Abstract
The present study reports the pretreatment of paddy straw by Trichoderma reesei MTCC 164 and Coriolus versicolor MTCC 138 to observe the changes in chemical composition and its correlation with change of surface structure, morphology and porosity of paddy straw. Compared with untreated straw, cellulose decreased by 15.9 and 19.3 % in T. reesei MTCC 164 and C. versicolor MTCC 138 pretreated paddy straw respectively. Lignin content increased by 41.4 % in T. reesei pretreated paddy straw whereas decreased by 19.1 % in C. versicolor pretreated straw. The microscopic structural changes were examined by scanning electron microscopy under reasonable conditions. Results showed that digestibility of paddy straw are increased by treating paddy straw with both the cultures. Both surface area and pore size of treated straw were increased partially due to solubilization of silica components.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Paddy straw is an abundant lignocellulosic waste consists of 35–40 % w/w cellulose, 25–30 % w/w hemicellulose, 10–15 % w/w lignin and 6–12 % w/w silica [1]. Traditionally, more than 70 % of paddy straw is burnt in the field itself, which causes environmental pollution problems. A limited amount is used as animal feed, feedstock for paper industry, substrate for mushroom cultivation and organic fertilizer. Most of the cellulose content in the paddy straw cannot be fully and economically utilized by these ways. By enzymatic hydrolysis, paddy straw can be converted to reducing sugars, which can be further fermented to target products such as ethanol, methane, lactic acid, single cell protein and hydrogen by suitable microorganisms.
Several pretreatment methods have been proposed to increase the paddy straw digestibility in the recent years [2, 3]. In each option, the biomass is reduced in size and its physical structure is opened. Each pretreatment method offers distinct advantages and disadvantages. The physical and chemical methods are expensive procedures with respect to cost and energy. Microbial pretreatment is the most suitable method for increasing paddy straw digestibility. In nature, there are many microorganisms, like bacteria and fungi that live on natural lignocellulose. Though the growth period of bacteria is shorter than that of fungi, their half-baked cellulase system makes them less useful in the industrial production of cellulase. On the contrary, cellulolytic and lignocellulolytic fungus have been extensively studied.
Keeping in view the importance of lignocellulolytic fungi in increasing paddy straw digestibility, this study focuses on pretreatment of paddy straw with Trichoderma reesei microbial type culture collection (MTCC) 164 and Coriolus versicolor MTCC 138. The changes in chemical composition were correlated with changes observed by scanning electron microscopy (SEM).
Materials and Methods
Procurement of the Materials
Fresh paddy straw was procured from the research field of Punjab Agricultural University, Ludhiana. It was chopped to 3–4 cm size with a Toka machine and was stored in poly bags at room temperature. Standard cultures of T. reesei MTCC 164 and C. versicolor MTCC 138 was procured from MTCC, Institute of Microbial Technology, Chandigarh.
Pretreatment of Paddy Straw
Paddy straw was pretreated with standard cultures namely T. reesei MTCC 164 and C. versicolor MTCC 138. The inoculum required for pretreatment of paddy straw was prepared on wheat grains. The wheat grains were washed and boiled for 20–30 min till tender. The excess water was drained off. The grains were then mixed with 2 % gypsum (CaSO4) and 4 % calcium carbonate (CaCO3). The grains were dispensed into empty glucose bottles (250 g/bottle) or autoclavable poly bags. The bottles were cotton plugged and autoclaved for 90 min. The poly bags were plugged by using plastic ring which supports the cotton plug. After cooling the bottles or poly bags were inoculated by placing two 1 cm bits of 7–8 days old culture on the opposite sides of bottle. The bottles/poly bags were incubated at 30 ± 2 °C, till the mycelium completely impregnates the wheat grains (8–10 days) [4].
Chopped paddy straw (250 g) was soaked overnight and excess water was drained off. The paddy straw was then inoculated with inoculum at the rate of 10 % w/w ratio (25 g in 250 g paddy straw) and incubated at 30 ± 2 °C for 25 days. After required incubation, pretreated straw was analyzed for change in proximate and chemical composition by standard method [5]. A control comprising un-inoculated soaked paddy straw was also run simultaneously.
