Abstract
Background and aims
Recent studies have debated the utility of beta-blockers to prevent variceal hemorrhage (V.H.) in cirrhosis patients with ascites. We aimed to evaluate the safety and efficacy of propranolol (PPL) compared to endoscopic variceal ligation (EVL) for V.H. primary prevention in patients with ascites.
Methods
Cirrhosis patients with ≥ grade 2 ascites and varices needing primary prophylaxis were randomly assigned to receive either PPL (n = 80) or EVL (n = 80). Patients were followed monthly until 12 months or transplant or death. The primary endpoint was 12-month transplant-free-survival (TFS). Secondary endpoints were the incidence of V.H., acute kidney injury (AKI), and control of ascites.
Results
Baseline characteristics were similar between the groups. PPL-group had a lower 12-month TFS (76.0% vs. 89.7%; p = 0.02) as compared with EVL-group. Mean arterial pressure ≤ 82 mmHg and MELD-sodium were the independent predictors of mortality. Incidence of VH was comparable between PPL and EVL-groups [6 (7.5%) vs. 2 (2.5%), p = 0.13]. In PPL vs. EVL-group, more patients had worsening of ascites (15% vs. 5%; p = 0.03), developed refractory ascites (13.7% vs.3.7%; p = 0.02), relapse of ascites (37.1% vs. 16.4%, p < 0.01), and AKI (26.2% vs. 12.5%; p = 0.02). Side effects were comparable between the two groups.
Conclusions
Primary VH-prophylaxis with PPL is associated with lower survival, poor control of ascites, and increased risk of AKI in cirrhosis patients with ≥ grade 2 ascites. PPL and EVL are equally effective in preventing V.H. Serial monitoring of blood pressures and renal functions is needed in cirrhosis patients with ascites on PPL (NCT02649335).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ascites is a frequent complication of cirrhosis and carries 15% 1-year mortality [1]. About 60% of cirrhosis patients with ascites have esophageal varices [2].The presence of ascites, poor liver functions, and large varices predict variceal hemorrhage (V.H.) in such patients [2]. The development of V.H. heralds a risk of acute-on-chronic liver failure, high morbidity, and mortality [3], and therefore, patients require V.H. prophylaxis.
Among the options for V.H. prophylaxis, endoscopic variceal ligation (EVL) controls only the variceal progression, whereas the nonselective beta-blockers (NSBB) improve the pathophysiology of portal hypertension and survival in cirrhosis patients [4, 5]. However, the use of NSBBs in patients with ascites is not entirely safe due to complex hemodynamic alterations. Original studies evaluating NSBBs for V.H. prophylaxis were heterogeneous and included compensated patients or a varying proportion of patients with ascites [6]. Further, literature review adds to an uncertainty about benefit of NSBBs in patients with ascites. A meta-analysis showed a lower reduction in risk of V.H. by NSBBs among patients with ascites [7]. Several observational studies have shown the harmful effects of NSBBs on survival, and renal functions in refractory ascites (R.A.), spontaneous bacterial peritonitis (SBP), and liver transplant (L.T.) waitlisted patients [8,9,10,11,12]. Conversely, retrospective cohort studies in patients with ascites have shown beneficial or lack of harmful effects of NSBB in these patients [13,14,15].
As per the “window hypothesis”, the beneficial effect of NSBB opens with the development of varices and closes with the development of R.A., SBP, or hepatorenal syndrome (HRS) [16]. But the closing of this window period is still debatable. We hypothesize that beneficial window of NSBBs closes in a subset of patients with significant ascites who otherwise require VH. prophylaxis.
Therefore, in this trial, we evaluated the effect of propranolol (PPL) versus EVL on survival, risk of V.H., and ascites control in cirrhosis patients with ≥ grade2 ascites requiring primary-VH-prophylaxis.
Methods
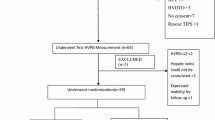
A single-center, open-label, parallel-group, randomized controlled trial was conducted at a tertiary care center between July 2015 and December 2016 (Fig. 1). Institute Ethics Committee approved the protocol (INT/IEC/2015/742). We registered the trial (NCT02649335), and adhered to the CONSORT, Declaration of Helsinki, and GCP guidelines.
Patients
We included consecutive patients with cirrhosis (Child-Turcotte-Pugh, CTP-B or C), age ≥ 18 years and ≤ 75 years, of any gender, and ≥ grade2 ascites with esophageal varices needing primary prophylaxis. The grade of ascites was defined as grade-I when ascites was detected only on ultrasonogram of abdomen, grade-II when there was a moderate symmetrical distension of abdomen, and grade-III when there was a marked abdominal distension. We excluded patients with active/recent infection within 2 weeks, hepatic encephalopathy (HE), renal dysfunction, hepatocellular carcinoma (HCC), portal vein thrombosis, active alcoholism, past-VH or NSBB use or EVL, R.A., pregnancy, HIV infection, contraindications for beta-blockers (severe chronic obstructive pulmonary disease, severe asthma, uncontrolled diabetes, bradyarrhythmia, peripheral vascular disease) and patients refusing to give consent. Demographic, clinical, and investigation-related data were noted. We measured the cardiac output (C.O.) at baseline by recording the diastolic mitral flow with duplex doppler during echocardiography (Vivid Five, Vingmed Technology, Japan) with an average value of a minimum of three cardiac cycles. Systemic vascular resistance was measured as mean arterial pressure (MAP)/C.O. MAP was measured as the sum of diastolic blood pressure and 1/3rd of pulse pressure and the average of three consecutive readings was analyzed.
Randomization and allocation
A statistician assigned patients to PPL or EVL groups in 1:1 ratio using computer-generated random numbers. Allocation concealment was done using sequentially numbered opaque sealed envelopes.
