Abstract
Objective
To explore the expression profile of miRNAs during differentiation of rat hepatic oval cells (HOCs) into hepatocellular carcinoma cells (HCC).
Methods
Proliferation of rat HOCs was induced by chemical carcinogen, 3′-methyl-4-dimethylaminoazobenzene (3′-Me-DAB) in male rats. By using Percoll density gradient centrifugation method, HOCs were isolated, followed by continuous cultivation in vitro. The isolated HOCs were identified via Thy-1 and C-kit detection under laser scanning confocal microscope. Total miRNA was then extracted from HOCs during cell differentiation for microarray hybridization. Differentially expressed miRNAs among the indicated time points were identified. The target genes of identified miRNAs were predicted using PicTar, Target-Scan, and miRanda; then the functions and pathways of the genes were enriched. Y chromosome-specific polymerase chain reaction (PCR) technique was utilized to trace the differentiation of the male HOCs in carcinogen-induced HCC of female rats.
Results
It was shown that isolated HOCs expressed stem cells markers of Thy-1 and C-kit in cytoplasm and membrane. Among 1,210 miRNAs identified, 22 were differentially expressed (P < 0.05, fold change ≥2), including 19 up-regulated and 3 down-regulated ones. The predicted target genes of these miRNAs were enriched in several functions, including axon guidance, angiogenesis, post-transcriptional protein modification, and small molecular metabolism. For PCR-based SRY detection, HCC genomic DNA of female rats from the experimental group displayed the same PCR product as that from normal male rat.
Conclusion
Differentially expressed miRNAs exerted important roles during the differentiation process of HOCs to HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MicroRNA (miRNA) is a small 21–22 nucleotide non-coding RNA molecule found in plants, animals, and some viruses, which functions via base pairing with complementary sequences within mRNA molecules, resulting in translation blocking or mRNA degradation [1]. Since the first discovery of miRNA deregulation in chronic lymphocytic leukemia [2], many miRNAs have been found to be related to several types of cancer. Lower level of miR-324a in non-small-cell lung carcinoma samples is a prognostic indicator of poor survival [3]. High miR-185 or low miR-133b levels are correlated with metastasis in colorectal cancer [4]. Recently, growing evidences show that a small subpopulation of cells in cancers possess stem cell properties, called cancer stem cells [5], and cancer cells are derived from cancer stem cells. Emerging evidence indicates that certain abnormal miRNA expression levels could trigger the dysregulation of cancer stem cell, resulting in unlimited self-renewal and cancer progression [6]. With high expression level in cancer stem cells of colonic adenoma and tumor, miR-135a and miR-135b are involved in the activation of Wnt signaling pathway by regulating the APC expression [7]. The let-7 expression is absent in the breast cancer stem cells, resulting in high HMGA2 expression level [8–10]. HMGA2 is functionally important for self-renewing of breast cancer stem cells. The low expression of miR-34 in pancreatic cancer could cause activated Bcl-2, HMGA2, and Notch signaling pathways, which inhibits stem cell functions [11].
Hepatocellular carcinoma (HCC) is the third cause of cancer-related death [12]. Hepatic oval cells (HOCs) are progenitor cell population in adult liver. Under chronic liver disease or hepatotoxin administration, oval cells would migrate from the biliary tree to the damaged parenchyma, giving rise to hepatocytes and biliary epithelial cells. On the other hand, HOCs have a close link with hepatocarcinogenesis [13, 14]. After isolation and hypodermic inoculation, the proliferated HOCs from p53−/− mice fed a choline-free and ethionine-supplemented diet progressed into tumors similar to HCC, indicating that a subset of HCC could have originated from HOCs [15]. Investigation with Solt–Farber HCC model showed that HOCs expressing Gpc3 were closely related to HCC [16].
