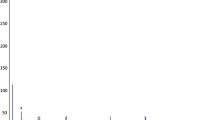Abstract
The present investigation is focused on the study of chemical composition of a bioactive compound derived from a rumen isolate Paracoccus pantotrophus FMR19 using GC–MS and to find out the antibacterial activity of the extracted crude bioactive compounds against multidrug resistant organisms (MDROs) and other clinical pathogens. GC–MS analysis revealed that P. pantotrophus FMR19 produced eight major compounds that have been reported to exhibit antimicrobial property. The main components identified from hexane fraction are long chain alkanes, fatty alcohols, fatty acid methyl ester and aromatic hydrocarbons. These molecules are not only active against clinical pathogens such as Salmonella sp. and Proteus sp. and also effective against MDROs such as Metallo β lactamase and Pan drug resistant bacterial strains and Methicillin resistant Staphylococcus aureus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bioactive compounds produced by plants and microbes exhibit pharmacological or toxicological effects in man and animals. Bacteria have been considered as one of the significant groups of microorganisms due to their ability to produce a wide array of secondary metabolites, such as antibiotics, antitumor agents, immunosuppressive agents, cosmetics, vitamins, nutritional materials, herbicides, pesticides, anti-parasitic agents and enzymes [1–3]. Around 23,000 bioactive secondary metabolites produced by microorganisms have been reported.
Several novel bioactive compounds have also been discovered from aquatic Actinomycetes [4, 5]: rifamycin, (Micromonospora sp.) [6]; salinosporamide-A, an anticancer metabolite (Salinispora sp.) [7]; marinomycins (Marinophilus sp.) [8]; abyssomicin-C (Verrucosispora sp.) and marino pyrroles (Streptomyces sp.) [6, 9]. The appearances of multidrug-resistant pathogenic strains caused substantial morbidity and mortality especially among the elderly and immune-compromised patients [10]. To overcome this situation, there is a need to improve or discover a novel class of antibiotics and antimicrobial compounds that have different mechanisms of action worldwide [11].
There have been a number of reports on the antibacterial activities of long-chain fatty alcohols [6]. It has been reported, the activity increases with the length of the carbon chain [12] and the water/octanol partition coefficient has been identified as an important determinant of activity [8, 13]. Other studies indicated that appropriate length of the carbon chain determines the effective activity [14, 15]. However, there is no consensus about the precise length of the carbon chain for the potential activity.
In India, approximately 1,50,000 tonnes of offals in the form of rawhide trimmings, limed animal fleshing, green animal fleshing, hide splits and chrome shavings are disposed, that are not utilized or underutilized thus creating a solid waste disposal problem in tanneries [16]. In anaerobic bacterial fermentation, the proteins and amino acids derived from degradation of animal fleshing results in branched chain fatty acid, accompanied by potentially important metabolites such as amines, phenolic compounds, and volatile sulfur compounds.
The present work focuses on the utilization of animal fleshing (ANFL), as a substrate for the microbial production of the bioactive compounds. The ability of microorganisms to grow and produce an appreciable level of bioactive compounds, using ANFL as substrate could offer tremendous potential for the development of biotechnological methods for the rapid hydrolysis of ANFL. The produced crude bioactive preparation was characterized using GC–MS analysis and used as an antibacterial agent against multidrug resistant organisms (MDROs) and clinical pathogens.
Materials and Methods
Isolation of Rumen Microbes
The rumen fluid collected from the slaughter house was filtered through double layer muslin cloth. One ml of filtrate was serially diluted and cultured by spread plate using Hungate’s medium (HiMedia) under anaerobic conditions. The isolated microbe was identified as Paracoccus pantotrophus and the 16S rRNA sequence obtained was submitted to NCBI GenBank with the assigned number as JX012237.