Scanning Electron Microscopy
The change in surface structures of pretreated paddy straw was observed by SEM. SEM of untreated (control) and pretreated paddy straw was done in Nano and Electron Microscopy Laboratory at PAU, Ludhiana. For SEM, the paddy straw was dried in oven at 60 °C for 24 h. Dried samples were treated with 10 % ethanol w/v, dried in air for 24 h and grind to powdered form. The samples were sticked carbon glue for inspection. And were observed using SEM at 10.8 mm and 2.5K magnification.
Results and Discussion
Results from Table 1 showed that showed potency index of standard cultures for different activities i.e. cellulase activity, remazol brilliant blue (RBB) activity and guaiacol activity. T. reesei MTCC 164 showed cellulase activity of potency index of 5.0, while C. versicolor MTCC 138 showed potency index of 3.5. C. versicolor MTCC 138 showed RBB and Guaiacol activity having potency index of 2.7 and 1.3 respectively. T. reesei MTCC 164 showed negative results on RBB and guaiacol plates, while C. versicolor MTCC 138 showed presence of redness zone on guaiacol plates indicating presence of lignin peroxidase. From the above results it was concluded that T. reesei MTCC 164 was cellulolytic whereas C. versicolor MTCC 138 was lignocellulolytic.
Results from Table 2 showed that in case in control (un-inoculated soaked paddy straw), there was very little or insignificant change in almost all components i.e. cellulose, hemicellulose, lignin and silica. In case of paddy straw pretreated with T. reesei MTCC 164, initially a decreasing trend of hemicellulose content was observed for a period of 15 days, however further increase in pretreatment period led to increase in hemicellulose content. Maximum decrease in hemicellulose content was observed on 10th day (20.5 %) which remained only 13.5 % on 15th day of incubation. Lignin content increased with the increase of incubation period reaching 11.6 % in 25 days treated sample, as T. reesei was cellulolytic only. A maximum of 30.0 % silica was removed after 25 days treatment. In C. versicolor MTCC 138 pretreated straw, the cellulose content kept on decreasing with the increasing incubation period, with the maximum reduction of 19.3 % after 25 days pretreatment. Lignin content decreased with the increase of incubation period with maximum reduction of 19.1 % in 25 days treated sample. Decrease in silica was smooth and significant with a maximum removal of 32.5 % after 25 days treatment. The content of hemicellulose increased due to partly removal of cellulose and lignin.
Similar results were reported by Kahlon and Dass [6] which states that Pleurotus ostreatus and Sporotrichum pulverulentum inoculated paddy straw is more digestible with significant reduction in lignin and utilization of cell wall constituents by the fungi. Shi et al. [7] pretreated the cotton stalks with Phanerochaete chrysosporium and found significant decrease in lignin i.e. 19.38 and 35.33 % for submerged and solid state cultivation respectively. Taniguchi et al. [8] compared the pretreatment effect of four white-rot fungi (P. chrysosporium, Trametes versicolor, Ceriporiopsis subvermispora and P. ostreatus) on rice straw hydrolysis and found P. ostreatus to be the most promising fungus as it caused significant decrease in lignin as compared to the other three fungi [9]. Lignin biodegradation by different microorganisms has been widely studied and it has been shown that inoculation with lignin-degrading microorganisms can accelerate the composting process and improve compost quality [9]. He also evaluated the relationships of lignin degradation and humification by fungi. Phutela et al. [4] too reported a maximum of 30 % silica removal in case of 25 days pretreated paddy straw with T. reesei MTCC 164.
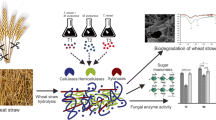
Because a large fraction of cellulose, lignin and silica was removed by pretreatment, there were some physical changes in the straw. For this reason, SEM pictures of pretreated and untreated paddy straw were produced. Figure 1a–c showed the longitudinal section of paddy straw before and after biological pretreatment i.e. (a) untreated paddy straw, (b) paddy straw treated with T. reesei MTCC 164 and (c) paddy straw treated with C. versicolor MTCC 138. The distinct changes in surface structure are visible in the basic tissue of paddy straw. The untreated paddy straw exhibited a rigid and highly compact structure whereas pretreated sample showed opening of the holo-cellulose fibrils due to creation of pores of different sizes. Microfibrils were separated from initial connected structure and are fully exposed, thus increasing the external surface area and porosity of paddy straw. Similar results were also reported by Zhang and Cai [10], who observed changes in histological structures of rice straw after 2 % NaOH treatment. Xu et al. [11] reported the changed structure and surface area of the pretreated straw by SEM that is in the favor of enzymatic hydrolysis. Silica nodules were also observed in pretreated straw (Fig. 1d, e). Energy dispersive analysis revealed that silica nodules had 90–94 % silica content. The frequent appearance of silica bodies indicated progressive consumption of organic matter by the fungus leaving behind the inorganic elements. Kuhad and Johri [12] too reported that the fermented straw showed strikingly prominent silica nodules attached to rib like structures. Yu et al. [13] reported that both morphological and structural characteristics are changed due to organic polar substances and inorganic silica partly dissolved which leaves higher surface area with more pores on acid treated plant residues.
