Abstract
Background
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a newly proposed definition of fatty liver disease (FLD) independent of excessive alcohol consumption (EAC) and hepatitis viral infection. Evidence on the mortality risk in different types of FLD [nonalcoholic FLD (NAFLD), alcoholic FLD (AFLD), and MAFLD] is sparse, hindering the identification of high-risk populations for preferential clinical surveillance.
Methods
A total of 11,000 participants in the Third National Health and Nutrition Examination Survey were enrolled. Participants were categorized into three groups [FLD( − ), MAFLD( − ), and MAFLD( +)] according to FLD and MAFLD criteria, and further categorized into six groups by EAC. Multivariate Cox proportional hazard model was used to estimate the risk of all-cause, cardiovascular-related, and cancer-related mortality.
Results
During a median follow-up of 23.2 years, a total of 3240 deaths were identified. Compared with FLD( − )/EAC( − ) participants, MAFLD( +) individuals had higher all-cause mortality risk [hazard ratio (HR) = 1.28, 95% confidence interval (CI) = 1.18–1.39] regardless of EAC status [MAFLD( +)/NAFLD: HR = 1.22, 95%CI = 1.11–1.34; MAFLD( +)/AFLD: HR = 1.83, 95%CI = 1.46–2.28], while not for MAFLD( − ) individuals. Furthermore, diabetes-driven-MAFLD had higher mortality risk (HR = 2.00, 95%CI = 1.77–2.27) followed by metabolic dysregulation-driven-MAFLD (HR = 1.30, 95%CI = 1.06–1.60) and overweight/obesity-driven-MAFLD (HR = 1.11, 95%CI = 1.00–1.22). Additionally, MAFLD( − ) participants with elevated fibrosis score were also associated with statistically significantly higher mortality risk (HR = 3.23, 95%CI = 1.63–6.40).
Conclusions
Utilizing a representative sample of the US population, we proved the validity of MAFLD subtype and fibrosis score, rather than the traditional definition (NAFLD and AFLD), in the risk stratification of FLD patients. These findings may be applied to guide the determination of surveillance options for FLD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a new definition of fatty liver disease (FLD) proposed in 2020, characterized by the presence of hepatic steatosis and accompanied by either type 2 diabetes mellitus (T2DM), overweight/obesity, or lean metabolic dysregulation [1]. MAFLD has been one of the common causes of liver dysfunction worldwide [2] and has affected approximately 20–30% of the US general population [3]. Meanwhile, MAFLD is closely linked to worse hepatic and extra-hepatic (cardiovascular and non-liver malignancies) adverse outcomes [4]. For the large population of MAFLD, it is important to target high-risk groups for priority surveillance.
As for the two main types of traditional fatty liver definition, non-alcoholic FLD (NAFLD) and alcoholic FLD (AFLD) share histopathological features and spectrums [5], but have different risks of morbidity and mortality [6]. Although most studies showed that participants with MAFLD were at a higher risk of death than those with NAFLD [7,8,9], there is a lack of evidence concerning mortality risk by alcohol intake and different types of FLD.
In addition, since MAFLD was a heterogeneous disease, the variation of the association between different MAFLD subtypes with mortality was not well described, with the available studies suggesting conflicting results [10,11,12,13]. A recent study based on 4718 adults aged 45–80 years in Austria suggested that the association between MAFLD and mortality was primarily driven by T2DM [11]. While another study in Korean aged 40–70 years showed different results, noting the highest mortality risk was observed in individuals with the lean metabolic dysregulation subtype [13].
Increasing evidence suggested that non-invasive fibrosis scores, consisting of easy-to-get clinical parameters and biomarkers, could be used to identify FLD patients at high risk for advanced fibrosis [14] and as a screening tool for primary care [15]. Recent evidence has also shown the validity of the non-invasive scores in identifying advanced fibrosis [16] and predicting prognosis [17] in MAFLD patients. However, little is known about the role of non-invasive scores in FLD individuals unfulfilling MAFLD criteria.
Therefore, utilizing a longitudinal study of a nationally representative sample of the US adults, we comprehensively evaluated the variation in long-term mortality risk of FLD individuals by the presence of MAFLD and its subtypes (T2DM, overweight/obesity, and lean metabolic dysregulation [1]). Furthermore, we provide evidence for the definition of FLD in high-risk populations based on the MAFLD subtype and fibrosis severity.