However, there is limited report on miRNA expression profiling during the differentiation of HOCs into HCC. Through microarray technology, we profiled miRNAs expression pattern among several indicated time points of the differentiation period. Among 1,210 miRNAs, 19 were up-regulated and 3 were down-regulated. Our results may provide comprehensive insights for the functional study of miRNA regulation in HCC cancer stem cell differentiation.
Materials and methods
Laboratory animals
Sixty Wistar rats (SPF, 20 females, 40 males, 120 ± 20 g, animal certificate number: 2002-2009, 2004B023, 2004A068) were provided by the Laboratory Animal Center of Southern Medical University. Rats were fed a regular diet and raised at 28 °C and 40–70 % humidity.
Separation and purification of differentiated HOC
Incised livers from the 3′-methyl-4-dimethylaminoazobenzene group (3′-Me-DAB, Tokyo Chemical Industry Co., Ltd) (rats were fed a diet containing 0.06 % 3′-Me-DAB for 28 days, n = 30) were washed twice with icy D-Hank’s buffer (Weijia Technologies, Co., Ltd, Guangzhou) and minced into 0.1 cm × 0.1 cm explants, which were then digested with 10 ml buffer [0.1 % collagenase (Sigma) and 0.025 % EDTA-DMEM (Gibco)] at 37 °C for 15 min. Sequentially, the upper liquid was filtered through a 100-mesh strainer, while the leftover tissue was digested twice as above. The hepatic cell pellet was obtained by two rounds of centrifugation (600 rpm for 5 min and 1,400 rpm for 10 min) at 4 °C. HOCs were purified from the separated hepatic cells by Percoll density gradient centrifugation [17] and cultured in DMEM/F12 (HyClone) containing 10 % fetal bovine serum (Weijia Technologies, Co., Ltd) and 0.2 % gelatin at 37 °C and 5 % CO2. The HOC viability was determined by 0.25 % Trypan blue staining (Gibco).
Identification of HOC using laser scanning confocal microscope
HOCs growing on glass coverslips were washed three times with 0.01 M phosphate buffer solution (PBS) for 1 min. Cells were then fixed in 4 % PFA at 4 °C for 15 min, followed by washing three times (0.01 M PBS for 3 min). Cells were then incubated with first antibodies [Thy-1 monoclonal antibody (mAb) (Santa Cruz, USA) and C-kit mAb (Santa Cruz, USA)] and FITC-labeled second Ab (ZhongshanJinqiao Biotechnology Co., Ltd., Beijing), consecutively. The washed slides were mounted at room temperature and stored in dark. Image capture, merging and analysis for FITC-labeled Ab signal were performed under laser scanning confocal microscope (LSCM) (excitation 488 nM, emission 504 nM). A green signal in cytoplasm and membrane was considered as positive. Passage 1 (P1), P2, P4, P6, P8, and P10 HOC were used for FITC–LSCM observation. FITC–LSCM detection and miRNA extraction were conducted every five passages since the 10th passage.
Malignant HOC tracing via HOC inoculation in rats
Twenty normally raised Wistar female rats (fed a regular diet) were injected with 25 mg/kg nembutal for intraperitoneal anesthesia and then divided into experimental and control groups. The newly isolated HOC suspension from male rats fed 3′-Me-DAB (106 cells per spot) was inoculated underneath the liver capsule of the rats of the experimental group. No injection was performed on the control group. To promote tumor growth, the two groups of Wistar rats were fed a diet containing 0.06 % 3′-Me-DAB for 14 weeks. 0.1 mg/(kg d) tarcrolimus (Astellas Pharma Inc.) was administrated to inhibit immunologic rejection.
miRNA extraction and labeling
A total of 19 passages of 3′-Me-DAB induced HOCs (P1, P2, P4, P6, P8, P10, and every 5th passage after P10) were harvested for total RNA extraction with one-step TRIzol method. RNAs were condensed with iso-propanol precipitating method and quantified using spectrophotometer. The total RNA quality was evaluated with formaldehyde denaturing gel electrophoresis. mirVana™ miRNA Isolation Kit (Ambion, USA) was used for miRNA extraction. The miRNA samples passing electrophoresis quality inspection (Agilent Bioanalyzer 2100) were labeled for microarray analysis.
