Abstract
Background & Aims
Despite increasing knowledge regarding the cellular and molecular mechanisms of liver fibrogenesis, there is currently no approved drug for the treatment of liver fibrosis. Mesenchymal stem cells (MSCs) are multipotent progenitor cells representing an attractive therapeutic tool for tissue damage and inflammation. This study was designed to determine the protective effect and underlying mechanism of human umbilical cord-derived MSCs (UC-MSCs) on thioacetamide-induced liver fibrosis.
Methods
Liver fibrosis was induced in mice by intraperitoneal injection of thioacetamide (TAA). Some mice were then given injection of UC-MSCs or UC-MSCs-derived exosomes (UC-MSCs-Exo) via the tail vein. Liver tissues were collected for histologic analysis.
Results
We found that administration of UC-MSCs significantly reduced serum alanine aminotransferase and aspartate aminotransferase levels, and attenuated hepatic inflammation and fibrosis. Moreover, the therapeutic effect of UC-MSCs-derived exosomes was similar to that of UC-MSCs. Intriguingly, UC-MSCs-Exo treatment downregulated the expression of smoothened (SMO), a fundamental component of Hedgehog signaling which plays a critical role in fibrogenesis, and subsequently inhibited the activation of hepatic stellate cells, a central driver of fibrosis in experimental and human liver injury. Furthermore, the anti-inflammatory and anti-fibrotic effects of UCMSCs- Exo was reversed by the SMO agonist SAG treatment in mice.
Conclusion
Our findings suggest that UC-MSCs-Exo exert therapeutic effects on liver fibrosis, at least in part, through inhibiting the Hedgehog/SMO signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic stimulation of multiple factors causes liver fibrosis, the main risk factors identified at present include viral infection, alcoholism, obesity-related steatohepatitis and so on [1, 2]. Liver fibrosis is the formation of a fibrous scar, which develops in response to acute or chronic cell injury resulting in the deposition of extracellular matrix (ECM) proteins such as collagen and fibronectin [2,3,4]. It is well documented that activated HSCs were the main source of ECM, and that HSC activation represents a central event during liver fibrogenesis [5, 6]. Further development of liver fibrosis can lead to cirrhosis and even liver cancer, imposing a heavy health and economic burden on many countries [4, 7]. However, there is currently no effective drug to treat liver fibrosis. Therefore, it is urgent and important to explore the pathogenesis of liver fibrosis and to find alternative treatment strategies for chronic liver diseases including liver fibrosis.
Mesenchymal stem cells (MSCs) are multipotent stem cells with self-renewal abilities, high proliferation rate and multipotent differentiation capacity [8]. The main sources of MSCs are the bone marrow MSCs (BM-MSCs), adipose-derived MSCs (AD-MSCs), and umbilical cord MSCs (UC-MSCs) [9]. Among of these, UC-MSCs are distinguished due to their easy collection, low immunogenicity, high paracrine potential rapid proliferation speed and fewer ethical issues [10]. Multiple lines of evidence has demonstrated that MSCs exert considerable promise for use in tissue repair due to their multilineage differentiation and immunomodulatory properties [11]. Our recent studies also indicated that MSCs alleviated ischemia/reperfusion and acetaminophen-induced acute liver injury by modulating macrophage Hippo/β-catenin signaling and AMPK/SIRT1 pathway, respectively [12, 13]. Although much progress has been made in the use of MSCs to treat various diseases including liver fibrosis [14], a number of phase III clinical trials of MSC-based cell therapy were failed to meet the primary endpoints [15]. Thus, exploring the underlying mechanisms by which MSCs exert their therapeutic effects emerges as one of the key challenges of MSC therapy.
Exosomes are shown to carry nucleic acids, proteins, lipids and metabolites to mediate near and long-distance intercellular communication in health and disease and affect various aspects of cell biology. Previous studies demonstrated the importance of the exosome-mediated cellular communication in liver fibrosis [16,17,18]. Exosomes are simpler than their parent cells, therefore they are easier to produce and store [19]. Importantly, they have no risk of tumor formation and are less immunogenic [20]. Therefore, the use of exosomal cell-free therapy is a more promising than the direct use of MSCs in the treatment of liver fibrosis. However, the efficacy and potential therapeutic mechanism of human UC-MSCs-Exo in the treatment of liver fibrosis has not been fully understood.