Production of Bioactive Compound
The rumen bacterial strain was inoculated in anaerobic basal medium namely Hungate’s broth containing the composition (g/L): (KH2PO4—0.02 g; K2HPO4—0.03 g; MgSO4—0.01 g; CaCl2—0.01 g; (NH4)2SO4—0.1 g; NaCl—0.1 g; Cysteine HCl—0.02 g; NaHCO3—0.5 g; resazurin—0.0001 g; cellulose—1.0 g; Trace element-1 ml/l). In addition to that, the medium was supported with 1:4 ratio of cellulose and leather industry solid waste animal flesh and incubated for 9 days at 37 °C to achieve the better degradation of animal flesh and thereby the high yield of bioactive compounds. The samples were centrifuged at 10,000×g in 10 min and the cell free supernatant was collected and extracted with chloroform. The hexane fraction that showed good antibacterial effect was subjected to GC–MS analysis to identify the nature of compounds. The chloroform was removed using rotary evaporator under reduced pressure to get crude extract and the crude extract was further purified by column chromatography. A glass column of 50 cm height and 3 cm diameter with silica gel (100–200 Mesh size) was used for the purification of the crude extract. The admixture is loaded at top of the column and the fractions were eluted using non polar solvents (hexane or ethyl acetate).
Antibacterial Bioassay
The antibacterial activity of isolated fractions was checked against clinical pathogens such as Proteus sp., Salmonella sp., Salmonella paratyphi A, Salmonella paratyphi B and Staphylococcus aureus. Activity was also tested against MDROs such as Metallo β-lactamase resistant organism (MBL)-Pseudomonas aeruginosa, Pan drug resistant organism (PDR)—Klebsiella pneumoniae and Methicillin Resistant S. aureus (MRSA). These cultures were obtained from Sri Muthukumaran Medical College, Chennai. The multidrug resistance was confirmed by agar well diffusion method. Briefly, the bacterial lawn (10−8 CFU/ml) of the each test organism was prepared in Mueller–Hinton agar plate. The agar plates were allowed to dry and wells of 10 mm were made with a sterile cork borer on the inoculated agar plates. 10 µg of the active fraction in Dimethyl sulfoxide (DMSO) was added into the well and the DMSO was used as control. The plates were incubated at 37 °C for 24 h and observed for the zone of inhibition around the wells and the zone of inhibition was measured using an antibiotic zone scale (Himedia, Mumbai).
GC–MS Analysis of the Crude Bioactive Compound in Hexane Fraction
The crude bioactive hexane fraction was subjected to GC–MS analysis (Fig. 1) and the conditions used for the GC–MS analysis are presented in Table 1. The spectrum of the crude component was compared with the spectrum of the known components in the National Institute Standard and Technology (NIST) library [17]. The name, molecular formula, weight and chemical structure of the components of the test materials were identified.
Results and Discussion
Production of Antibacterial Compound and GC–MS Analysis
Paracoccus pantotrophus FMR19 produced eight major compounds, identified from hexane fraction by GC–MS analysis as long chain alkanes [E-15 Heptadecanol], fatty alcohols [n-Nonadecanol-1, 7.956 %; n-Tridecan-1-ol, 6.861 %; n-tetracosanol-1, 4.641 %; 1-Heptacosanol, 2.738 %], fatty acid methyl ester [Octadecanoic acid, 9,10-dichloro-, methyl ester, 4.166 %] and aromatic hydrocarbon [2,6-bis(1,1-dimethylethyl)phenol, 1.115 %] and other minor compounds are also represented in Table 2. These major compounds may be responsible for antibacterial activity [6, 7, 12, 15].
Retention time at 65.818 min corresponds to the compound E-15-Heptadecenol with peak area 10.555 %. Long chain alkanes such as Hexadecane have also been reported to have antibacterial and antioxidant activities [15].
The n-Nonadecanol-1 compound appeared at 75.674 min with a peak area 7.956 % and has been proven to have cytotoxic property and antibacterial activity [1]. The compound 1-Heptacosanol appeared at 100.572 min with a peak area of 2.738 % are reported to have antimicrobial and antioxidant activity [2].