Microarray analysis
miRNA Complete Labeling and Hyb Kit (Agilent) was used for miRNA labeling and hybridization. The labeled miRNA samples from both 3′-Me-DAB induced and normal HOCs were hybridized with Agilent Rat miRNA chip [Agilent rat miRNA (8*15 K)V16.0 Kit, encompassing 676 rat miRNAs probes]. The hybridization signal was scanned with Agilent Microarray Scanner (Agilent) and chip data were analyzed with Feature Extraction software 10.7 (Agilent). The raw data were subjected to preprocessing, background signal removal, and median normalization, respectively. The mean signal value of the four probes for each miRNA was designated as the miRNA abundance and used for Si/N ratio calculation. T test was used for statistical comparison of miRNA level between two samples. Up-regulated miRNAs (ratio >2, P < 0.001) and down-regulated miRNAs (ratio <0.5, P < 0.001) were selected.
Real-time quantitative PCR (qPCR)
RNA samples extracted from P56 3′-Me-DAB-induced HOCs and normal HOCs were reverse-transcribed into cDNA according to the user’ manual (Reverse transcription kit: Fermantas). SYBR Green real-time qPCR kit from Bio-TOYOBO Company was used for two-step amplification. The qPCR reaction was conducted in a 10 μl system containing 5 μl 2× SYBR Real-time PCR Pre-mixture, 0.3 μl forward primer (10 μM), 0.3 μl reverse primer (10 μM), cDNA 2 μl, and dH2O 2.4 μl. The PCR program was: 95 °C pre-denaturation for 2 min, 95 °C denaturation for 15 s, 55 °C annealing at 25 s and 30 cycles. A melting curve was generated between 65 and 95 °C. miRNA primers were purchased from Yingrun Biology Corporation. For qPCR data analysis: \( \Delta C_{\text{t}} = C_{{{\text{t}}_{{_{\text{miRNA}} }} }} - C_{{{\text{t}}_{{_{\text{U6}} }} }} \) . \( \Delta \Delta C_{\text{t}} = \Delta C_{{{\text{t}}_{{_{\text{P56}} }} }} - \Delta C_{{{\text{t}}_{\text{control}} }} \). Fold changes = \( 2^{{ - \Delta \Delta C_{\text{t}} }} \). The primer sequences for qPCR are listed in Table 1.
Bio-informatics analysis
The target genes in the noncoding regions for most of the differentially expressed miRNAs were predicted using PicTar, Target-Scan, and miRanda software, respectively. The software of miRGator was used for the target genes prediction for the rest of the miRNAs. For each miRNA, only genes predicated by each of the software were designated as target genes. GO analysis and pathway analysis were performed for the identified target genes. The target gene number of each pathway was used for hypergeometric distribution analysis and the P values indicating target gene enrichment in a particular pathway were calculated to find out the related biological pathway. A P value <0.05 and a false positive rate (FDR) <0.05 were considered as statistically significant.
PCR-based male rat HOC tracing
Sex-determining region of the Y (SRY) is the dominant sex-determining gene located on the short arm of the Y chromosome [18]. PCR-based SRY detection method was utilized to trace the HCC cells originating from male Wistar rat HOCs. The induced HCC tissues of female rats from both experimental and control groups were used for DNA extraction (genomic DNA extraction kit: Dingguo Biotech, Co., Ltd, Beijing). SRY primers were designed as SRY 68F: 5′AGGCGCAAGTTGGCTCAAC3′ and SRY 168R: 5′GGCCTTTTTTCGGCTTCTG3′. The PCR reaction was performed in a 25 μl system containing 5 μl 5× PCR buffer, 0.5 μl 10 mM dNTP, 1 μl SRY 68F, 1 μl SRY 68R, 0.15 μl Taq DNA polymerase (1.667 × 108 nkat/l), 1 μl genomic DNA, and 16.35 μl dH2O. The PCR program was: 93 °C pre-denaturation for 3 min, 93 °C denaturation for 30 s, 55 °C annealing for 25 s, 72 °C elongation for 45 s, 30 cycles, and a final 72 °C elongation for 10 min. For DNA electrophoresis (20 g/l agarose gel), 8 μl PCR product was used. The DNA image was captured under ultraviolet light.