Materials and methods
Study population
A retrospective analysis of a population-based sample was conducted based on the Third National Health and Nutrition Examination Survey (NHANES III) (1988–1994) in the US [18]. In-person interview, physical examination, and laboratory tests were conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention. A total of 13,856 adults aged 20–74 years from NHANES III completed hepatic steatosis ultrasound examination (HSUE). We excluded participants with missing data (N = 2518) on FLD definition (N = 2077), fibrosis score (N = 270), mortality status (N = 37), and covariables (N = 134). We also excluded participants with positive serum hepatitis viral antibody (N = 338) in the main analysis. Finally, a total of 11,000 participants were included (Supplementary Fig. 1). The NHANES III survey was approved by the institutional review board of the NCHS (https://www.cdc.gov/nchs/nhanes/irba98.htm), and written informed consent was obtained from all participants.
Assessment of clinical and laboratory test
Clinical information was collected by questionnaires, physical measurements, and laboratory tests. Excessive alcohol consumption (EAC) was defined as individuals had an average alcohol intake of > 3 drinks/day in males and > 2 drinks/day in females [8]. T2DM was defined as either the clinical diagnosis of diabetes, current use of anti-diabetic medication, fasting blood glucose ≥ 126 mg/dl, and/or hemoglobin A1c ≥ 6.5%. Other definitions were described in Supplementary Methods.
Ascertainment of different types of fatty liver disease and advanced fibrosis
Hepatic steatosis was ascertained by HSUE using Toshiba Sonolayer SSA-90A (Tustin, CA) [19]. Based on five parameters (liver to kidney contrast, parenchymal brightness, deep beam attenuation, bright vessel walls, and gallbladder wall definition), the ultrasonographic assessment was classified as normal, mild, moderate, or severe hepatic steatosis. Mild to severe hepatic steatosis was considered as FLD [20]. Results in intra-rater and inter-rater reliability (% agreement) of radiologic evaluations were 91.3% (Kappa = 0.77) and 88.7% (Kappa = 0.70), respectively [20]. NAFLD was defined as the presence of FLD without EAC. AFLD was defined as the presence of FLD with EAC. For both NAFLD and AFLD groups, individuals with viral hepatitis were excluded [8, 9]. MAFLD was defined as the evidence of hepatic steatosis accompanied by one of three following features: T2DM, overweight/obesity (defined as BMI ≥ 25 kg/m2), or lean metabolic dysregulation (Supplementary Methods) [1].
Regarding advanced fibrosis, three scores [aspartate aminotransferase to platelet ratio index (APRI), fibrosis-4 (FIB-4) index, and NAFLD fibrosis score (NFS)] were calculated (Supplementary Methods).
In the present analysis, we first classified all participants into the following three groups based on FLD and MAFLD status: FLD( − ), MAFLD( − ), and MAFLD( +). Then, by incorporating EAC status, participants were further classified into the following six groups: FLD( − )/EAC( − ), FLD( − )/EAC( +), MAFLD( − )/NAFLD, MAFLD( − )/AFLD, MAFLD( +)/NAFLD, and MAFLD( +)/AFLD.
Two kinds of subtype analyses were conducted among MAFLD( +) participants. First, all MAFLD( +) participants were categorized as T2DM-driven-MAFLD, overweight/obesity-driven-MAFLD (participants free of T2DM and with BMI ≥ 25.0 kg/m2), or metabolic dysregulation-driven-MAFLD (lean individuals with at least 2 metabolic risk factors among non-diabetic participants) [10]. Second, according to the specific criteria of MAFLD, participants were categorized into the following seven groups: BMI( +), T2DM( +), MD( +), BMI( +)&MD( +), BMI( +)&T2DM( +), MD( +)&T2DM( +), and BMI( +)&MD( +)&T2DM( +). More details were described in the Supplementary Methods.
Ascertainment of mortality
The public-use linked mortality files from the National Death Index (NDI) provided mortality follow-up data from the date of survey participation through December 31, 2015. Causes of death were recorded by the International Classification Disease-Ninth/Tenth Revision (ICD-9/10). Participants were followed from the date of examination at the mobile examination center until death or December 31, 2015. In this study, the main outcomes were all-cause mortality, cardiovascular-related mortality (I00–I09, I11, I13, I20–I51, and I60–I69), and cancer-related mortality (C00–C97).