Emerging evidence has demonstrated the important role of Hedgehog pathway in the progression of liver fibrosis. Hedgehog signaling can be simplified into four fundamental components: (i) the ligand Hedgehog (Sonic hedgehog, Indian hedgehog, and Desert hedgehog), (ii) the cell surface receptor Patched (Ptc), (iii) the signal transducer Smoothened (SMO), and (iv) the effector transcription factor Glioblastoma (Gli) family (Gli1, Gli2, and Gli3) [21]. Hedgehog signaling is initiated by the ligands which interact with Ptc expressed on target cells of Hedgehog signaling. This interaction de-represses the activity of SMO and permits the propagation of intracellular signals that culminate in the nuclear localization of Gli and consequently regulates the expression of Gli-target genes [22]. In healthy adult livers, Hedgehog pathway expression is relatively dormant with low production of ligands robust expression of Hedgehog inhibitors [2]. During fibrogenesis, Hedgehog signaling promotes transdifferentiation of HSCs by activating Yes-associated protein 1 (YAP) [23]. Blocking Hedgehog pathway and YAP activation prevented HSCs from transdifferentiating into activated HSCs [24]. However, it remains largely unknown as to whether UC-MSCs-Exo can inhibit fibrosis by targeting the Hedgehog signaling.
In the present study, using thioacetamide (TAA)-induced mouse liver fibrosis model and in vitro co-culture experiments, we investigated the therapeutic effects and underlying mechanism of UC-MSCs-Exo-based protection against hepatic fibrosis. Our findings suggest that UC-MSCs-Exo inhibits HSC activation and attenuates liver fibrosis through targeting the Hedgehog/SMO signaling.
Methods and materials
Mouse liver fibrosis model and treatment
The C57BL/6 mice (6–7 weeks old) purchased from Hubei Provincial Center for Disease Control and Prevention (Wuhan, China) were bred at a temperature of 30 ± 2 °C with a 12 h light/dark cycle. Animal welfare and experimental procedures were performed according to the guide for the Care and Use of Laboratory animals.
Mice were randomly divided into six groups: normal control (NC) group, saline + UC-MSCs-Exo group, saline + UC-MSCs group, liver fibrosis + PBS group, liver fibrosis + UC-MSCs-Exo group, and liver fibrosis + UC-MSCs group (n = 5/group). The fibrosis group mice were fasted for 12 h before modeling and were intraperitoneally injected with 150 mg/kg of TAA for 3 times/week. Other mice were treated with the same amount of normal saline. Four weeks after liver fibrosis induction, the saline + UC-MSCs-Exo group and the liver fibrosis + UC-MSCs-Exo group mice were intravenously injected with 75 μg/200 μL UC-MSCs-Exo. For the saline + UC-MSCs group and the liver fibrosis + UC-MSCs group, 1.0 × 106/200 μL UC-MSCs were intravenously injected into mice. The liver fibrosis + PBS group mice were intravenously injected with 200 μL PBS. The NC group was untreated to serve as blank control. Some mice were injected with the SMO agonist SAG (20 mg/kg body weight, i.p., SML1314, Sigma Aldrich, USA) 24 h before UC-MSCs-Exo administration. Mice were sacrificed 6 weeks after TAA injection.
Statistical analysis
All data were presented as mean ± SD by GraphPad Prism 5 software (GraphPad Software Inc., California, USA) and analyzed by SPSS 19 statistical software (SPSS Inc., Chicago, Illinois). Multiple group comparisons were performed using one-way analysis of variance (ANOVA). T test was used for two-group comparisons. p value < 0.05 was considered as statistically significant.
Additional methods
Further detailed methodology is described in the supplemental materials.