Statistical analysis
All data were expressed as mean ± SD. SPSS17.0 was used for statistical analysis. For chip analysis, T test was used for pairwise comparison.
Results
HOC identification and morphology observation
The FITC–LSCM results showed that the isolated HOCs displayed the stem cell markers Thy-1 and C-kit in both cytoplasm and membrane (Fig. 1a, b), which indicated that the HOCs in our system were qualified. Under normal growth conditions, the HOCs were polygon, oval, or round in shape (Fig. 1c, d). There was continuous growth of HOCs during the in vitro culturing period (Fig. 1c–f). More cytoplasm was found in cells from later passages. HOCs gradually differentiated into round HCC cells and slender spindle-like bile duct cells (Fig. 1e, f).
HOC identification and morphology observation. a, b Isolated HOCs expressing stem cell markers Thy-1 and C-kit. Images were captured under LSCM [×400 power field (PF)]. c–f Cell shape observation under optical microscope. c Freshly isolated HOCs (×400 PF). d HOCs 10 h post-isolation (×100 PF). e Cultured HOCs 10 weeks post-isolation (×100 PF). f Cultured HOCs 15 weeks post-isolation (×400 PF). The red arrows indicate bile duct cells, while the blue arrows indicate HCCs
Microarray hybridization and analysis
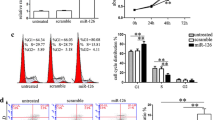
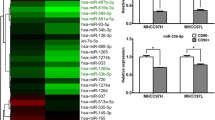
All the RNA samples underwent purity and integrity inspection. The ultraviolet spectrophotometer results showed that A260/280 of all RNA samples fell in the range of 1.89–2.02. For each RNA sample, 18S and 28S bands were clearly seen from the gel electrophoresis image (Fig. 2a). RNA samples with high purity and integrity were used for microarray analysis. The representative miRNA chip hybridization images showed evenly distributed fluorescent signal, full signal spots, and low background, which indicated ideal hybridization quality. The chip images were loaded into Feature Extraction software 10.7 for data reading and 1,210 miRNAs were identified. The data were furthered analyzed by Gene Spring Software 11.0 and 22 differentially expressed miRNAs were obtained (P ≤ 0.001, fold change ≥2), of which 19 were up-regulated and 3 were down-regulated (Fig. 2b). To confirm the microarray data, five representative miRNAs were chosen for qPCR validation and U6 was utilized as internal reference. The qPCR results showed that compared with control normal HOCs, the P56 3′-Me-DAB-induced HOCs displayed elevated expression of has-miR-302, has-miR-92b, and has-miR-195 and down-regulated has-miR-214 and has-miR-18 (Fig. 2c), consistent with the microarray data.
Differentially expressed miRNAs identification during differentiation of HOCs into HCCs. a Gel electrophoresis image for total RNA sample. b Cluster analysis for 22 differentially expressed miRNAs. The red and green signals indicate down- and up-regulations, respectively. c Validation of microarray results by qPCR
Biological informatics analysis
Based on Sanger database, 1,295 target genes were predicted for the 22 differentially expressed miRNAs. The target genes have been annotated in the Gene Ontology database. Based on their biological functions, these target genes have been classified into several groups, including axon guidance, angiogenesis, post-transcriptional protein modification, small molecule metabolism, O-glycan processing, insulin receptor signal transduction pathway, hypoxia response, and so on (Fig. 3). The target genes of the differentially expressed miRNAs were also subjected to pathway annotation in the KEGG database. The related pathways are cancer pathway, focal adhesion, hedgehog signaling pathway, MAPK pathway, O-oligosaccharide biosynthesis, insulin signaling pathway, endocytosis, adhesion connection, ECM receptor interaction, steroid biosynthesis, and so on.