The crude bioactive compound produced in the study contains fatty acid methyl esters. Only few studies have been reported that the crude bioactive compound showed the antimicrobial activity against Bacillus subtilis and Sarcina lutea, but there is no antimicrobial activity against the growth of S. aureus and Pectobacterium carotovorum at a concentration of 2000 μg/ml. Whereas the compound extracted in our study showed antibacterial activity against S. aureus (4 mm) proving to be even more effective at a concentration of 10 μg/ml. Fatty acid methyl esters from microalgae were also reported to possess antimicrobial properties [18, 19]. Also, few aromatic hydrocarbons are shown to have antibacterial activity against the microbial pathogens [20, 21]. Hence, all those components eluted in hexane might be attributed to the antimicrobial activity of the MDROs and clinical pathogens.
Antibacterial Activity of the Bioactive Molecule Extracted in Hexane
The clinical pathogens Proteus sp. (7 mm), Salmonella sp. (5 mm), Pseudomonas aeruginosa (5 mm), Salmonella paratyphi-B (6 mm), S. aureus (4 mm), Salmonella paratyphi-A (5 mm) showed zone of inhibition. The bioactive hexane fraction showed 8, 7 and 4 mm zones of inhibition against metallo β lactamase resistant (MBL) bacterial strain, pan-drug resistant (PDR) bacterial strain and methicillin-resistant S. aureus (MRSA) respectively. The results showed that the hexane fraction is more active against MDROs than the clinical pathogens (Figs. 2, 3).
Naoko et al. [22] reported that the long chain fatty acids 1-Dodecanol, n-Nonadecanol-1, and 1-tridecanol had the highest antibacterial activity against S. aureus. Chandrasekar et al. [23] showed that NimbapatradiChoornam had strong antimicrobial activity against Klebsiella pneumoniae, S. aureus, E. coli and Candida albican. The presence of phenol, 2,4-bis[1,1-dimethylethyl]-derivative, isopropyl myristate, eicosane, octadecanoic acid and hexadecanoic acid compounds were responsible for the antimicrobial activity of Nimbadipatrachoornam.
Rahbar et al. [24] had done investigation on leaf and stem extracts of Origanumvulgare L. sp. and found good antimicrobial activity of tridecane, 9, 12, 15-octadecatrienoic acid [ω-3], tetradecane, hexadecanoic acid and pentadecane against seven Gram-positive and Gram-negative bacteria (Bacillus subtilis, Enterococcus faecalis, S. aureus, Staphylococcus epidermidis, Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumoniae), as well as three fungi (Candida albicans, Saccharomyces cerevisiae and Aspergillus niger).
References
Atta HM, Dabour SM, Desoukey SG (2009) Sparsomycin antibiotic production by Streptomyces sp. AZ-NIOFD1: taxonomy, fermentation, purification and biological activities. Am Eurasian J Agric Environ Sci 5:368–377
Imada C (2005) Enzyme inhibitors and other bioactive compounds from marine Actinomycetes. Antonie Van Leeuwenhoek 87:59–63. doi:10.1007/s10482-004-6544-x
Valli S, Suvathi SS, Aysha OS, Nirmala P, Vinoth KP, Reena A (2012) Antimicrobial potential of Actinomycetes species isolated from marine environment. Asian Pac J Trop Biomed 2:469–473. doi:10.1016/S2221-1691(12)60078-1
Solanki R, Khanna M, Lal R (2008) Bioactive compounds from marine Actinomyces. Indian J Microbiol 48:410–431. doi:10.1007/s12088-008-0052-z
Kalia VC (2013) Quorum sensing inhibitors: an overview. Biotechnol Adv 31:224–245. doi:10.1016/j.biotechadv.2012.10.004
