCLC. Therefore, the efficiency of PKC inhibitors still need to be confirmed by more large-sample, multicenter, randomized, controlled trials.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
D’Addario G, Fruh M, Reck M, Baumann P, Klepetko W, Felip E, et al. Metastatic non-small-cell lung cancer. ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii56–vii64.
Soon YY, Stockler MR, Askie LM, Boyer MJ. Duration of chemotherapy for advanced non-small-cell lung cancer: a systematic review and meta-analysis of randomized trials. J Clin Oncol. 2009;27(20):3277–83.
Clark AS, West KA, Blumberg PM, Dennis PA. Altered protein kinase C (PKC) isoforms in non-small cell lung cancer cells: PKCdelta promotes cellular survival and chemotherapeutic resistance. Cancer Res. 2003;63(4):780–6.
Martiny-Baron G, Fabbro D. Classical PKC isoforms in cancer. Pharmacol Res. 2007;55(6):477–86.
Winegarden JD, Mauer AM, Gajewski TF, Hoffman PC, Krauss S, Rudin CM, et al. A phase II study of bryostatin-1 and paclitaxel in patients with advanced non-small cell lung cancer. Lung Cancer. 2003;39(2):191–6.
Edelman MJ, Bauer KS Jr, Wu S, Smith R, Bisacia S, Dancey J. Phase I and pharmacokinetic study of 7-hydroxystaurosporine and carboplatin in advanced solid tumors. Clin Cancer Res. 2007;13(9):2667–74.
Mack PC, Gandara DR, Lau AH, Lara PN Jr, Edelman MJ, Gumerlock PH. Cell cycle-dependent potentiation of cisplatin by UCN-01 in non-small-cell lung carcinoma. Cancer Chemother Pharmacol. 2003;51(4):337–48.
Monnerat C, Henriksson R, Le Chevalier T, Novello S, Berthaud P, Faivre S, et al. Phase I study of PKC412 (N-benzoyl-staurosporine), a novel oral protein kinase C inhibitor, combined with gemcitabine and cisplatin in patients with non-small-cell lung cancer. Ann Oncol. 2004;15(2):316–23.
Vansteenkiste J, Canon JL, Riska H, Pirker R, Peterson P, John W, et al. Randomized phase II evaluation of aprinocarsen in combination with gemcitabine and cisplatin for patients with advanced/metastatic non-small cell lung cancer. Invest New Drugs. 2005;23(3):263–9.
Socinski MA, Raju RN, Stinchcombe T, Kocs DM, Couch LS, Barrera D, et al. Randomized, phase II trial of pemetrexed and carboplatin with or without enzastaurin versus docetaxel and carboplatin as first-line treatment of patients with stage IIIB/IV non-small-cell lung cancer. J Thorac Oncol. 2010;5(12):1963–9.
Mansfield AS, Fields AP, Jatoi A, Qi Y, Adjei AA, Erlichman C, et al. Phase I dose escalation study of the PKCι inhibitor aurothiomalate for advanced non-small-cell lung cancer, ovarian cancer, and pancreatic cancer. Anticancer Drugs. 2013;24(10):1079–83.
Nakajima E, Helfrich B, Chan D, Zhang Z, Hirsch FR, Chen V, et al. Enzastaurin, a protein kinase C-beta-selective inhibitor, inhibits the growth of SCLC and NSCLC cell lines. J Clin Oncol. 2006;24:612s (abstract 13138).
Giovannetti E, Honeywell R, Hanauske AR, Tekle C, Kuenen B, Sigmond J, et al. Pharmacological aspects of the enzastaurin-pemetrexed combination in non-small cell lung cancer (NSCLC). Curr Drug Targets. 2010;11(1):12–28.
Carducci MA, Musib L, Kies MS, Pili R, Truong M, Brahmer JR, et al. Phase I dose escalation and pharmacokinetic study of enzastaurin, an oral protein kinase C beta inhibitor, in patients with advanced cancer. J Clin Oncol. 2006;24:4092–9.
Villalona-Calero MA, Ritch P, Figueroa JA, Otterson GA, Belt R, Dow E, et al. A phase I/II study of LY900003, an antisense inhibitor of protein kinase C-alpha, in combination with cisplatin and gemcitabine in patients with advanced non-small cell lung cancer. Clin Cancer Res. 2004;10:6086–93.
