Abstract
Background and aims
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease worldwide. In this study, we aimed to investigate the role and regulatory mechanism of Annexin A2 (ANXA2) in the pathogenesis of NAFLD.
Methods
Histological analyses and ELISA were used to illuminate the expression of ANXA2 in NAFLD and healthy subjects. The role of ANXA2 was evaluated using high-fat diet (HFD)-fed mice via vein injection of adeno-associated viruses (AAV) knocking down ANXA2 or non-targeting control (NC) shRNAs. Moreover, HepG2 and LO2 cells were employed as in vitro hepatocyte models to investigate the expression and function of ANXA2.
Results
ANXA2 was confirmed to be one of three hub genes in liver injury, and its expression was positively correlated with NAFLD activity score (NAS) and macrophage infiltration in NAFLD. Moreover, ANXA2 was significantly upregulated in NAFLD patients and HFD-fed mice. LPS/TLR4 pathway strongly upregulated ANXA2 expression, which is mediated by direct ANXA2 promoter binding by TLR4 downstream NF-κB p65 and c-Jun transcription factors. Increased ANXA2 expression was correlated with decreased autophagy flux and autophagy was activated by the depletion of ANXA2 in the models of NAFLD. Furthermore, ANXA2 interference led to the activation of AMPK/mTOR signaling axis, which may play a causal role in autophagy flux and the amelioration of steatosis.
Conclusions
ANXA2 is a pathological predictor and promising therapeutic target for NAFLD. ANXA2 plays a crucial role in linking inflammation to hepatic metabolic disorder and injury, mainly through the blockage of AMPK/mTOR-mediated lipophagy.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide [1]. Because of sedentary lifestyles and Western diet, the prevalence of NAFLD in Asia has risen to an alarming rate of 29.62% over the past decades [2]. NAFLD is characterized by excessive lipid accumulation in the liver and can progress to non-alcoholic steatohepatitis (NASH), connotes progressive liver injury that can lead to cirrhosis and eventually hepatocellular carcinoma (HCC) [3]. Currently, there is no effective pharmacological treatment for NAFLD. It is urgent to explore new biomarkers and therapeutic targets for this severe disease.
Autophagy plays a critical role in cellular physiology and dysregulation in autophagy-related processes can initiate various human diseases, including neurodegenerative, cardiovascular, musculoskeletal, pulmonary, kidney, metabolic disorders, and cancers [4]. Autophagic degradation of lipid droplets, termed lipophagy, plays a pivotal role in liver lipid catabolism, and the amelioration of NAFLD (29,109,982). During the development of NAFLD, the autophagic flux is compromised, partially contributing to the aberrant increase in hepatic lipid accumulation [5]. Based on human clinical studies, the regulation of autophagy can be a promising strategy for multiple human disorders, especially NAFLD [4].
Annexin A2 (ANXA2), a member of a super-family of Ca2+ dependent membrane binding proteins, is involved in a range of vital biological process, including cell proliferation, apoptosis, angiogenesis, and metastasis. ANXA2 is widely distributed in extracellular matrix, plasma membrane and endosomal compartments [6, 7]. Notably, ANXA2 plays a critical role in endocytic and extracellular trafficking, multi-vesicular biogenesis, and autophagy, and regulates the transportation and recycling of various macromolecules, including oligonucleotides, cellular receptors and lipids [6, 8, 9]. Accordingly, due to its role in cellular trafficking, ANXA2 has been reportedly involved in the regulation of various human diseases such as pulmonary fibrosis, severe acute pancreatitis, osteoporosis, and autoimmune disease [10,11,12]. Of importance, recent evidence indicates that the upregulation of ANXA2 was associated with methionine-choline deficient (MCD)-induced steatosis, inflammation and fibrosis in the liver [13]. Besides, ANXA2 is considered one of differential genes in steatotic livers of human subjects [14]. However, the underlying mechanism by which ANXA2 regulates the pathogenesis of NAFLD remains unknown.