SEM of paddy straw before and after biological pretreatment, a longitudinal section of paddy straw before treatment, b longitudinal section of paddy straw after treatment with T. reesei MTCC 164, c longitudinal section of paddy straw after treatment with C. versicolor MTCC 138, d T. reesei MTCC 164 treated paddy straw showing silica nodules and e C. versicolor MTCC 138 treated paddy straw showing silica nodules
Conclusions
Thus from above studies it is concluded that the digestibility of paddy straw can be increased significantly by treating paddy straw with fungal cultures like T. reesei MTCC 164 and C. versicolor MTCC 138. Reduction in silica content in both cases seems to be the important factor in opening up of compact microfibrils and significant morphological changes. The distorted tissue, appearance of silica nodules, separated and fully exposed microfibrils increased the external surface area and the porosity of the paddy straw, thus facilitating enzymatic hydrolysis.
References
Thygesen A, Thomsen AB, Schmidt AS, Jorgensen H (2003) Production of cellulose and hemicelluloses degrading enzymes by filamentous fungi cultivated on wet oxidized wheat straw. Enzym Microbiol Technol 32:606–615
Saratale GD, Chen SD, Lo YC, Saratale RG, Chang JS (2008) Outlook of biohydrogen production from lignocellulosic feedstock using dark fermentation-a review. J Sci Ind Res 67:962–979
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100:10–18
Phutela UG, Sahni N, Sooch SS (2011) Fungal degradation of paddy straw for enhancing biogas production. Indian J Sci Technol 4:660–665
AOAC (2000) Association of Official Analytical Chemists, official methods of analysis, 17th edn. AOAC, Gaithersburg
Kahlon SS, Dass SK (1987) Biological conversion of paddy straw into feed. Biol Wastes 22:11–21
Shi J, Shivappa RRS, Chinn M, Howell N (2009) Effect of microbial pretreatment on enzymatic hydrolysis and fermentation of cotton stalks for ethanol production. Biomass Bioenergy 33:88–96
Taniguchi M, Suzuki H, Watanabe D, Sakai K, Hoshino K, Tanaka T (2005) Evaluation of pretreatment with Pleurotus ostreatus for enzymatic hydrolysis of rice straw. J Biosci Bioeng 100:637–643
Lopez MJ, Vargas G, Suarez-Estrela F, Moreno J (2006) Biodelignification and humification of horticultural plant residues by fungi. Int Biodeterior Biodegrad 57:24–30
Zhang Q, Cai W (2008) Enzymatic hydrolysis of alkali pretreated rice straw by Trichoderma reesei ZM4-F3. Biomass Bioenergy 32:1130–1135
Xu Z, Wang Q, Jiang Z, Yang X, Ji Y (2007) Enzymatic hydrolysis of pretreated soybean straw. Biomass Bioenergy 31:162–167
Kuhad RC, Johri BN (1992) Fungal decomposition of paddy straw: light and scanning electron microscopic study. Indian J Microbiol 32:255–258
Yu CT, Chen WH, Men LC, Huang WS (2009) Microscopic structure features changes of rice straw treated by boiled acid solution. Ind Crops Prod 29:308–315
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phutela, U.G., Sahni, N. Microscopic Structural Changes in Paddy Straw Pretreated with Trichoderma reesei MTCC 164 and Coriolus versicolor MTCC 138. Indian J Microbiol 53, 227–231 (2013). https://doi.org/10.1007/s12088-012-0321-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-012-0321-8