Statistical analysis
We accounted for the complex NHANES survey design, survey nonresponse, poststratification, and oversampling by applying appropriate sample weights in all statistical analyses, according to the analytical guidelines published by NCHS. Therefore, the estimates from our analyses were representative of the entire US non-institutionalized population. Continuous data were presented as mean ± standard errors and categorical data were presented as total numbers (percentages). Analysis of variance and chi-square test adjusting for sampling weights were used to compare basic characteristics for continuous and categorical variables. We applied survey-weight adjusted multivariable Cox proportional hazards regression model to estimate hazard ratios of all-cause and cardiovascular-related, and cancer-related mortality. Multivariable model 1 was adjusted for sex (male or female), age (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, or other), educational level (< 9th grade, 9–11th grade, high school or higher), marital status (married, divorced/widowed, or never married), smoking status (never, past smoker, or current smoker), and sedentary lifestyle (yes or no). Model 2 was adjusted for alanine aminotransferase (continuous), and total cholesterol (continuous) in addition to model 1. Model 3 was adjusted for dietary intake of meats (yes or no), vegetables (yes or no), and fruits (yes or no) in addition to model 2.
Three sensitivity analyses were conducted. First, participants who died within the first three years of follow-up were excluded to minimize potential reverse causation. Second, participants with viral hepatitis were included to explore the impact of viral infection on mortality risk in FLD individuals. Third, we further adjusted metabolic factors in the MAFLD subtypes analysis to exclude possible confounding. All analyses were conducted using R v4.0.3. Two-sided p < 0.05 was considered statistically significant.
Results
Basic characteristic
A total of 11,000 adults, comprising 7030 participants without steatosis [i.e., FLD( − ) group, including 5661 EAC( − ) participants and 1369 EAC( +) participants], 563 FLD participants unfulfilling MAFLD criteria [i.e., MAFLD( − ) group, including 416 NAFLD participants and 147 AFLD participants], and 3407 FLD participants meet MAFLD criteria [i.e., MAFLD( +) group, including 2737 NAFLD participants and 670 AFLD participants] were included in the present analysis. Compared with FLD( − ) group, MAFLD( +) group were older (mean age 47.8 years), more likely to be male (55.7%), overweight participants (88.5%), with T2DM (16.8%), hypertension (51.0%), sedentary lifestyle (23.9%), and have elevated level of total cholesterol (214.6 mg/dl) and fasting glucose (107.5 mg/dl). Meanwhile, MAFLD( +) individuals were less likely to be current smokers (23.5%) and with lower high-density lipoprotein cholesterol (HDL-C) levels (44.4 mg/dl). Moreover, among the 59.7% (691/1206) T2DM patients who reported their diagnostic time, the mean duration (from the first diagnosis to the baseline survey) was 7 years (Table 1 and Supplementary Table 1).
Mortality risk in individuals stratified by MAFLD
During a median follow-up of 23.2 years (interquartile range: 21.6–25.0), a total of 3240 deaths (861 cardiovascular-related deaths and 801 cancer-related deaths) were documented. Compared with FLD( − ) group, MAFLD( +) individuals had significantly higher risk of all-cause mortality [hazard ratio (HR) = 1.26, 95% confidence interval (CI) = 1.16–1.36], cardiovascular-related mortality (HR = 1.26, 95%CI = 1.06–1.50), and cancer-related mortality (HR = 1.34, 95%CI = 1.05–1.72). However, MAFLD( − ) individuals were not significantly associated with mortality risk. (Fig. 1, Supplementary Table 2).
Association of fatty liver disease with all-cause, cardiovascular-related, and cancer-related mortality stratified by MAFLD status. aAdjusted for sex, age, race/ethnicity, education, marital status, smoking status, sedentary lifestyle, alanine aminotransferase, total cholesterol, intake of meats, fruits, and vegetables. FLD fatty liver disease, MAFLD metabolic dysfunction-associated fatty liver disease, HR hazard ratio, CI confidence interval
Based on the above three groups [FLD( − ), MAFLD( − ), or MAFLD( +)], participants were further categorized into six groups by EAC status (Fig. 2). Compared with participants in FLD( − )/EAC( − ) group, those in FLD( − )/EAC( +) group were associated with higher all-cause mortality (HR = 1.25, 95%CI = 1.06–1.47) and cancer-related mortality (HR = 1.54, 95%CI = 1.04–2.27). Of note, neither MAFLD( − )/NAFLD participants (HR = 1.00, 95%CI = 0.65–1.52) nor MAFLD( − )/AFLD participants (HR = 1.39, 95%CI = 0.88–2.19) were found to have significantly higher all-cause mortality risk (all p > 0.05). Contrarily, both MAFLD( +)/NAFLD participants (HR = 1.22, 95%CI = 1.11–1.34) and MAFLD( +)/AFLD participants (HR = 1.83, 95%CI = 1.46–2.28) were at elevated all-cause mortality risk (all p < 0.05). The same trend was observed in the results for both cardiovascular-related mortality and cancer-related mortality.