Casey EM, Harb W, Bradford D, Bufill J, Nattam S, Patel J, et al. Randomized, double-blinded, multicenter, phase II study of pemetrexed, carboplat in, and bevacizumab with enzastaurin or placebo in chemonaïve patients with stage IIIB/IV non-small cell lung cancer: Hoosier Oncology Group LUN06-116. J Thorac Oncol. 2010;5(11):1815–20.
Chiappori A, Bepler G, Barlesi F, Soria JC, Reck M, Bearz A, et al. Phase II, double-blinded, randomized study of enzastaurin plus pemetrexed as second-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2010;5(3):369–75.
Paz-Ares L, Douillard JY, Koralewski P, Manegold C, Smit EF, Reyes JM, et al. Phase III study of gemcitabine and cisplatin with or without aprinocarsen, a protein kinase C-alpha antisense oligonucleotide, in patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2006;24(9):1428–34.
Lee SH, Chen T, Zhou J, Hofmann J, Bepler G. Protein kinase C-beta gene variants, pathway activation, and enzastaurin activity in lung cancer. Clin Lung Cancer. 2010;11:169–75.
Ritch P, Rudin CM, Bitran JD, Edelman MJ, Makalinao A, Irwin D, et al. Phase II study of PKC-alpha antisense oligonucleotide aprinocarsen in combination with gemcitabine and carboplatin in patients with advanced non-small cell lung cancer. Lung Cancer. 2006;52(2):173–80.
Körner A, Mudduluru G, Manegold C, Allgayer H. Enzastaurin inhibits invasion and metastasis in lung cancer by diverse molecules. Br J Cancer. 2010;103(6):802–11.
Kuo WL, Liu J, Mauceri H, Vokes EE, Weichselbaum R, Rosner MR, et al. Efficacy of the multi-kinase inhibitor enzastaurin is dependent on cellular signaling context. Mol Cancer Ther. 2010;9(10):2814–24.
Regala RP, Thompson EA, Fields AP. Atypical protein kinase C iota expression and aurothiomalate sensitivity in human lung cancer cells. Cancer Res. 2008;68(14):5888–95.
Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, Fields AP. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell. 2014;25(2):139–51.
Kim ES, Neubauer M, Cohn A, Schwartzberg L, Garbo L, Caton J, et al. Docetaxed or pemetrexed with of without cetuximab in recurrent or progressive non-small cell lung cancer after platinum-based therapy: a phase 3, open-label, randomised trial. Lancet Oncol. 2013;14(13):1326–36.
Dy GK, Mandrekar SJ, Nelson GD, Meyers JP, Adjei AA, Ross HJ, et al. A randomized phase II study of gemcitabine and carboplatin with or without cediranib as first-line therapy in advanced non–small-cell lung cancer, north central cancer treatment group study N0528. J Thorac Oncol. 2013;8(1):79–88.
Harper MT, Poole AW. Diverse functions of protein kinase C isoforms in platelet activation and thrombus formation. J Thromb Haemost. 2010;8(3):454–62.
Fisher MD, D’Orazio A. Phase II and III trials: comparison of four chemotherapy regimens in advanced non small-cell lung cancer (ECOG 1594). Clin Lung Cancer. 2000;2(1):21–2.
Gridelli C, de Marinis F, Pujol JL, Reck M, Ramlau R, Parente B, et al. Safety, resource use, and quality of life in paramount: a phase III study of maintenance pemetrexed versus placebo after induction pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol. 2012;7(11):1713–21.
Acknowledgments
This study was supported by the Grants from Tianjin Medical University Science Foundation (No. 2012kyq16), Application Foundation and Front Technology Research Program of Tianjin (No. 13JCQNJC12500), High School Science and Technology Fund Planning Project of Tianjin (No. 20130112).
Conflict of interest
All authors declare no conflict of interest of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L.L., Cao, F.F., Wang, Y. et al. The protein kinase C (PKC) inhibitors combined with chemotherapy in the treatment of advanced non-small cell lung cancer: meta-analysis of randomized controlled trials. Clin Transl Oncol 17, 371–377 (2015). https://doi.org/10.1007/s12094-014-1241-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-014-1241-3