In this study, integrative analysis of differentially expressed genes (DEGs), weighted gene co-expression network analysis (WGCNA), and ClueGO in CRA000931 and GSE126848 datasets revealed the upregulation of ANXA2 as a biomarker of NAFLD progression. Furthermore, bioinformatic analysis demonstrated the mRNA level of ANXA2 was positively correlated with NAFLD activity score (NAS) and fibrosis score, and nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPK) pathways were enriched in the high-expression group of ANXA2. We found that ANXA2 was markedly upregulated in NAFLD patients and mice, as well as in oleic acid (OA) treated hepatocytes. Knockdown of ANXA2 significantly alleviated the deregulation of lipid accumulation and liver steatosis in vivo and in vitro via the activation of AMP-activated protein kinase (AMPK)/mechanistic target of rapamycin (mTOR) pathway, highlighting ANXA2 as a novel target for the treatment of NAFLD.
Materials and methods
Detailed methods are presented in the supplementary materials and methods
Human liver samples
Liver samples were collected from patients who had undergone liver biopsy in Affiliated Hospital of Nantong University during 2015–2020. Participants with excessive alcoholic consumption, chronic viral hepatitis (hepatitis B and hepatitis C), drug-induced liver injury, Wilson’s disease, and autoimmune liver diseases were excluded. The human study was approved by the Human Research Ethics Committee of the Affiliated Hospital of Nantong University and conducted in accordance with all relevant ethical regulations. Informed consent was obtained from each participant.
Animal experiments
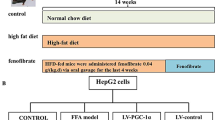
C57BL/6 male mice of 8 weeks were obtained from the Laboratory Animal Center of Nantong University. Mice were fed with normal chow diet (NCD) or high-fat diet (HFD, 45% of calories from fat) for 16 weeks. The HFD group was injected with associated virus vector (AAV)-non-targeting control (NC) or AAV-shANXA2 for 8 weeks. The mice were housed at room temperature and maintained at 23 °C (± 2 ℃) with a 12 h/12 h light/dark cycle, and ad libitum access to food and water. All animal protocols were approved by the Animal Care and Use Committee of Nantong University.
Cell culture
HepG2 cells were obtained from BeNa Culture Collection (BNCC, Beijing, China; #338070). LO2 cells were gifted from Shanghai Institute of Cell Biology. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin (Gibco, Thermo Scientific, Shanghai) at 37 °C in a humidified incubator containing 5% CO2. Cells were treated with 0.5 mM OA (CAS: 112-80-1, Sangon Biotech) and 1 μg/ml lipopolysaccharide (LPS) (L4391; Sigma). Small interfering RNA (siRNA)-ANXA2 was purchased from GenePharma Biotechnology Co. (Shanghai, China). All transfections were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) using the manufacturer's instructions. 24 h after transfection, cells were exposed to OA for the indicated periods of time.
Statistical analysis
All statistical analyses in this study were performed using GraphPad Prism 9.0 software. Mann–Whitney U-test (non-normal distribution) or unpaired t test (normal distribution) were used to analyze the significance between the two groups. ANOVA was used to analyze the significance of differences among more than two groups. The statistical significance was indicated as follows: *; #, p < 0.05, **; ##, p < 0.01, ***; ###, p < 0.001. Correlations were analyzed usingPearson’s correlation test. Data in tables were presented as means ± standard deviation (SD) or proportions. Binary unconditional logistic regression models were used to estimate the odds ratios (ORs) of NAFLD-related factors and corresponding 95% confidence intervals (CIs). Potential confounders including age and sex were adjusted. Multivariate analyses with stepwise logistic regression were used to determine the independent factors associated with NAFLD. All statistical tests were two sided. p < 0.05 was considered statistically significant.
Results
Integrative analysis of novel liver injury gene set during NAFLD progression
As a hallmark of NAFLD progression, progressive liver injury is a key determinant of the extent of NAFLD prognosis. However, the gene set underpinning liver injury and NAFLD deterioration remains poorly understood. To dissect pivotal genes underlying liver injury and their contributions in the development of NAFLD, we compiled a NAFLD-associated liver injury dataset to identify key genes and corresponding regulatory networks (Fig. 1a). Integrative analysis of human hepatic transcriptome signatures (NAFLD dataset, GEO accession number GSE126848), the diethylnitrosamine (DEN) + HFD-induced chronic liver injury (CLI) model dataset (DEN dataset, BIG Data Center (http://bigd.big.ac.cn) accession number CRA000931) and the acetaminophen (APAP)-induced acute liver injury (ALI) model dataset (APAP dataset) was conducted to interrogate the gene signatures critically involved in liver injury, with the flowchart depicted in Fig. 1a and the method section.