Forest plot of association between difference types of FLD and all-cause, cardiovascular-related, and cancer-related mortality. aAdjusted for sex, age, race/ethnicity, education, marital status, smoking status, and sedentary lifestyle. bAdjusted for alanine aminotransferase and total cholesterol in addition to model 1. cAdjusted for intake of meats, fruits, and vegetables in addition to model 2. FLD fatty liver disease, EAC excessive alcohol consumption, MAFLD metabolic dysfunction-associated fatty liver disease, NAFLD nonalcoholic fatty liver disease, AFLD alcoholic fatty liver disease, HR hazard ratio, CI confidence interval
Subtype analysis for MAFLD individuals
Subgroup analysis concerning the three main criteria used for defining MAFLD was conducted. Our results showed that MAFLD participants with T2DM had the highest risk of all-cause mortality (HR = 2.00, 95%CI = 1.77–2.27), cardiovascular-related mortality (HR = 2.35, 95%CI = 1.91–2.89), and cancer-related mortality (HR = 2.04, 95%CI = 1.42–2.93) than individuals with lean metabolic dysregulation and those with overweight/obesity (Fig. 3). The overweight/obesity subtype was associated with all-cause mortality (HR = 1.11, 95%CI = 1.00–1.22) and cancer-related mortality (HR = 1.32, 95%CI = 1.00–1.73), while lean metabolic dysregulation subtype was only associated with all-cause mortality (HR = 1.30, 95%CI = 1.06–1.60) (Supplementary Table 3).
Association of fatty liver disease with all-cause, cardiovascular-related, and cancer-related mortality stratified by MAFLD criteria. aFLD( − )/EAC( − ) group was used as a reference, since Fig. 2 showed that the FLD( − )/EAC( +) group was significantly associated with higher mortality risk. bAdjusted for sex, age, race/ethnicity, education, marital status, smoking status, sedentary lifestyle, alanine aminotransferase, total cholesterol, intake of meats, fruits, and vegetables. *p < 0.05. FLD fatty liver disease, EAC excessive alcohol consumption, MAFLD metabolic dysfunction-associated fatty liver disease, T2DM type 2 diabetes mellitus
After further categorizing the population into seven subgroups, we found that individuals who met all three criteria of MAFLD had the highest risk, i.e., 2.1-fold, 2.4-fold, and 2.1-fold increase in all-cause (HR = 2.05, 95%CI = 1.80–2.34), cardiovascular-related (HR = 2.41, 95%CI = 1.93–2.99), and cancer-related (HR = 2.10, 95%CI = 1.46–3.02) mortality (Supplementary Table 4). Limited by insufficient sample size in the T2DM( +) group, the association of T2DM with mortality could not be explicated independently, so inter-group comparisons were adopted instead. T2DM( +)&BMI( +) individuals had a higher all-cause mortality risk (HR = 0.58, 95%CI = 0.20–1.70) than those with solely BMI( +) (HR = 0.52, 95%CI = 0.29–0.94). Similarly, MD( +)&T2DM( +) individuals had higher all-cause mortality risk (HR = 1.83, 95%CI = 1.24–2.70) than those with only MD( +) (HR = 1.30, 95%CI = 1.06–1.61), which suggested the elevated risk of comorbid T2DM (Supplementary Table 4).
Non-MAFLD individuals with fibrosis and mortality
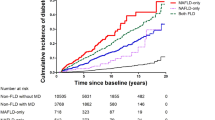
A total of 563 MAFLD( − ) individuals, including 532 (95.6%) individuals with low APRI and 31 (4.4%) individuals with intermediate-high APRI were included in this analysis. MAFLD(-) individuals with intermediate-high APRI were strongly associated with a significant 2.9-fold, 1.9-fold, and 10.8-fold higher risk of all-cause mortality (HR = 2.91, 95%CI = 1.46–5.77), cardiovascular-related mortality (HR = 1.88, 95%CI = 1.24–2.85), and cancer-related mortality (HR = 10.74, 95%CI = 4.35–26.51) after adjusting for demographic factors. In further multivariable model adjusting for laboratory indicators and dietary intake, the association of elevated APRI with all-cause (HR = 3.23; 95%CI = 1.63–6.40), cardiovascular-related (HR = 1.99, 95%CI = 1.21–3.27), and cancer-related (HR = 17.08, 8.80–33.18) mortality remain persisted. However, MAFLD( − ) participants with low APRI were not at elevated risk of neither all-cause nor disease-specific mortality. Meanwhile, MAFLD( +) individuals had a significantly higher risk of all-cause and disease-specific mortality independent of APRI level (all p < 0.05) (Fig. 4). Additionally, neither FIB-4 nor NFS was associated with mortality risk among MAFLD( − ) individuals (all p > 0.05) (Supplementary Fig. 2, Supplementary Fig. 3).