Hughes CC, Prieto-Davo A, Jensen PR, Fenical W (2008) The marinopyrroles, antibiotics of an unprecedented structure class from a marine Streptomyces sp. Org Lett 10:629–631. doi:10.1021/ol702952n
Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W (2003) Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew Chem Int Ed Engl 42:355–357. doi:10.1002/anie.200390115
Jensen PR, Gontang E, Mafnas C, Mincer TJ, Fenical W (2005) Culturable marine Actinomycete diversity from tropical Pacific Ocean sediments. Environ Microbiol 7:1039–1048. doi:10.1111/j.1462-2920.2005.00785.x
Riedlinger J, Reicke A, Zahner H, Krismer B, Bull AT, Maldonado LA, Ward AC, Goodfellow M, Bister B, Bischoff D, Sussmuth RD, Fiedler HP (2004) Abyssomicins, inhibitors of the para-aminobenzoic acid pathway produced by the marine Verrucosispora strain AB-18-032. J Antibiot 57:271–279. doi:10.1038/ja.2007.54
Koul S, Jyotsana P, Anjali M, Kalia VC (2016) Potential emergence of multi-quorum sensing inhibitor resistant (MQSIR) bacteria. Indian J Microbiol 56:1–18. doi:10.1007/s12088-015-0558-0
Mukherjee K, Tribedi P, Mukhopadhyay B, Sil AK (2013) Antibacterial activity of long-chain fatty alcohols against Mycobacteria. FEMS Microbiol Lett 338:177–183. doi:10.1111/1574-6968.12043
Kabelitz N, Santos PM, Heipieper HJ (2003) Effect of aliphatic alcohols on growth and degree of saturation of membrane lipids in Acinetobacter calcoaceticus. FEMS Microbiol Lett 220:223–227. doi:10.1016/S0378-1097(03)00103-4
Willis AT (2014) Anaerobic bacteriology: clinical and laboratory practice. Butterworth-Heinemann, London
Rajalakshmi S, Mahesh N (2014) Production and characterization of bioactive metabolites isolated from Aspergillus terreus in rhizosphere soil of medicinal plants. Int J Curr Microbiol Appl Sci 3:784–798
Kalpana Devi V, Shanmugasundaram R, Mohan VR (2012) GC–MS analysis of ethanol extracts of Entada pursaetha dc seed. Biosci Discover 3:30–33
Yogeswari S, Ramalakshmi S, Neelavathy R, Muthumary J (2012) Identification and comparative studies of different volatile fractions from Monochaetia kansensis by GCMS. Glob J Pharmacol 6:65–71
Chaudhary R, Tripathy A (2015) Isolation and identification of bioactive compounds from Irpex Lacteus Wild Fleshy Fungi. J Pharm Sci Res 7:424–434
Salem MZ, Ali HM, Mansour MM (2014) Fatty acid methyl esters from air-dried wood, bark, and leaves of Brachychiton diversifolius R. Br: antibacterial, antifungal, and antioxidant activities. Bioresources 9:3835–3845
Suresh A, Praveenkumar R, Thangaraj R, Oscar FL, Baldev E, Dhanasekaran D, Thajuddin N (2014) Microalgal fatty acid methyl ester a new source of bioactive compounds with antimicrobial activity. Asian Pac J Trop Dis 4:979–984. doi:10.1016/S2222-1808(14)60769-6
Boussaada O, Ammar S, Saidana D, Chriaa J, Chraif I, Daami M, Helal AN, Mighri Z (2008) Chemical composition and antimicrobial activity of volatile components from capitula and aerial parts of Rhaponticum acaule DC growing wild in Tunisia. Microbiol Res 163:87–95. doi:10.1080/10412905.2009.9700142
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253. doi:10.1016/j.ijfoodmicro.2004.03.022
Naoko T, Akiko S, Miki N, Keisuke M, Kazutoyo E, Hajime H, Yoshihiro I (2007) Antibacterial activity of long-chain fatty alcohols against Staphylococcus aureus. Molecule 12:139–148. doi:10.3390/12020139
Chandrasekar T, Rao MRK, Kumar RV, Prabhu K, NandhaKumar V, Divya D (2015) GC–MS analysis, antimicrobial, antioxidant activity of an Ayurvedic medicine. Nimbapatradi Choornam. J Chem Pharm Res 7:124–136. doi:10.1155/2014/694934