Integrative analysis of novel liver injury gene set during NAFLD progression. a The overarching framework and research methodology of this study. b The upper one indicates DEGs between ALI-VS-CON and CLI-VS-CON comparisons, while the below displays the number of shared DEGs between two comparisons. c Heatmap of the correlation between the module eigengenes and clinical traits of liver injury models; liver injury genes were identified by three module eigengenes. d Left: network analysis for the biological process functions of 210 liver injury genes; right: genes that acted as high-connection node (connected GOGroups > = 4) in the network. e The three hub genes were jointly identified by shared DEGs from (b) and high-connection nodes from (d)
In total, 55,357 genes were detected in the above datasets across 30 samples. Principal component analysis (PCA) suggested the existence of batch effects in the DEN and APAP datasets. The statistical differences between an experimental group and the control group were distinguished after the removal of batch effects (Fig. S1a, b). Following the removal of batch effects, the datasets were subjected to differential expression gene (DEG) analysis. Based on the selection criteria of adjusted p-value < 0.01 and FoldChange (FC) ≥ 1.4, 1829 DEGs were identified between CLI and control (CON), including 1473 up-regulated and 356 down-regulated genes (Fig. 1b, up, left). Likewise, we identified 774 DEGs between ALI and CON, including 539 up-regulated and 235 down-regulated genes (Fig. 1b, up, right). Notably, we observed 205 overlapping DEGs between ALI-VS-CON and CLI-VS-CON comparisons (Fig. 1b, down). Thereafter, we calculated the Median Absolute Deviation (MAD) of each gene and selected the top 5000 genes based on the MAD for WGCNA of the DEN dataset, APAP dataset and the combined data (DEN + APAP) [15]. The results showed that the turquoise module genes may be co-expressed in CLI group (Fig. S1c; Fig. 1c, up, left), while genes in the turquoise module were predominately expressed in ALI samples (Fig. S1d; Fig. 1c, up, right), as well as genes in the blue module of CON group (Fig. S1e; Fig. 1c, down, left). Lastly, we found 210 genes that met the criteria in all of the three modules of liver-injury conditions and referred to them as liver-injury genes (Fig. 1c, down, right).
ANXA2 is significantly upregulated in inflammation-related liver disease dataset
Furthermore, liver-injury genes were subjected to Gene Ontology (GO) pathway analysis, which revealed significant enrichments of genes involved in cell cycle regulation, cell proliferation, cell migration, phagocytosis, extracellular matrix regulation, regulation of lipase activity and DNA replication (Fig. 1d). We then calculated the numbers of GO functions (GO Terms) and the numbers of GO groups (GO Groups) connected by each gene, and selected genes with GO Groups ≥ 4 as central node genes (Fig. 1d). Three hub genes (ANXA2, LOX, CDKN1A) were identified both in DEGs and central node genes (Fig. 1e). To further explore the roles of these hub genes in NAFLD, we examined their expression profiles in a clinical NAFLD dataset (GSE126848), and revealed significantly increased expression of ANXA2 (p = 9.7E−10) and CDKN1A (p = 1.6E−06) in the NAFLD group, as compared to the controls (Fig. 2a). Of great intrigue, while the role of p21CDKN1A in hepatic injury and death has been well recognized, the involvements of ANXA2 in liver injury and NAFLD development remain to be investigated.