Results
Identification and characterization of UC-MSCs and UC-MSCs-Exo
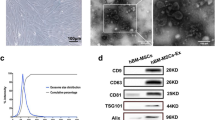
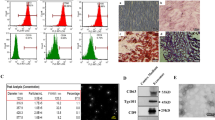
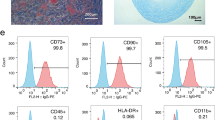
We first characterized UC-MSCs that were isolated from human umbilical cord tissues. Our data showed that the cultured UC-MSCs exhibited a spindle-shaped morphology and plastic-adherent characteristic (Fig. 1A). Biological effectiveness experiments confirmed that UC-MSCs could be differentiated into adipogenic, osteogenic, and chondrogenic phenotypes (Fig. 1B). Flow cytometry analysis confirmed that UC-MSCs were positive for CD90 (98.03%), CD105 (96.71%) and CD73 (99.91%), and negative for CD34 (0.07%), CD19 (0.07%), CD45 (0.07%) and HLA-DR (0.07%) (Fig. 1C).
Identification and characterization of UC-MSCs and UC-MSCs-Exo. A Morphological appearance of cultured UC-MSCs. scale bar: 100 µm. B Differentiation abilities of UC-MSCs were detected by cellular staining. The order from left to right: adipogenesis using Oil red O staining, osteogenesis using Alizarin red staining, and chondrogenesis using Alcian blue staining. Scale bar, 40 µm. C Specific surface markers of cells were examined by flow cytometry. The UC-MSCs associated with markers were positive for CD90, CD105, and CD73 and were negative for CD34, CD19, CD45, and HLA-DR. D Morphological analysis of UC-MSCs-Exo by transmission electron microscopy. Scale bar, 200 nm. E Nanoparticle tracking analysis (NTA) of size distribution of UC-MSCs-Exo. F Western blot assay indicated the positive expression of Alix and CD9, and negative expression of Calnexin in UC-MSCs-Exo. Data are presented as the mean ± SD
UC-MSCs-Exo were successfully isolated from UC-MSCs using the differential centrifugation method. The representative morphology of purified exosomes was observed by transmission electron microscopy. As shown in Fig. 1D, the exosomes exhibited a characteristic saucer-like shape. In addition, the exosome, was limited by a lipid bilayer, had an average diameter ranging from 30 to 150 nm (Fig. 1E). Western blotting confirmed that UC-MSCs-Exo expressed and enriched the known exosome markers Alix and CD9, and did not express Calnexin (Fig. 1F).
UC-MSCs and UC-MSCs-Exo treatment reduces TAA-induced hepatic inflammation and improves liver function in mice
The effectiveness of isolated UC-MSCs-Exo to alleviate TAA-induced liver fibrosis was assessed in a mouse model. H&E staining showed that inflammatory cell infiltration were distinctly increased in liver tissues of TAA-administered mice compared with control mice. The livers from UC-MSCs or UC-MSCs-Exo-treated mice obviously had less inflammatory cell infiltration than the livers from PBS-treated controls (Fig. 2A). Biochemical analysis showed the restoration of liver function after UC-MSCs and UC-MSCs-Exo treatment. The serum levels of ALT and AST were significantly reduced in the treated groups compared to the controls (Fig. 2B, C).
UC-MSCs and UC-MSCs-Exo treatment reduces TAA-induced liver inflammation and improves liver function in mice. A Representative H&E-stained liver sections. Scale bar, 100 µm. B Serum ALT levels were analyzed. C Serum AST levels were analyzed. D Quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR)-assisted detection of Tnf-α in liver tissues (n = 5 samples/group). E qRT-PCR-assisted detection of Il-6 in liver tissues. F qRT-PCR–assisted detection of Mcp-1 in liver tissues. G qRT-PCR–assisted detection of Adgre1 in liver tissues. n = 5 per group, data are presented as the mean ± SD. **p < 0.01
Next, we detected the expression of proinflammatory mediators in liver tissue by qRT-PCR. Compared to the PBS-treated controls, UC-MSCs and UC-MSCs-Exo treatment reduced the mRNA levels of proinflammatory cytokines and chemokines including Tnf-α, Il-6, and Mcp-1 in livers tissues (Fig. 2D–F). Meanwhile, the expression of Adgre1 in the UC-MSCs and UC-MSCs-Exo-treated livers were greatly reduced when compared to the controls (Fig. 2G). Therefore, both UC-MSCs and UC-MSCs-Exo treatment reveal the improvement of liver function and reduction of hepatic inflammation.