The classification of target genes based on biological functions. The circle sectors represent the target genes, and the functions shared by certain gene sets are marked outside the sectors. The colors changing from ENPEP to SLITRK4 are used to sort the target genes according to the regulatory miRNAs
PCR-based SRY gene tracing
The capacity of the isolated HOCs to develop in vivo into HCC was validated in a rat model. All the female Wistar rats from both experimental and control groups developed nodular or cauliflower tumor after 14 weeks of 3′-Me-DAB treatment. For PCR-based SRY detection, HCC genomic DNA of female rats from the experimental group displayed the same PCR product as that of genomic DNA from normal male rat (Fig. 4), indicating that the HCC of the experimental group originated from injected HOCs. HCC genomic DNA of the control female group generated different DNA lanes from normal male rats (Fig. 4).
PCR-based SRY tracing demonstrated that HCC cells of female rats of the experimental group were derived from inoculated male HOCs. Maker 100 bp DNA ladder, ranging from 100 to 2,000 bp. M gDNA from normal male rats, F gDNA of HCC cells from the control female group, T1, T2, and T3 are gDNAs of HCC cells from the experimental female group
Discussion
Cancer stem cells have been reported in many human tumors and are thought to be responsible for tumor initiation, progression, metastasis, therapy resistance, and relapse. miRNA plays critical roles in normal stem cell functions during developmental events [19, 20] and are also important regulators of cancer stem cells [21]. To unveil the expression profile of miRNAs during the differentiation of rat HOCs to HCC, total miRNAs were isolated from HOCs induced by 3′-Me-DAB for miRNA chip hybridization. At least 22 miRNAs were found differentially expressed and 1,295 corresponding target genes were predicted. Our findings are strong evidences for the close link between cancer stem cell and miRNAs network.
During the transformation of HOC to HCC, we identified 19 up-regulated and 3 down-regulated miRNAs, including miR-302, miR-92b, miR-195, miR-214, and miR-139. miR-302 could reprogram human skin cancer cells into a pluripotent ES-cell-like state [22]. It is also reported that Oct4/Sox2 could promote the expression of miRNA-302 leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells [23]. A former report indicates that the miR-92b is overexpressed during hepatocarcinogenesis, resulting in enhanced proliferation and differentiation arrest of hepatic progenitors by targeting CCAAT/enhancer binding protein beta [24]. Our results showed elevated expression of miR-302 and miR-92b, which is consistent with previous reports. An interesting research in human embryonic stem cells (hESC) showed that miR-195, whose target is WEE1, a negative G2/M kinase modulator of the CycB/CDK complex, regulates hESC cell proliferation [25]. miR-195 was reported to suppress tumorigenity and regulate G1/S transition of human HCCs [26]. Our miRNA profiling data also showed high level of miR-195 during the transformation of HOCs to HCCs. Future work should be undertaken to dissect the function of miR-195 in cell cycle regulation of cancer stem cells.
In our study, miR-214 and miR-139 were found to be down-regulated during the transition of HOCs to HCCs. The down-regulation of miR-214 in HCC has been reported [27]: miR-214 regulates β-catenin pathways directly to suppress invasion, stem-like traits, and recurrence of human HCC [28]. miR-139 was reported to suppress metastasis and progression in HCC and colorectal cancer by targeting Rho-kinase 2 [29] and type I insulin-like growth factor receptor [30], respectively. Our findings provide novel clues for the functional roles of miR-214 and miR-139 in cancer stem cell development.