ANXA2 expression is positively correlated with NAFLD severity, fibrosis score and inflammatory infiltration. a The mRNA expression levels of three hub genes between NAFLD (n = 31) and control (n = 14) groups in GSE126848 dataset. b Point plots for the mRNA expression levels of ANXA2 in GSE135251 (NAFLD = 206 and control = 10) in which patients were stratified by NAS or fibrosis score. Linear regression was performed. c Volcano plot of DEGs between ANXA2-high group and ANXA2-low group in GSE135251. Inflammation and fibrosis-related DEGs were shown. d Inflammation and fibrosis-related pathways were significantly enriched in the ANXA2-high group by KEGG pathway enrichment analysis. e Relevance analysis between the mRNA expression levels of ANXA2 and CD68, CCL2 or CCR2 in GSE174478 (NAFLD = 94). f Inflammatory pathways were significantly enriched in the ANXA2-high group by GSEA in GSE174478. g GSEA showed the enrichment of NF-κB and MAPK pathways in the livers of ANXA2-high group VS ANXA2-low group
ANXA2 expression is positively correlated with NAFLD severity, fibrosis score and inflammatory infiltration
To decipher the role of ANXA2 in the regulation of liver injury and NAFLD pathogenesis, we analyzed the correlation among ANXA2 expression and NAFLD severity. Analysis of transcriptome dataset GSE135251 (206 patients with NAFLD and 10 control patients) from the Gene Expression Omnibus, revealed a significant positive correlation among ANXA2 mRNA expression, the NAS and fibrosis score (Fig. 2b). Moreover, the patients were divided into ANXA2-high and ANXA2-low groups according to the median of ANXA2 expression. Differential analysis and KEGG enrichment analysis showed that inflammation and fibrosis-related pathways were significantly enriched in the ANXA2-high group (Fig. 2c, d). Analysis of transcriptome dataset GSE174478 (94 patients with NAFLD), showed that patients with high ANXA2 expression exhibited worsened macrophage infiltration, compared with those with low ANXA2 expression (Fig. 2e). Likewise, Gene Set Enrichment Analysis (GSEA) validated that NAFLD-associated inflammatory pathways were significantly enriched in the ANXA2-high group (Fig. 2f), including the NF-κB (Fig. 2g) and MAPK (Fig. 2h) pathways.
Multivariate analysis reveals that ANXA2 level in serum is an independent and strong predictive factor of NAFLD
Next, we examined whether ANXA2 expression is indicative of NAFLD diagnosis using univariate and multivariate analyses [16, 17]. A total of 148 NAFLD patients and 70 control subjects were included in the analysis. Univariate analysis uncovered that body mass index (BMI), alanine aminotransferase (ALT), total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), glycosylated hemoglobin type A1c (HbA1c), fasting blood glucose (FBG), ANXA2 and non-significant differences in aspartate transaminase (AST) and high-density lipoprotein (HDL) levels may be predictive for NAFLD occurrence (Table S1). According to the multivariate analysis, BMI, ALT, TG and ANXA2 were independent predictors for NAFLD. When ANXA2 level in serum increased by 1, the risk of developing NAFLD increased by 292.3% (OR 3.923; 95% CI 1.411–10.909; p = 0.009) (Table S2).
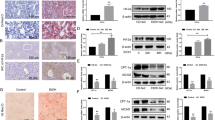
In NAFLD, NF-κB p65 and c-Jun are activated which can increase the transcriptional activity of ANXA2 and result in the overexpression of ANXA2
Immunohistochemistry and immunofluorescence of clinical liver tissues revealed that ANXA2 was obviously overexpressed in NAFLD patients, as compared to the tissues of healthy subjects (Fig. 3a). In line with these data, the protein level of ANXA2 was significantly upregulated in the liver of HFD-fed mice (Fig. 3b, c). Moreover, we examined the regulation of ANXA2 expression using HepG2 hepatoma as an in vitro hepatocyte model. quantitative polymerase chain reaction (qPCR) and Western blot analyses revealed that ANXA2 expression was increased in OA-exposed hepatocytes (Fig. 3d, e). In parallel, we showed that ANXA2 expression was upregulated by LPS/TLR4 pathway, as the upregulation of ANXA2 mRNA and protein levels were coincidentally accompanied by the activation of TLR4 downstream NF-κB p65 and c-Jun pathways (Fig. 3f, g). The results were in line with our previous finding that NF-κB and MAPK pathways were activated in the ANXA2-high group. Furthermore, we revealed that LPS exposure increased the occupancy of p65 and c-Jun on the promoter of ANXA2, suggesting that ANXA2 is a direct transcriptional target of TLR4-mediated NF-κB p65 and c-Jun signaling (Fig. 3h).