Forest plot of association between fatty liver disease and all-cause, cardiovascular-related, and cancer-related mortality stratified by MAFLD status and APRI. aFLD( − )/EAC( − ) group was used as a reference, since Fig. 2 showed that the FLD( − )/EAC( +) group was significantly associated with higher mortality risk. bAdjusted for sex, age, race/ethnicity, education, marital status, smoking status, and sedentary lifestyle. cAdjusted for alanine aminotransferase and total cholesterol in addition to model 1. dAdjusted for intake of meats, fruits, and vegetables in addition to model 2. FLD fatty liver disease, EAC excessive alcohol consumption, MAFLD metabolic dysfunction-associated fatty liver disease, APRI aspartate aminotransferase to platelet ratio index, HR hazard ratio, CI confidence interval
In sensitivity analyses, when we excluded participants who died within 3 years of follow-up (N = 219) or included participants with viral hepatitis (N = 338) the main findings remained consistent with our primary analyses (Supplementary Tables 5, 6). After further adjusting for metabolic factors, the highest mortality risk among MAFLD participants with T2DM persisted (Supplementary Table 7).
Discussion
In this large US population-based prospective study, compared with FLD( − )/EAC( − ) participants, individuals fulfilling MAFLD criteria (MAFLD[ +]) had a 28% higher mortality risk, which was mainly driven by T2DM subtype, and independent of traditional FLD types (22% in MAFLD( +)/NAFLD, and 83% in MAFLD( +)/AFLD, respectively). Additionally, for FLD Individuals unfulfilling MAFLD criteria [MAFLD( − )] but with elevated APRI, a statistically significantly higher mortality risk (223%) was observed. By incorporating MAFLD subtype and APRI, individuals could be stratified into the high-risk group (14.5%), moderate-risk group (68.0%), and low-risk group (17.5%), which may be applicable in guiding surveillance priority for FLD-related outcomes (Fig. 5).
MAFLD and APRI were suitable for extracting high-risk population with all-cause mortality from FLD individuals. ap < 0.05. bThe cut-offs of 1.00 and 2.00 for hazard ratio were used to categorize FLD individuals as low-risk group, moderate-risk group, and high-risk group. FLD fatty liver disease, EAC excessive alcohol consumption, MAFLD metabolic dysfunction-associated fatty liver disease, NAFLD non-alcoholic fatty liver disease, AFLD alcoholic fatty liver disease, T2DM type 2 diabetes mellitus, APRI aspartate aminotransferase to platelet ratio index
Consistent with prior studies [5, 21], our analyses found that all-cause (25%) and cancer-related mortality (54%) risk were higher among FLD( − )/EAC( +) individuals than FLD( − )/EAC( − ) individuals, which suggested the long-term health damage of alcohol in individuals even without FLD, whereas for FLD individuals, our study found for the first time that MAFLD( +) individuals had higher mortality risk regardless of EAC status. After the MAFLD definition was proposed lately, multi-studies confirmed it showed more practical for identifying high-risk FLD patients with disease progression [22, 23]. However, prior studies lacked a comparison of the mortality risk from alcohol intake and different types of FLD. Through the comprehensive analysis, our study directly corroborated the correctness of the MAFLD definition (incorporating metabolic markers without considering the presence of EAC).
Till now, only four studies focusing on the association of MAFLD subtype with overall mortality showed the inconsistent direction of results [10,11,12,13]. Consistent with our study, two papers included 4718 adults in Austria [11] and 152,139 adults in China [12] suggested that the mortality risk in MAFLD was mainly driven by T2DM subtype. Similar results were observed in a study based on the US population [10], in which T2DM subtype had the highest mortality risk adjusting for demographic variables, and comparable risk to lean metabolic dysregulation subtype by additionally adjusting for laboratory indicators. A contrary result was observed in 8919 Korean adults [13] suggesting that individuals with lean metabolic dysregulation subtype were associated with higher mortality risk without adjusting for dietary intake, which has been reported to be closely associated with FLD [24] and mortality [25]. The differences between the existing studies may be due to the variation in population and covariates. Besides, the present study filled a gap in the evidence linking T2DM subtype to elevated cardiovascular and cancer-related mortality risk. Meanwhile, it was the only study that observed the elevated mortality risk of T2DM subtype in our detailed seven subgroups’ analysis in MAFLD( +) individuals of the US.