Rahbar N, Shafagha A, Salimi F (2012) Antimicrobial activity and constituents of the hexane extracts from leaf and stem of Origanum vulgare L. sp. Viride (Boiss.) Hayek. Growing wild in Northwest Iran. J Med Plants Res 6:2681–2685. doi:10.5897/JMPR11.1768
Raj Kumar K, Brindha Priyadarisini V, Ranjith Kumar M (2012) Isolation and identification of bioactive compounds from Bacillus Megateriume5 from the south east coastal region of India against urinary tract infectious pathogens. Int J Phar Biol Archives 3:842–847
Usha Nandhini S, Sangareshwari S, Lata K (2015) Gas chromatography-mass spectrometry analysis of bioactive constituents from the marine Streptomyces. Asian J Pharm Clin Res 8:244–246
Girija S, Veeramuthu D, Pandi Suba K, Hariprasad G, Raghuraman R (2014) Chromatographic characterization and GC-MS evaluation of the bioactive constituents with antimicrobial potential from the pigmented ink of Loligo duvauceli. Int Sch Res Notices. doi:10.1155/2014/820745
Al-Youssef HM, Hassan WHB (2015) Antimicrobial and antioxidant activities of Parkinsonia aculeata and chemical composition of their essential oils. Merit Res J Med Med Sci 3:147–157
Olubunmi A, Gabriel OA, Stephen AO, Scott FO (2011) Antioxidant and antimicrobial activity of cuticular wax from Kigelia Africana. FABAD J Pharm Sci 34:187–194
Garaniya N, Bapodra A (2014) Ethno botanical and Phytophrmacological potential of Abrus precatorius L.: a review. Asian Pac J Trop Biomed 4:27–34. doi:10.12980/APJTB.4.2014C1069
Al-Abd NM, Mohamed ZN, Mansor M, Azhar F, Hasan MS, Kassim M (2015) Antioxidant, antibacterial activity, and phytochemical characterization of Melaleuca cajuputi extract. BMC Complement Altern Med 15:1–13. doi:10.1186/s12906-015-0914-y
Nahar N, Rahman S, Rahman SM, Moniruzzaman M (2016) GC-MS analysis and antibacterial activity of Trigonella foenumgraecum against bacterial pathogens. Free Radical Antioxid 6:109–114. doi:10.5530/fra.2016.1.13
Hussain AZ, Kumaresan S (2014) GC-MS studies and phytochemical screening of Sesbania grandiflora L. J Chem Pharm Res 6:43–47
Afolayan AJ, Ashafa AOT (2009) Chemical composition and antimicrobial activity of the essential oil from Chrysocoma ciliata L. leaves. J Med Plant Res 3:390–394
Kuppuswamy MK, Jonnalagadda B, Arockiasamy S (2013) GC-MS analysis of chloroform extract of Croton bonplandianum. Int J Pharm Bio Sci 4:613–617
Bhardwaj A, Shakil NA, Jha V, Gupta RK (2014) Screening of nutritional, phytochemical, antioxidant and antibacterial activity of underutilized seeds of Scirpus articulatus: the basis of Khubahi Ramdana industry. J Pharma Phytochem 3:311–320
Sivakumar SR (2014) GC-MS analysis and antibacterial potential of white crystalline solid from red algae Portieria hornemannii against the plant pathogenic bacteria Xanthomnas axonopodis pv. citri (Hasse) Vauterin et al. and Xanthomonas campestris pv. malvacearum (smith 1901) dye 1978b. Int J of Adv Res 2:174–183
Acknowledgments
The author I. Faridha Begum, Senior research Fellow is grateful for the financial assistance by University Grant Commision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faridha Begum, I., Mohankumar, R., Jeevan, M. et al. GC–MS Analysis of Bio-active Molecules Derived from Paracoccus pantotrophus FMR19 and the Antimicrobial Activity Against Bacterial Pathogens and MDROs. Indian J Microbiol 56, 426–432 (2016). https://doi.org/10.1007/s12088-016-0609-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-016-0609-1