In NAFLD, NF-κB p65 and c-Jun are activated which can increase the transcriptional activity of ANXA2 and result in the overexpression of ANXA2. a Representative images of immunohistochemical (IHC) staining and Immunofluorescence for ANXA2 in liver sections from Normal (n = 3) and NAFLD patients (n = 6). Original magnification: 400X, 200X. b Immunohistochemical staining for ANXA2 in liver tissues of NCD-fed mice and HFD-fed mice. Original magnification: 400×. c Western blot analysis of ANXA2 in the livers of NCD-fed mice and HFD-fed mice were determined by western blot and normalized to GAPDH (n = 3 per group). d The mRNA levels of ANXA2 in HepG2 cells treated with or without OA (500 μM) were measured by qPCR. e Western blot analysis of ANXA2 in HepG2 cells treated with or without OA (500 μM) for 24 h. f Western blot analysis of ANXA2, p-p65, p65, p–c-Jun, c-Jun in HepG2 cells treated with LPS (1 μg/ml) for 0 h, 3 h, 6 h, 12 h, 24 h, and 48 h. *, compared with 0 h. g The mRNA levels of ANXA2 in HepG2 cells treated with LPS (1 μg/ml) for 0 h, 3 h, 12 h, and 48 h were measured by qPCR. *, compared with 0 h. h Chromatin immunoprecipitation (ChIP) assay confirmed that p65 and c-Jun could bind to the promoter region of ANXA2 in HepG2 cells and their binding ability was enhanced by LPS treatment. *, compared with IgG; #, compared with CON
Depletion of ANXA2 alleviates NAFLD progression by activating autophagy flux
To further assess the mechanism by which ANXA2 participates in NAFLD development, we established an HFD-mediated NAFLD model in C57BL/6 mice and then interfered with hepatic ANXA2 expression via intravenous injection of AAV-shANXA2 (Fig. 4a, b). The body weight and liver volume were significantly decreased after ANXA2 depletion, compared with the AAV-NC group (Fig. 4c, d). ANXA2 reduction notably attenuated HFD-induced hepatic lipid accumulation (Fig. 4e). Furthermore, transmission electron microscopes (TEM) analysis indicated that NCD-fed mice exhibited significantly increased number of autophagosomes in liver tissues compared with HFD-fed mice (Fig. 4f). Notably, treatment with AAV-shANXA2 restored the number of autophagosomes and decreased the number of lipid droplets in HFD-fed mice (Fig. 4f). To further evaluate the involvement of ANXA2 in autophagic regulation, we examined the levels of LC3-II/I ratio and autophagosome adapter p62. Western blot analysis revealed that HFD feeding reduced the LC3-II/I ratio and enhanced the hepatic level of p62 (Fig. 4g). In contrast, these alterations were reversed after ANXA2 interference in the livers of HFD-fed mice (Fig. 4h).
Depletion of ANXA2 alleviates NAFLD progression by activating autophagy flux. a Experimental outline. b Western blot analysis of ANXA2 in HFD-fed mice injected with AAV-NC or AAV-shANXA2. c Liver images of mice in different groups. d Body weight of mice in different groups (n = 10 per group). e The images of hematoxylin and eosin (H&E) staining and Oil Red O staining from different groups (n = 5 per group). *, compared with NCD + AAV-NC; #, compared with HFD + AAV-NC. f Autophagosomes in hepatocytes in the liver tissues from different groups were observed by TEM. The red arrows indicate autophagosomes, the yellow arrows indicate lipid droplets. Original magnification: 2000× (n = 5 per group). g Western blot analysis of p62, LC3-I, LC3-II in the livers of NCD-fed mice and HFD-fed mice (n = 3 per group). h Western blot analysis of p62, LC3-I, LC3-II in HFD-fed mice injected with AAV-NC or AAV-shANXA2 (n = 3 per group)
ANXA2 blocks autophagy and aggravates OA-induced lipid deposition
Thereafter, we investigated the involvement of ANXA2 in the regulation of autophagic flux using hepatic cell cultures. We screened three ANXA2 small interfering RNA (siRNA) oligos and found that siRNA-ANXA2-512 effectively depleted ANXA2 in hepatocytes (Fig. 5a). As predicted, Oil Red O staining assay showed that the increase of lipid droplets in OA-treated hepatocytes was reversed by the interference of ANXA2 (Fig. 5b; Fig. S2a). To validate the regulatory role of ANXA2 in autophagic flux, we employed autophagic flux inhibitor chloroquine (CQ), which blocks lysosomal degradation of LC3-II and causes its accumulation following autophagic flux. Downregulation of ANXA2 significantly increased the LC3-II/I ratio and decreased p62 expression both in the presence or absence of CQ (Fig. 5c; Fig. S2b). TEM analysis further revealed that ANXA2 depletion increased autophagosomes and decreased lipid droplets both in OA-treated and -untreated cells (Fig. 5d; Fig. S2c).