In terms of the mechanism of diabetes-driven-MAFLD, the livers of patients with NAFLD might release a variety of proatherogenic, proinflammatory, and diabetogenic mediators that had important roles in the development of both cardiovascular disease and T2DM [26]. Additionally, persistent exposure to hyperglycemia and elevated concentrations of circulating insulin stimulated cancer progression [27]. No consensus for the treatment of MAFLD has been reached, and regular physical activity and weight loss remain the main treatment methods [28]. With its high level of severity and easy access to information, diabetes is of the greatest concern in terms of public health implications. In the context of limited health resources, the risk of death can be reduced by focusing on surveillance and interventions among the T2DM subtype population.
Being overweight has been identified to be strongly associated with higher overall mortality through a meta-analysis of 239 prospective studies [29]. However, some studies also suggested that a slight excess of adipose tissue may serve as an energy reserve, thus showing a protective effect against death [12]. Hence, individuals with solely BMI( +) associated with decreased mortality risk may be reasonable in our study. Besides, lean metabolic dysregulation individuals may have greater liver damage and cardiovascular risk [30] due to more ectopic fat accumulation (primarily in the visceral distribution) [31]. Similar to another study [13], we did not find the lean metabolic dysregulation subtype to be associated with mortality risks for cardiovascular and cancer events. Future research, including sufficient sample size and diversified characteristics of the population are needed to confirm our result and to explore the heterogeneity of the effect on the health and treatment outcomes of extrahepatic organs among different groups of MAFLD.
Based on 7761 US adults with MAFLD, Kim, et al. found that FLD individuals who unfulfilling MAFLD criteria did not have an increased mortality risk [8]. However, other researchers pointed out that FLD individuals without MAFLD had a significantly higher risk for cardiovascular events [32] and in-depth analysis was needed to clarify the risk of this unneglectable subpopulation (accounting for approximately 18.3% of the FLD population) [33]. Our study demonstrated for the first time that for individuals unfulfilling MAFLD criteria, APRI could effectively identify a subpopulation at elevated death risk (223%), which additionally provided high-risk population targeting for surveillance. Compared with other advanced fibrosis scores (i.e., NFS and FIB-4), APRI required only two readily available laboratory indicators (AST and platelet count), which guaranteed its feasibility of application in primary care. In addition, some novel biomarkers and scores including N-terminal propeptide of type 3 collagen (PRO-C3) and ADAPT score have been suggested to identify advanced fibrosis in MAFLD patients [16]. More studies are needed to further confirm the performance of more novel scores.
To our knowledge, this is the first comparative study of the association between AFLD and NAFLD categories based on the newly proposed MAFLD terminology. Meanwhile, our study is the first to identify a high-risk population from non-MAFLD steatosis individuals. However, some limitations should be paid attention to when interpreting our results. First, history of ultrasound examination and secondary causes of hepatic steatosis other than alcohol and viral infection (e.g., autoimmune liver disease) could not be identified in this study. Second, hepatic steatosis and advanced fibrosis were diagnosed by the hepatic ultrasound and serum markers rather than liver biopsy. However, considering the satisfactory diagnostic performance of ultrasonography [34] and non-invasive fibrosis scores [16] in clinical practice and the potential complications of invasive biopsy examination [35], the present results may be more applicable for general practice. Third, the liver-related mortality risk could not be analyzed since the restricted data were not available. Finally, mortality risk in the viral-infected population was not estimated because not all participants had available viral infection data. However, the sensitivity analysis we conducted by re-including participants with explicit viral infection suggested the stability of the primary results. In addition, these limitations may be balanced by the benefits of a nationally representative population-based sample, adequate follow-up period, and the ability to generalize findings to the US population.
In conclusion, utilizing a large population-based study, we found that MAFLD criteria rather than the traditional definition (NAFLD and AFLD) could effectively identify high-risk FLD individuals, which was mainly determined by T2DM subtype. Meanwhile, APRI was a useful predictor of mortality risk in FLD individuals unfulfilling MAFLD criteria, which may be a useful complement. Given the high prevalence of FLD worldwide, the MAFLD and APRI-based risk stratification strategy may be immediately applied to guide the surveillance options for FLD patients, hence potentially preventing more life-threatening adverse clinical outcomes.
Data availability
The Third National Health and Nutrition Examination Survey (NHANES III) dataset are publicly available at National Center for Health Statistics of the Center for Disease Control and Prevention and the link to the database is https://wwwn.cdc.gov/nchs/nhanes/Nhanes3/Default.aspx.