UC-MSCs and UC-MSCs-Exo treatment alleviates liver fibrosis
Histopathological examination using Sirius Red and Masson’s trichrome staining confirmed the successful establishment of an animal model of liver fibrosis after TAA administration. However, the collagen deposition was significantly reduced in UC-MSCs and UC-MSCs-Exo-treated mice compared to the controls (Fig. 3A, B). Moreover, α-SMA is a specific marker involved in the development of liver fibrosis and HSC activation [5], therefore we examined the expression level of α-SMA. Immunofluorescence staining showed that the expression of α-SMA was markedly decreased after UC-MSCs and UC-MSCs-Exo treatment (Fig. 3C). Meanwhile, we detected the mRNA levels of genes encoding fibrotic factors, including Acta2, collagen type1-α1 (Col1α1), collagen type III α1 (Col3α1) and tissue inhibitor of metalloproteinase-1 (Timp1) in liver tissue by qRT-PCR. These expressions were significantly reduced in UC-MSCs and UC-MSCs-Exo-treated livers (Fig. 3D–G). These results suggest that treatment with either UC-MSCs or UC-MSCs-Exo distinctly alleviates TAA-induced liver fibrosis in mice.
UC-MSCs and UC-MSCs-Exo treatment alleviates liver fibrosis. A Representative pictures of Sirius red staining in liver sections. Scale bar, 100 µm. B Representative pictures of Masson staining in liver sections. Scale bar, 100 µm. C Representative immunofluorescence staining of α-SMA in liver sections (red). Nuclei were labeled with DAPI (blue). Scale bar, 50 µm. D qRT-PCR-assisted detection of Acta2 in liver tissues. E qRT-PCR-assisted detection of Col1α1 in liver tissues. F qRT-PCR-assisted detection of Col3α1 in liver tissues. G qRT-PCR-assisted detection of Timp1 in liver tissues. n = 5 per group, data are presented as the mean ± SD. *p < 0.05, **p < 0.01
UC-MSCs and UC-MSCs-Exo treatment inhibits the expression of SMO in fibrotic livers
It has been reported that Hedgehog signaling plays an important role in liver diseases, including liver fibrosis [25]. Moreover, previous studies suggest that the Hedgehog/SMO pathway may act as a potential therapeutic target for liver fibrosis [23, 26]. We analyzed the GEO database (GEO: GSE173961) and found that the mRNA levels of SMO in the liver tissue of the TAA-induced mice liver fibrosis model was significantly higher than that in the control groups. However, SMO expression was markedly downregulated in the recovered livers (Fig. 4A). Therefore, we analyzed the correlation between SMO level and degree of TAA-induced liver fibrosis in mice. As expected, the mRNA level of SMO was positively correlated with the mRNA levels of Col1α1 and Acta2 (Fig. 4B, C).
UC-MSCs and UC-MSCs-Exo treatment inhibits the expression of SMO in fibrotic liver tissues. A The expression of SMO in normal liver tissues and TAA-induced fibrotic liver tissues by the GEO database. B, C The correlation between Col1α1/Acta2 and SMO mRNA expression in livers after TAA injection (n = 15 mice). The correlation coefficient was calculated by the Pearson correlation test. D Western blot analysis of SMO expression in liver tissue. E Representative immunofluorescence staining of SMO in liver sections (green). Nuclei were labeled with DAPI (blue). Scale bar, 50 µm
Next, we examined the protein levels of SMO in liver tissues by western blot. Obviously, the expression of SMO in the liver was increased after TAA administration. However, SMO expression was dramatically downregulated after UC-MSCs and UC-MSCs-Exo treatment (Fig. 4D). Immunofluorescence staining of liver tissue further confirmed that the expression of SMO was significantly suppressed by UC-MSCs and UC-MSCs-Exo treatment (Fig. 4E). These results suggest that UC-MSCs and UC-MSCs-Exo inhibit the expression of SMO, a key mediator of liver fibrogenesis.