ANXA2 blocks autophagy and aggravates OA-induced lipid deposition. a Western blot analysis of ANXA2 of HepG2 cells transfected with control or different ANXA2 siRNAs to screen for the appropriate siRNA-ANXA2. *, compared with si-NC. b The images of HepG2 cells transfected with control or siRNA-ANXA2 and were exposed to OA or not OA for 24 h by Oil Red O staining. c HepG2 cells transfected with control or siRNA-ANXA2 were exposed to OA for 24 h in the presence or absence of CQ, ANXA2, p62, LC3-I, LC3-II proteins were assessed by western blot and normalized to GAPDH. *, compared with si-NC + DMSO; #, compared with siANXA2 + CQ. d Autophagosomes in hepatocytes transfected with control or siRNA-ANXA2 in the presence or absence of OA were observed by TEM. The red arrows indicate autophagosomes, and the yellow arrows indicate lipid droplets. Original magnification: 5000×
ANXA2 inhibits autophagy related to the AMPK-mTOR pathway in vivo and in vitro
Western blot analysis showed that HFD significantly reduced hepatic p-AMPK/AMPK ratio and increased p-mTOR/mTOR ratio, compared with the chow diet (Fig. 6a). In agreement with a beneficial effect of ANXA2 interference on lipid metabolism and autophagic flux, ANXA2 interference increased p-AMPK/AMPK ratio and decreased p-mTOR/mTOR ratio in HFD-fed mice (Fig. 6b). Similar trends were found in OA-treated cells (Fig. 6c, d; Fig. S2d, e). These data imply that ANXA2 may inhibit the phosphorylation of AMPK, which in turn leads to mTOR activation and autophagy flux blockade.
ANXA2 inhibits autophagy related to the AMPK-mTOR pathway in vivo and in vitro. a Western blot analysis of p-AMPK, AMPK, p-mTOR and mTOR in NCD-fed mice and HFD-fed mice. b Western blot analysis of p-AMPK, AMPK, p-mTOR and mTOR in HFD-fed mice injected with AAV-NC or AAV-shANXA2. c Western blot analysis of p-AMPK, AMPK, p-mTOR and mTOR in HepG2 cells treated with or without OA. d Western blot analysis of p-AMPK, AMPK, p-mTOR and mTOR in OA-treated HepG2 cells transfected with control or siRNA-ANXA2
Discussion
In the present study, we demonstrated the critical role of ANXA2 in the development of NAFLD using integrative bioinformatics analysis and functional validation. ANXA2 was markedly upregulated in serum and liver tissues of NAFLD subjects, and its depletion protected mice from lipid accumulation and liver injury. ANXA2 exacerbates lipid metabolism and accumulation by the blockade of AMPK/mTOR signaling-mediated autophagy flux. Of great importance, we revealed that ANXA2 expression is initiated by LPS/TLR4-mediated NF-κB and c-Jun signaling. These studies highlight ANXA2 as an important intermediary protein linking aberrant inflammation to hepatic metabolic disorder and liver injury, suggesting a potential target for NAFLD therapy.
Liver injury, which represents the pivotal instigator of the pathogenesis underlying various liver diseases including NAFLD, initiates a chain of pathological events characterized by chronic inflammation, stress events, and repairing processes [18]. During the progression of NAFLD, liver injury is mostly attributed to lipotoxicity, pro-inflammatory cytokines and oxidative stress. In this regard, inflammation has been well documented to play an indispensable role in hepatic damage and death. TLR4 signaling plays an integral role in metabolic disorders and liver injury during NAFLD development, via its role in the regulation of downstream targets involved in lipid metabolism and stress events (26,172,853, 26,113,297). Using integrative bioinformatic analysis, we showed that the expression of ANXA2, along with CDKN1A, is indicative of NAFLD progression and liver injury. Moreover, our study for the first time revealed that ANXA2 expression is strongly initiated by LPS/TLR4 signaling, suggesting that ANXA2 upregulation may result from pro-inflammatory environment during NAFLD development, underscoring the potential of employing ANXA2 as an indicator of NAFLD-associated inflammatory infiltration and liver injury.