Abbreviations
- MAFLD:
-
Metabolic dysfunction-associated fatty liver disease
- FLD:
-
Fatty liver disease
- NAFLD:
-
Nonalcoholic fatty liver disease
- AFLD:
-
Alcoholic fatty liver disease
- T2DM:
-
Type 2 diabetes mellitus
- NHANES:
-
National health and nutrition examination survey
- NCHS:
-
National center for health statistics
- HSUE:
-
Hepatic steatosis ultrasound examination
- EAC:
-
Excessive alcohol consumption
- APRI:
-
Aspartate aminotransferase to platelet ratio index
- FIB-4:
-
Fibrosis-4
- NFS:
-
NAFLD fibrosis score
- NDI:
-
National death index
- ICD-9:
-
International classification disease-ninth
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- BMI:
-
Body mass index
- HDL-C:
-
High-density lipoprotein cholesterol
- CRP:
-
C-reactive protein
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
References
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209
Prasoppokakorn T, Pitisuttithum P, Treeprasertsuk S. Pharmacological therapeutics: current trends for metabolic dysfunction-associated fatty liver disease (MAFLD). J Clin Transl Hepatol. 2021;9(6):939–946
Le P, Chaitoff A, Rothberg MB, McCullough A, Gupta NM, Alkhouri N. Population-based trends in prevalence of nonalcoholic fatty liver disease in US adults with type 2 diabetes. Clin Gastroenterol Hepatol. 2019;17(11):2377–2378
Lin H, Zhang X, Li G, Wong GL, Wong VW. Epidemiology and clinical outcomes of metabolic (dysfunction)-associated fatty liver disease. J Clin Transl Hepatol. 2021;9(6):972–982
Chang Y, Cho YK, Cho J, Jung HS, Yun KE, Ahn J, et al. Alcoholic and nonalcoholic fatty liver disease and liver-related mortality: a cohort study. Am J Gastroenterol. 2019;114(4):620–629
Wild SH, Walker JJ, Morling JR, McAllister DA, Colhoun HM, Farran B, et al. Cardiovascular disease, cancer, and mortality among people with type 2 diabetes and alcoholic or nonalcoholic fatty liver disease hospital admission. Diabetes Care. 2018;41(2):341–347
Huang Q, Zou X, Wen X, Zhou X, Ji L. NAFLD or MAFLD: which has closer association with all-cause and cause-specific mortality?-Results from NHANES III. Front Med. 2021;8: 693507
Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75(6):1284–1291
Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential Clinical characteristics and mortality outcomes in persons with NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. 2021;19(10):2172–81.e6
Chen X, Chen S, Pang J, Tang Y, Ling W. Are the different MAFLD subtypes based on the inclusion criteria correlated with all-cause mortality? J Hepatol. 2021;75(4):987–989
Semmler G, Wernly S, Bachmayer S, Leitner I, Wernly B, Egger M, et al. Metabolic dysfunction-associated fatty liver disease (MAFLD)-rather a bystander than a driver of mortality. J Clin Endocrinol Metab. 2021;106(9):2670–2677
Wang X, Wu S, Yuan X, Chen S, Fu Q, Sun Y, et al. Metabolic dysfunction-associated fatty liver disease and mortality among Chinese adults: a prospective cohort study. J Clin Endocrinol Metab. 2021;107(2):e745–e755
Moon JH, Kim W, Koo BK, Cho NH. Metabolic dysfunction-associated fatty liver disease predicts long-term mortality and cardiovascular disease. Gut Liver. 2022;16(3):433-442. https://doi.org/10.5009/gnl210167
Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68(2):305–315
Wang Z, Bertot LC, Jeffrey GP, Joseph J, Garas G, de Boer B, et al. Serum fibrosis tests guide prognosis in metabolic dysfunction-associated fatty liver disease patients referred from primary care. Clin Gastroenterol Hepatol. 2021;S1542–3565(21):01055–01057
Tang LJ, Ma HL, Eslam M, Wong GL, Zhu PW, Chen SD, et al. Among simple non-invasive scores, Pro-C3 and ADAPT best exclude advanced fibrosis in Asian patients with MAFLD. Metabolism. 2021;128: 154958
Decraecker M, Dutartre D, Hiriart JB, Irles-Depé M, Chermak F, Foucher J, et al. Long-term prognosis of patients with metabolic (dysfunction)-associated fatty liver disease by non-invasive methods. Aliment Pharmacol Ther. 2022;55(5):580–592
CDC/NCHS. Analytic and reporting guidelines: the third National Health and Nutrition Examination Survey NHANES III (1988–94). Hyattsville: National Center for Health Statistics Centers for Disease Control and Prevention; 1996
Third National Health and Nutrition Examination Survey. Hepatic/Gallbladder Ultrasound and Hepatic Steatosis (HGUHS). Available at: https://wwwn.cdc.gov/nchs/Data/Nhanes3/34A/HGUHS.htm. Accessed October 14, 2020.