UC-MSCs-Exo inhibits HSC activation through suppressing the Hedgehog/SMO pathway in vitro
Next, we further explored the association between the anti-fibrosis effect of UC-MSCs-Exo and the Hedgehog/SMO pathway. Western blot analysis revealed that the expression of α-SMA was obviously upregulated after TGF-β treatment in LX2 cells. However, UC-MSCs-Exo treatment significantly inhibited α-SMA expression (Fig. 5A). Meanwhile, we tested the effects of UC-MSCs-Exo on the Hedgehog/SMO pathway. The expression of SMO was decreased after UC-MSCs-Exo treatment (Fig. 5B). Gli1, which is the transcription factor for Hedgehog/SMO signaling pathway, was also significantly downregulated by UC-MSCs-Exo treatment (Fig. 5C). Consistent with these results, immunofluorescence staining confirmed that UC-MSCs-Exo treatment inhibited α-SMA, SMO and Gli1 expression in LX2 cells (Fig. 5D). In addition, the mRNA levels of Hedgehog target genes including Ptch1, Gli1 and Cyclin D1 were decreased by UC-MSCs-Exo treatment (Fig. 5E). To further confirm that UC-MSCs-Exo inhibited HSC activation through suppressing the Hedgehog/SMO pathway, we pretreated LX2 cells with the SMO agonist SAG. As expected, UC-MSCs-Exo-mediated inhibition of HSC activation was reversed by SAG treatment (Fig. 5F).
UC-MSCs-Exo depresses SMO expression and inhibits HSC activation in vitro. A Western blot analysis of α-SMA expression in LX2 cells. B Western blot analysis of SMO expression in LX2 cells. C Western blot analysis of Gli1 expression in LX2 cells. D Representative immunofluorescence staining of α-SMA, SMO, and Gli1 in LX2 cells. Scale bar, 100 µm. E qRT-PCR-assisted detection of Ptch1, Gli1 and Cyclin D1 in LX2 cells. F LX2 cells were pretreated with 500 nM of SAG for 6 h, followed by MSC-Exo treatment. Representative immunofluorescence staining of α-SMA in LX2 cells. Scale bar, 100 µm. Representative of three experiments
The therapeutic effect of UC-MSCs-Exo was reversed by SAG treatment in vivo
To further confirm that UC-MSCs-Exo alleviated liver fibrosis through targeting the Hedgehog/SMO signaling, TAA-induced fibrotic mice were injected with the SMO agonist SAG before UC-MSCs-Exo administration. H&E staining and biochemical analysis of serum ALT/AST levels showed that liver damage was increased in SAG + UC-MSCs-Exo-treated mice compared with UC-MSCs-Exo-treated mice (Fig. 6A, B), which was accompanied by increased expressions of proinflammatory mediators including Tnf-α, Il-6, Mcp-1, and Adgre1 after SAG treatment (Fig. 6C). In addition, Sirius Red and Masson’s trichrome staining revealed that SAG treatment reversed the anti-fibrotic effect of UC-MSCs-Exo in TAA-induced fibrotic mice (Fig. 6D). Meanwhile, we detected the expressions of genes encoding fibrotic factors, including Acta2, Col1α1, Col3α1 and Timp1 in liver tissue. As expected, these mRNA levels were significantly upregulated in SAG + UC-MSCs-Exo-treated mice compared with UC-MSCs-Exo-treated mice (Fig. 6E). These results suggest that UC-MSCs-Exo attenuates hepatic fibrosis through targeting the Hedgehog/SMO signaling.