While the role of ANXA2 in facilitating tumor progression has been intensively investigated, its involvement in metabolic diseases remains poorly understood [13]. In addition, aberrant expression of ANXA2 may serve as a potential predictive factor and diagnostic biomarker in assorted cancer types [19]. A recent study showed that ANXA2 was highly expressed in insulin-resistant epididymal adipose tissues and silencing ANXA2 was shown to ameliorate palmitic acid (PA)-induced insulin resistance in differentiated 3T3L1 cells [20]. ANXA2 has also been reportedly involved in the development of hepatic fibrosis via the initiation of caspase-1-mediated hepatocyte pyroptosis (36,324,154). ANXA2 exhibited the most significant correlation with severity of the liver inflammation and fibrosis [21]. Overexpression of S100A11/ANXA2 has been documented as a biomarker of poor prognosis in liver fibrosis and HCC [13]. Wang et al. reported that ANXA2 ameliorated liver fibrosis in NASH via regulating proinflammatory factors [22]. Despite the fact that ANXA2 has been demonstrated as a key player in inflammatory regulation and hepatic injury, the association between ANXA2 and lipid metabolism remains unclarified. Our study showed that depleting ANXA2 using ANXA2 siRNA oligos could lead to reduced lipid accumulation and liver injury, and meanwhile an increase in autophagic flux in NAFLD mice and OA-treated hepatocyte cells. Moreover, we reported that ANXA2 inhibited autophagy to facilitate lipid metabolism via regulating the AMPK/mTOR pathway. These findings underscore the potential of targeting ANXA2 as a general intervening strategy against both metabolic disorders and hepatic injury during the progression of NAFLD and NASH. Therefore, the development of highly selective and potent ANXA2 pharmacological inhibitors will be important for the management of metabolic liver diseases, including NAFLD and NASH.
Collectively, the present work employed liver injury datasets to deduce key genes in the pathological process of liver injury in NAFLD and showed an important role of ANXA2 in NAFLD progression. We showed that ANXA2 expression was increased in the liver and serum of patients and mice with NAFLD. The initiation of ANXA2 expression by the pro-inflammatory TLR4 pathway underscores the importance of ANXA2 as a mediary protein linking inflammatory environment to metabolic disorders and liver injury. Importantly, the depletion of ANXA2 ameliorated HFD-induced lipid accumulation and liver injury, highlighting the therapeutic potential of targeting ANXA2 in NAFLD. We further identified that ANXA2 negatively regulates autophagy flux and hence lipid catabolism by regulating the AMPK/mTOR pathway. Taken together, these data indicate that ANXA2 is a pivotal driver of inflammation-associated NAFLD progression and a novel target for the treatment of NAFLD-related lipid accumulation and liver injury.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739–752
Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389–398
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922
Klionsky DJ, Petroni G, Amaravadi RK, Baehrecke EH, Ballabio A, Boya P, et al. Autophagy in major human diseases. EMBO J. 2021;40: e108863
Allaire M, Rautou PE, Codogno P, Lotersztajn S. Autophagy in liver diseases: time for translation? J Hepatol. 2019;70:985–998
Valapala M, Vishwanatha JK. Lipid raft endocytosis and exosomal transport facilitate extracellular trafficking of annexin A2. J Biol Chem. 2011;286:30911–30925
Zhang HT, Zeng Q, Wu B, Lu J, Tong KL, Lin J, et al. TRIM21-regulated Annexin A2 plasma membrane trafficking facilitates osteosarcoma cell differentiation through the TFEB-mediated autophagy. Cell Death Dis. 2021;12:21
Wang S, Sun H, Tanowitz M, Liang XH, Crooke ST. Annexin A2 facilitates endocytic trafficking of antisense oligonucleotides. Nucleic Acids Res. 2016;44:7314–7330
de Graauw M, Cao L, Winkel L, van Miltenburg MH, le Devedec SE, Klop M, et al. Annexin A2 depletion delays EGFR endocytic trafficking via cofilin activation and enhances EGFR signaling and metastasis formation. Oncogene. 2014;33:2610–2619
Wang K, Zhang T, Lei Y, Li X, Jiang J, Lan J, et al. Identification of ANXA2 (annexin A2) as a specific bleomycin target to induce pulmonary fibrosis by impeding TFEB-mediated autophagic flux. Autophagy. 2018;14:269–282