National Health; and Nutrition Examination Survey (NHANES) III. Hepatic Steatosis Ultrasound Images Assessment Procedures Manual 2010. Available at: https://www.cdc.gov/nchs/data/nhanes/nhanes3/hepatic_steatosis_ultrasound_procedures_manual.pdf. Accessed October 15, 2020.
Pearson MM, Kim NJ, Berry K, Moon AM, Su F, Vutien P, et al. Associations between alcohol use and liver-related outcomes in a large national cohort of patients with cirrhosis. Hepatol Commun. 2021;5(12):2080–2095
Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40(9):2082–2089
Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH, et al. An observational data meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. 2021;S1542–3565(21):01276–01283
Zelber-Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: evidence and plausible mechanisms. Liver Int. 2017;37(7):936–949
Schwedhelm C, Boeing H, Hoffmann G, Aleksandrova K, Schwingshackl L. Effect of diet on mortality and cancer recurrence among cancer survivors: a systematic review and meta-analysis of cohort studies. Nutr Rev. 2016;74(12):737–748
Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10(6):330–344
Shahid RK, Ahmed S, Le D, Yadav S. Diabetes and cancer: risk, challenges, management and outcomes. Cancers. 2021;13(22):5735
Dongiovanni P, Valenti L. A nutrigenomic approach to non-alcoholic fatty liver disease. Int J Mol Sci. 2017;18(7):1534
Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, Global BMI Mortality Collaboration, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–786
Ampuero J, Aller R, Gallego-Durán R, Banales JM, Crespo J, García-Monzón C, et al. The effects of metabolic status on non-alcoholic fatty liver disease-related outcomes, beyond the presence of obesity. Aliment Pharmacol Ther. 2018;48(11–12):1260–1270
Stefan N, Schick F, Häring HU. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab. 2017;26(2):292–300
Lee H, Lee YH, Kim SU, Kim HC. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol. 2021;19(10):2138–47.e10
Wong VW, Lazarus JV. Prognosis of MAFLD vs NAFLD and implications for a nomenclature change. J Hepatol. 2021;75(6):1267–1270
Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54(3):1082–1090
Mózes FE, Lee JA, Selvaraj EA, Jayaswal ANA, Trauner M, Boursier J, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut. 2022;71(5):1006–1019
Acknowledgements
We would like to thank the participants and staff of the National Health and Nutrition Examination Survey (NHANES) for their valuable contributions. The authors assume full responsibility for analyses and interpretation of these data.
Funding
This work was supported by the National Key R&D Program of China (Grant No. 2021YFC2500400), the Beijing-Tianjin-Hebei Basic Research Cooperation Special Project (20JCZXJC00090), the Tianjin Municipal Commission of Health and Wellness Project (TJWJ2021MS008), and the Doctoral Fund of Tianjin Medical University Cancer Institute and Hospital (B2013). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
KXC has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript. Study concept and design: YCZ, ZYL, and KXC. Acquisition of data: YCZ, ZYL, BM, LML, WW, and CS. Analysis and interpretation of data: YCZ, ZYL, CS, HJD, YBH, BM, LML, and WW. Drafting of the manuscript: YCZ, ZYL, and KXC. Critical revision of the manuscript for important intellectual content: YCZ, ZYL, WW, HJD, YBH, FFS, FJS, and KXC. Obtained funding: ZYL, YBH, and KXC. Administrative, technical, or material support: HJD, YBH, FFS, FJS, and KXC. Study supervision: HJD, YBH, FFS, FJS, and KXC.
Corresponding author
Ethics declarations
Conflict of interest
The authors (Ya‑Cong Zhang, Zhang‑Yan Lyu, Bing Ma, Li‑Min Li, Wei Wang, Chao Sheng, Hong‑Ji Dai, Yu‑Bei Huang, Fang‑Fang Song, Feng‑Ju Song, Ke‑Xin Chen) declare that they have no conflict of interest.
Animal research
Not applicable.
Consent to participate
The Third National Health and Nutrition Examination Survey (NHANES III) was approved by the institutional reviews board of the National Center for Health Statistics (https://www.cdc.gov/nchs/nhanes/irba98.htm), and written informed consent was obtained from all individual participants in the study.
Consent to publish
All authors of this manuscript have read and approved the final submitted version and are aware that they are listed as an author on this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, YC., Lyu, ZY., Ma, B. et al. A new risk stratification strategy for fatty liver disease by incorporating MAFLD and fibrosis score in a large US population. Hepatol Int 16, 835–845 (2022). https://doi.org/10.1007/s12072-022-10362-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10362-3