The therapeutic effect of UC-MSCs-Exo was reversed by SAG treatment in vivo. Mice were injected with the SMO agonist SAG (20 mg/kg body weight, i.p.) 24 h before UC-MSCs-Exo administration. A Representative H&E-stained liver sections. Scale bar, 100 µm. B Serum ALT/AST levels were analyzed. C qRT-PCR-assisted detection of Tnf-α, Il-6, Mcp-1 and Adgre1 in liver tissues (n = 5 samples/group). D Representative pictures of Sirius red staining and Masson staining in liver sections. Scale bar, 100 µm. E qRT-PCR-assisted detection of Acta2, Col1α1, Col3α1 and Timp1 in liver tissues (n = 5 samples/group). Data are presented as the mean ± SD. *p < 0.05, **p < 0.01
Discussion
In this study, we investigated the therapeutic effects and potential mechanisms of UC-MSCs and UC-MSCs-Exo treatment in alleviating TAA-induced liver fibrosis. Our findings indicated that administration of UC-MSCs significantly improved liver function, reduced liver inflammation and attenuated hepatic fibrosis, and that the therapeutic effect of UC-MSCs-Exo was similar to that of UC-MSCs. Importantly, we demonstrated that UC-MSCs-Exo reduced HSC activation by inhibiting the Hedgehog/SMO pathway. Furthermore, the anti-inflammatory and anti-fibrotic effects of UC-MSCs-Exo was reversed by the SMO agonist SAG treatment in vivo. Our data highlight the importance of the Hedgehog/SMO signaling as a key anti-liver fibrosis target, and shed new light on the application of UC-MSCs-based cell-free therapeutic strategies for liver fibrosis.
The classical Hedgehog signaling is a conserved, highly complex pathway with important and diverse roles throughout animal development and adult tissue homeostasis [27]. Hedgehog signaling has been shown to modulate wound-healing responses in many adult tissues, including the liver [28]. It has been reported that Hedgehog is involved in the development of liver cirrhosis through metabolic reprogramming of HSCs [29], and conditionally ablation in Hedgehog/SMO signaling suppressed the myofibroblastic HSC phenotype [23]. Although some data showed that canonical Hedgehog inhibitor failed to alleviate pulmonary fibrosis, renal fibrosis, or myelofibrosis, the direct inhibition of Gli proteins can improve fibrosis [30]. Additionally, previous studies reported that cyclopamine, a Hedgehog signaling pathway inhibitor that inhibits SMO activity by altering the spatial structure of SMO, could dramatically decrease mitochondrial respiration and cell growth of myofibroblastic HSCs, thereby improving rat liver fibrosis [23]. In keeping with these studies, our results revealed that the expression of SMO was remarkedly upregulated in fibrotic livers, and was significantly positively correlated with the severity of liver fibrosis. Collectively, these findings suggest that the Hedgehog/SMO signaling might be an important target for treating liver fibrosis. Indeed, our in vivo and in vitro data demonstrated that UC-MSCs-Exo reduced HSC activation and hepatic fibrosis through suppressing the Hedgehog/SMO signaling.
MSCs contribute to tissue repair due to their immunomodulatory and regenerative potential [11]. MSCs are commonly used in clinical trials [9] owing to the fact that they are easy to collect, exhibit stronger proliferation capacity in vitro, and rarely raise ethical concerns [10, 31]. Accumulating evidence demonstrates that most of the beneficial effects mediated by MSCs can be attributed to the function of their exosomes [32,33,34,35,36]. Previous studies have demonstrated that MSC-Exo could prevent CCl4-induced acute liver injury by inhibiting hepatocellular siderosis [36]. In this study, our data demonstrated that UC-MSCs-Exo treatment can effectively alleviate TAA-induced HSC activation and liver fibrosis in mice. It is consistent with a previous report that BM-MSCs-Exo treatment ameliorated CCl4-induced liver fibrosis via inhibition of HSC activation [37]. In addition, inflammation is one of the important factors leading to fibrosis [38]. Consistently, our results indicated that UC-MSCs-Exo could obviously reduce hepatic inflammation. Interestingly, to some extent, UC-MSCs-Exo exerts the better therapeutic effect than their parent cells. It is consistent with the previous report that the therapeutic effect of BM-MSCs-Exo against liver damage was greater than that of BM-MSCs [37]. It has been reported that exosomes have similar functions to their parent cells. Moreover, exosomes may offer several advantages over their parent cells [39, 40]: (1) exosomes are smaller in nanoscale size and less complex than their parent cells; (2) exosomes are less immunogenic than their parent cells; (3) exosomes are safer cell-free reagents than their parent cells. Based on these findings, we speculate that UC-MSCs-Exo may serve as a more promising therapeutic strategy for liver fibrosis.