The target genes and their biological functions of the differentially expressed miRNAs were explored in this study; however, detailed functional studies for these miRNAs in HCC tumorigenesis are needed. Besides, although rat (Rattus rattus) is a mammalian animal model, the differentially expressed miRNAs obtained during the transformation process of HOC to HCC cell in rat system need to be validated in human cells. The spatio-temporal expression pattern of different miRNAs is important for us to understand the miRNA regulatory network in cancer development.
Nakanishi et al. [31] developed a relatively complicated and time-consuming method, tamoxifen-induced Cre-mediated cell tracking strategy, to trace the differentiation of progenitor cells. Y chromosome is a special male structure which is stable in spite of time and cell morphology changes [32]. A previous report utilized the described Y chromosome fluorescence in situ hybridization method for bone marrow-derived stem cell tracing [33]. In this study, PCR-based SRY detection method was utilized to trace the HCC cells originating from the male Wistar rat HOCs. It is simple and straightforward. HCC genomic DNA of female rats of the experimental group displayed the same PCR product as that of normal male rats, which indicates that HOC is capable of differentiating into HCC cells.
In conclusion, we identified 22 differentially expressed miRNAs in the differentiation process of rat HOCs to HCC cells. This in vivo study proved that HOCs identified from mutagen fed male rats have the potential of developing HCC cells in female rats. The target genes of these miRNAs were also predicted and subjected for bio-informatics analysis. Our finding revealed the relationships between miRNAs and cancer stem cells, which might be beneficial for the finding of novel therapeutic targets for HCC.
References
Khare S, Zhang Q, Ibdah JA. Epigenetics of hepatocellular carcinoma: role of microRNA. World J Gastroenterol. 2013;19(33):5439.
Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353(17):1793–801.
Shi Y, Liu C, Liu X, Tang DG, Wang J. The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC) growth and the CD44hi stem-like NSCLC cells. PLoS One. 2014;9(3):e90022.
Özata DM, Xie H, Lindforss U, Olivecrona H, Lui W-O. miR-185 and miR-133b deregulation is associated with overall survival and metastasis in colorectal cancer. Int J Oncol. 2011;39:311–8.
Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol. 2012;44(12):2144–51.
Xing F, Wu K, Watabe K. MicroRNAs in cancer stem cells: new regulators of stemness. Curr Pharm Des. 2014;28:28.
Nagel R, le Sage C, Diosdado B, van der Waal M, Vrielink JAO, Bolijn A, et al. Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Cancer Res. 2008;68(14):5795–802.
Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315(5818):1576–9.
Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21(9):1025–30.
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131(6):1109–23.
Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4(8):e6816.
Wong R, Frenette C. Updates in the management of hepatocellular carcinoma. Gastroenterol Hepatol. 2011;7(1):16.
Mishra L, Banker T, Murray J, Byers S, Thenappan A, He AR, et al. Liver stem cells and hepatocellular carcinoma. Hepatology. 2009;49(1):318–29.
Sell S. On the stem cell origin of cancer. Am J Pathol. 2010;176(6):2584–94.
Dumble ML, Croager EJ, Yeoh GC, Quail EA. Generation and characterization of p53 null transformed hepatic progenitor cells: oval cells give rise to hepatocellular carcinoma. Carcinogenesis. 2002;23(3):435–45.
Grozdanov PN, Yovchev MI, Dabeva MD. The oncofetal protein glypican-3 is a novel marker of hepatic progenitor/oval cells. Lab Invest. 2006;86(12):1272–84.
Sirica AE, Cihla HP. Isolation and partial characterizations of oval and hyperplastic bile ductular cell-enriched populations from the livers of carcinogen and noncarcinogen-treated rats. Cancer Res. 1984;44(8):3454–66.
Sinclair A, Berta P, Palmer M, Hawkins J, Griffiths B, Palmer M. A gene from the human sex-determining region encodes a protein with. Nature. 1990;346(6281):240–4.