Tang G, Yu C, Xiang K, Gao M, Liu Z, Yang B, et al. Inhibition of ANXA2 regulated by SRF attenuates the development of severe acute pancreatitis by inhibiting the NF-kappaB signaling pathway. Inflamm Res. 2022;71:1067–1078
Weiss R, Bitton A, Ben Shimon M, Elhaik Goldman S, Nahary L, Cooper I, et al. Annexin A2, autoimmunity, anxiety and depression. J Autoimmun. 2016;73:92–99
Sobolewski C, Abegg D, Berthou F, Dolicka D, Calo N, Sempoux C, et al. S100A11/ANXA2 belongs to a tumour suppressor/oncogene network deregulated early with steatosis and involved in inflammation and hepatocellular carcinoma development. Gut. 2020;69:1841–1854
Rezaei Tavirani M, Rezaei Tavirani M, Zamanian AM. ANXA2, PRKCE, and OXT are critical differentially genes in Nonalcoholic fatty liver disease. Gastroenterol Hepatol Bed Bench. 2019;12:131–137
Lee KH, Bosco A, O’Sullivan M, Song Y, Metcalfe J, Yu K, et al. Identifying gene network patterns and associated cellular immune responses in children with or without nut allergy. World Allergy Organ J. 2022;15: 100631
Mohamed RZ, Jalaludin MY, Anuar ZA. Predictors of non-alcoholic fatty liver disease (NAFLD) among children with obesity. J Pediatr Endocrinol Metab. 2020;33:247–253
Seo YB, Han AL. Association of the serum uric acid-to-creatinine ratio with nonalcoholic fatty liver disease diagnosed by computed tomography. Metab Syndr Relat Disord. 2021;19:70–75
Clemens MM, McGill MR, Apte U. Mechanisms and biomarkers of liver regeneration after drug-induced liver injury. Adv Pharmacol. 2019;85:241–262
Li Z, Yu L, Hu B, Chen L, Jv M, Wang L, et al. Advances in cancer treatment: a new therapeutic target, Annexin A2. J Cancer. 2021;12:3587–3596
Wang Y, Cheng YS, Yin XQ, Yu G, Jia BL. Anxa2 gene silencing attenuates obesity-induced insulin resistance by suppressing the NF-kappaB signaling pathway. Am J Physiol Cell Physiol. 2019;316:C223–C234
Staten NR, Welsh EA, Sidik K, McDonald SA, Dufield DR, Maqsodi B, et al. Multiplex transcriptional analysis of paraffin-embedded liver needle biopsy from patients with liver fibrosis. Fibrogenesis Tissue Repair. 2012;5:21
Wang G, Duan J, Pu G, Ye C, Li Y, Xiu W, et al. The Annexin A2-Notch regulatory loop in hepatocytes promotes liver fibrosis in NAFLD by increasing osteopontin expression. Biochim Biophys Acta Mol Basis Dis. 2022;1868: 166413
Funding
This work was supported by the National Natural Science Foundation of China (No. 82070624, No. 82272624, No. 81401985, No. 31801113 and No. 82273206), China Postdoctoral Science Foundation (No. 2018M632830, No. 2020M670039ZX and No. 2021M701793), Key Project of Health Commission of Jiangsu Province (No. ZDB2020006), Natural Science Foundation of Jiangsu Province (No. BK20211105), Social Development Foundation of Nantong City (No. MS12021073, No. MS22022005, No. JCZ21061 and No. MS22022014), Science and technology project of Nantong City (No. MS22022109 and No. JC12022026), Jiangsu Provincial Research Hospital (No. YJXYY202204).
Author information
Authors and Affiliations
Contributions
Conceptualization, Methodology and Project administration: JL, QZ. Investigation: MZ. Formal analysis: MZ, HW, JQ. Resources: XW, HW. Data Curation: YX, ML, SC, QT, QW. Writing-Original Draft: MZ, HW. Validation: LQ, QZ, CL, MX. Writing-Review & Editing: JL, LQ, QZ, BH. Visualization: MZ, HW, JQ. Funding acquisition: JL.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflicts of interest.
Ethics approval
The human study was approved by the Human Research Ethics Committee of the Affiliated Hospital of Nantong University and conducted in accordance with all relevant ethical regulations. All animal use and viral protocols were approved by the Committee on the Use of Live Animals in Teaching and Research, Nantong University.
Patient consent statement
Written consent was obtained from all the participants involved in this study.
Permission to reproduce material from other sources
No reproduction material from other sources was included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, H., Zhou, M., Jin, Q. et al. The upregulation of Annexin A2 by TLR4 pathway facilitates lipid accumulation and liver injury via blocking AMPK/mTOR-mediated autophagy flux during the development of non-alcoholic fatty liver disease. Hepatol Int 18, 1144–1157 (2024). https://doi.org/10.1007/s12072-023-10622-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10622-w