Although the present study demonstrated that UC-MSCs-Exo alleviated TAA-induced liver fibrosis through inhibition of the Hedgehog/SMO signaling, the exact mechanism by which UC-MSCs-Exo regulates the Hedgehog/SMO signaling remains to be determined. Further studies are required to identify the crucial components (eg, protein and no-coding RNA) of UC-MSCs-Exo which are involved in regulating Hedgehog/SMO signaling. In addition, future studies are required to compare the difference of therapeutic effects by UC-MSCs-Exo in mice with good and poor outcomes, and address whether the poor treatment outcomes are associated with the dysregulation of Hedgehog/SMO signaling. This will further confirm that UC-MSCs-Exo do indeed exert their function through the Hedgehog/SMO signaling. Since cell-free-based therapies avoid the potential tumorigenicity, immunogenicity, emboli formation, undesired differentiation, and infection transmission of cell transplantation [40], our study demonstrates the translational potential of MSCs-based cell-free therapy for liver fibrosis. It should be noted that the variability in isolation, characterization, and administration protocols for UC-MSCs and their exosomes across different studies poses a challenge to the reproducibility and comparability of results [41]. Therefore, standardization of these protocols is necessary to facilitate accurate interpretation and validation of findings in the future.
In conclusion, the present study demonstrated that UC-MSCs-Exo reduced hepatic inflammation, inhibited HSC activation, and attenuated liver fibrosis, at least in part, through targeting the Hedgehog/SMO signaling. This study extends our current understanding of the mechanisms of MSCs-mediated anti-liver fibrosis, and would pave the way for the use of cell-free therapy for liver fibrosis.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- MCP1:
-
Monocyte chemoattractant protein-1
- IL-6:
-
Interleukin-6
- IL-1β:
-
Nterleukin-1β
- TNF-α:
-
Tumor necrosis factor α
- Acta2:
-
α-Smooth muscle actin
- Col1α1:
-
Collagen type1-α1
- Col3α1:
-
Collagen type III α1
- Timp1:
-
Tissue inhibitor of metalloproteinase-1
- MSCs:
-
Mesenchymal stem cells
- UC-MSCs:
-
Umbilical cord-derived MSCs
- UC-MSCs-Exo:
-
UC-MSCs-exosome
- SMO:
-
Smoothened
- ECM:
-
Extracellular matrix
- Gli:
-
Glioblastoma
- TAA:
-
Thioacetamide
References
Parola M, Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37–55
Yan Y, et al. Extra- and intra-cellular mechanisms of hepatic stellate cell activation. Biomedicines. 2021;9(8):1014
Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18(3):151–166
Friedman SL. Liver fibrosis–from bench to bedside. J Hepatol. 2003;38(1):S38-53
Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14(7):397–411
Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27–42
Lackner C, Tiniakos D. Fibrosis and alcohol-related liver disease. J Hepatol. 2019;70(2):294–304
Weng Z, et al. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J Hematol Oncol. 2021;14(1):136
Zhang W, et al. Comparison of therapeutic effects of mesenchymal stem cells from umbilical cord and bone marrow in the treatment of type 1 diabetes. Stem Cell Res Ther. 2022;13(1):406
Kern S, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301
Wang Y, et al. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–1016
Li C, et al. Hippo signaling controls NLR family pyrin domain containing 3 activation and governs immunoregulation of mesenchymal stem cells in mouse liver injury. Hepatology. 2019;70(5):1714–1731
Yu M, et al. Notch-activated mesenchymal stromal/stem cells enhance the protective effect against acetaminophen-induced acute liver injury by activating AMPK/SIRT1 pathway. Stem Cell Res Ther. 2022;13(1):318
El Agha E, et al. Mesenchymal stem cells in fibrotic disease. Cell Stem Cell. 2017;21(2):166–177
Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11–22