Eisenreich A, Leppert U. The impact of microRNAs on the regulation of tissue factor biology. Trends Cardiovasc Med. 2013;24(3):129–32.
Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–79.
DeSano JT, Xu L. MicroRNA regulation of cancer stem cells and therapeutic implications. AAPS J. 2009;11(4):682–92.
Lin S-L, Chang DC, Chang-Lin S, Lin C-H, Wu DT, Chen DT, et al. Mir-302 reprograms human skin cancer cells into a pluripotent ES-cell-like state. RNA. 2008;14(10):2115–24.
Bourguignon LY, Wong G, Earle C, Chen L. Hyaluronan-CD44v3 interaction with Oct4–Sox2–Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem. 2012;287(39):32800–24.
Qian N-S, Liu W-H, Lv W-P, Xiang X, Su M, Raut V, et al. Upregulated MicroRNA-92b regulates the differentiation and proliferation of EpCAM-positive fetal liver cells by targeting C/EBPß. PLoS One. 2013;8(8):e68004.
Qi J, Yu J-Y, Shcherbata HR, Mathieu J, Wang AJ, Seal S, et al. microRNAs regulate human embryonic stem cell division. Cell Cycle (Georgetown, Tex). 2009;8(22):3729.
Xu T, Zhu Y, Xiong Y, Ge YY, Yun JP, Zhuang SM. MicroRNA-195 suppresses tumorigenicity and regulates G1/S transition of human hepatocellular carcinoma cells. Hepatology. 2009;50(1):113–21.
Li W, Xie L, He X, Li J, Tu K, Wei L, et al. Diagnostic and prognostic implications of microRNAs in human hepatocellular carcinoma. Int J Cancer. 2008;123(7):1616–22.
Xia H, Ooi LLP, Hui KM. MiR-214 targets β-catenin pathway to suppress invasion, stem-like traits and recurrence of human hepatocellular carcinoma. PLoS One. 2012;7(9):e44206.
Wong CCL, Wong CM, Tung EKK, Au SLK, Lee JMF, Poon RTP, et al. The microRNA miR-139 suppresses metastasis and progression of hepatocellular carcinoma by down-regulating Rho-kinase 2. Gastroenterology. 2011;140(1):322–31.
Shen K, Liang Q, Xu K, Cui D, Jiang L, Yin P, et al. MiR-139 inhibits invasion and metastasis of colorectal cancer by targeting the type I insulin-like growth factor receptor. Biochem Pharmacol. 2012;84(3):320–30.
Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet. 2013;45(1):98–103.
Jangravi Z, Alikhani M, Arefnezhad B, Sharifi Tabar M, Taleahmad S, Karamzadeh R, et al. A fresh look at the male-specific region of the human Y chromosome. J Proteome Res. 2012;12(1):6–22.
Eglitis MA, Mezey É. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci. 1997;94(8):4080–5.
Acknowledgments
This project was supported by Grants from the Natural Science Foundation of Hainan Province (No. 309063); the National Natural Science Foundation of China (No. 81260349).
Conflict of interest
The authors have declared that no competing interests exist.
Author information
Authors and Affiliations
Corresponding authors
Additional information
R. Xu and L. Zheng have contributed equally to this work.
The Publisher and Editor retract this article in accordance with the recommendations of the Committee on Publication Ethics (COPE). After a thorough investigation we have strong reason to believe that the peer review process was compromised.
About this article
Cite this article
Xu, R.H., Zheng, L.Y., He, D.L. et al. RETRACTED ARTICLE: Profiling of differentially expressed microRNAs (miRNAs) during differentiation of rat hepatic oval cells (HOCs) into hepatocellular carcinoma (HCC) cells. Clin Transl Oncol 17, 230–237 (2015). https://doi.org/10.1007/s12094-014-1218-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-014-1218-2