Wan T, et al. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci Adv. 2022;8(37):eabp9435
Xie Z, et al. Exosome-delivered CD44v6/C1QBP complex drives pancreatic cancer liver metastasis by promoting fibrotic liver microenvironment. Gut. 2022;71(3):568–579
Hou X, et al. Myeloid-cell-specific IL-6 signaling promotes microRNA-223-enriched exosome production to attenuate NAFLD-associated fibrosis. Hepatology. 2021;74(1):116–132
Vlassov AV, et al. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940–948
Greening DW, et al. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72–81
Kinoshita K, et al. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut. 2007;56(5):706–714
Omenetti A, et al. Hedgehog signaling in the liver. J Hepatol. 2011;54(2):366–373
Du K, et al. Hedgehog-YAP signaling pathway regulates glutaminolysis to control activation of hepatic stellate cells. Gastroenterology. 2018;154(5):1465–1479
Swiderska-Syn M, et al. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology. 2016;64(1):232–244
Machado MV, Diehl AM. Hedgehog signalling in liver pathophysiology. J Hepatol. 2018;68(3):550–562
Tong G, et al. Fibroblast growth factor 18 attenuates liver fibrosis and HSCs activation via the SMO-LATS1-YAP pathway. Pharmacol Res. 2022;178:106139
Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14(7):416–429
Bhave VS, et al. Regulation of liver growth by glypican 3, CD81, hedgehog, and Hhex. Am J Pathol. 2013;183(1):153–159
Chen Y, et al. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143(5):1319–1329
Kramann R, Schneider RK. The identification of fibrosis-driving myofibroblast precursors reveals new therapeutic avenues in myelofibrosis. Blood. 2018;131(19):2111–2119
Huang Y, et al. Single cell transcriptomic analysis of human mesenchymal stem cells reveals limited heterogeneity. Cell Death Dis. 2019;10(5):368
Lin Y, et al. Huc-MSC-derived exosomes modified with the targeting peptide of aHSCs for liver fibrosis therapy. J Nanobiotechnol. 2022;20(1):432
Heo JS, Kim S. Human adipose mesenchymal stem cells modulate inflammation and angiogenesis through exosomes. Sci Rep. 2022;12(1):2776
Dong L, et al. hUCMSC-extracellular vesicles downregulated hepatic stellate cell activation and reduced liver injury in S. japonicum-infected mice. Stem Cell Res Ther. 2020;11(1):21
Yan Y, et al. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther. 2017;25(2):465–479
Lin F, et al. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury. Cell Death Dis. 2022;13(3):271
Rong X, et al. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019;10(1):98
Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356(6342):1026–1030
Lotfy A, AboQuella NM, Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther. 2023;14(1):66
Hu C, et al. Mesenchymal stem cell-based cell-free strategies: safe and effective treatments for liver injury. Stem Cell Res Ther. 2020;11(1):377
Wu D, et al. Physical modulation of mesenchymal stem cell exosomes: a new perspective for regenerative medicine. Cell Prolif. 2024. https://doi.org/10.1111/cpr.13630
Funding
This work was supported by grants from the Natural Science Foundation of Hubei Province (2023AFB611), the Wuhan Municipal Science and Technology Bureau (2020020601012210), and the Scientific and Technological Project of Hubei Province in 2022 (2022BCE059).
Author information
Authors and Affiliations
Contributions
RZ and YZ performed in vitro and in vivo experiments, and data analysis; CJ and JP performed in vivo experiments; YY, WS, LK, YH, and YL performed in vitro experiments; CL contributed to the study concept and research design; RZ and CL wrote the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zong, R., Zheng, Y., Yan, Y. et al. Mesenchymal stem cells-derived exosomes alleviate liver fibrosis by targeting Hedgehog/SMO signaling. Hepatol Int (2024). https://doi.org/10.1007/s12072-024-10717-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12072-024-10717-y