Treatment arms
Long-acting PPL (Ciplar LA, Cipla Limited, India) was initiated orally at 40 mg/day, with weekly dose titration with a target heart rate of 55–60 beats/min or 20–25% reduction or maximum tolerated dose. The dose was reduced by half in case of side effects with the initial dose. Temporary stoppage or down-titration of PPL was allowed in situations such as hypotension (Systolic blood pressure, SyBP < 90 mmHg), hyponatremia, SBP, renal dysfunction (creatinine > 1.5 mg/dL), and acute V.H. Compliance with PPL was assessed by monthly pill counts and/or self-reporting.
Patients in EVL-group underwent regular sessions of EVL using a multi-band ligation device (six shooter; Wilson–Cook Inc.) till variceal eradication every 4 weeks followed by 3 monthly for the initial 6 months and 6 monthly thereafter. Variceal eradication was defined by the absence of varices. Recurrent esophageal varices were banded till eradication. Gastric varices were planned to be treated with cyanoacrylate glue injections only if they bled. A shift of patients from one group to other due to treatment intolerance was permitted. However, the patient was withdrawn/censored from the study.
Standard therapy
Patients were treated with a low sodium diet (2 g/day) and a combination of furosemide (20–160 mg/day) and spironolactone (50–400 mg/day) with dose escalation by one step at a time. The dose of diuretics was escalated when there was lack of mobilization defined as < 0.8 kg of weight loss over 4 days. Large-volume paracentesis (LVP) was done for symptomatic tense ascites with intravenous albumin (8 g/L ascites removed). Patients who developed R.A. on follow-up were treated with a low sodium diet, diuretics, albumin, and repeated LVP.
Follow-up
Patients were followed up monthly or as clinically indicated for 12 months. Clinical, biochemical parameters and side-effects were assessed at each visit. Alcohol abuse was determined by interviewing patients and relatives, where appropriate. Patients developing acute V.H. on follow-up in either group were hospitalized and treated with EVL within 12 h, intravenous terlipressin 2 mg 4 hourly, oral lactulose, and intravenous ceftriaxone 1gm once daily for 3–5 days. These patients were continued in the allocated group with close monitoring after discharge. Patients were subjected to L.T. as per willingness and feasibility.
Endpoints
The primary endpoint was 12-month transplant-free-survival (TFS). The patients lost to follow-up and withdrawn from the study were censored. Mortality attributable to V.H. and other causes were analyzed. Secondary endpoints were the incidence of V.H., acute kidney injury (AKI), and control of ascites. VH-related mortality was defined as death within 6 weeks of the index variceal bleed. Complete control of ascites was defined as the elimination of ascites by clinical and ultrasonographic examination. Partial response was defined as ascites not requiring paracentesis. Worsening of ascites was labeled when ascites increased from grade 2–3 or needed LVP. Relapse of ascites was defined as the reappearance of grade ≥ 2 ascites during follow-up in the absence of diuretics. A MAP cut-off of 82 mmHg was used to discern the impact of therapy in both arms based on previous studies showing poor survival in cirrhosis with MAP < 82 mmHg [17, 18].
Sample size
We calculated the sample size based on the 12-month survival of 85% in patients with ascites [1]. Assuming the survival difference of 20% at 1 year between PPL and EVL-group [11], 72 patients in each arm were required at a power of 80% and α-error of 5%. Therefore, adjusting for 10% dropouts, we included 80 subjects in each arm.
Statistical analysis
Data were analyzed using SPSS Inc., Chicago, IL, version 22.0 for Windows according to the intention-to-treat (ITT) principle unless otherwise stated. As appropriate, categorical data were represented as a number (percentage) and quantitative data as mean ± standard deviation or median (interquartile range). Kolmogorov–Smirnov test was used for data distribution. Student t test and Mann–Whitney U-test were applied to compare between two groups, as appropriate. Proportions were compared using Chi-square or Fisher's exact test. Survival analysis was done by the Kaplan–Meier method, and groups were compared by Log-Rank test. Cox-Proportional regression analysis was done to find independent predictors of survival, where variables in univariable analysis with p ≤ 0.10 were entered into the stepwise forward multivariable model. All tests were two-sided at a significance level of p < 0.05 and adjusted for sub-group comparisons.
All authors had access to the data and reviewed and approved the final manuscript. Individual participant data will not be shared.
Results
Baseline characteristics
Of 290 patients screened (Figure S1), 160 were randomized to PPL-group (n = 80) or EVL-group (n = 80). Most patients were in the sixth decade, 60% being males and 50% with alcoholic etiology. Baseline characteristics were comparable between the two groups (Table 1). Alcohol abstinence and recidivism were balanced between the two groups (p = 0.78). Sixteen patients with hepatitis B received antivirals and achieved negative viral DNA during the follow-up. Fifteen patients in the PPL group and 14 in EVL-group had hepatitis C who received directly acting antivirals with no difference in sustained virological response.
The dose of PPL to achieve the target heart rate was 40 mg/day (range 20–80) within 4.4 ± 2.0 weeks. Maintenance dose of PPL was 20 mg/day (n = 5, 6.2%), 40 mg/day (n = 49, 61.2%), 60 mg/day (n = 21, 26.2%), and 80 mg/day (n = 2; 2.5%). There was a significant reduction in heart rate, SyBP, diastolic blood pressure, and MAP after dose titration (p < 0.01; for each) in PPL-group (Table S1). Further, heart rate was reduced by 28% (range 4.3–52.5) after titration. More than a 20% rate reduction was noted in 64 (80%) patients. Five (6.2%) patients were non-compliant to PPL for more than one week, where PPL was restarted and re-titrated. Twenty-two (27.5%) patients in PPL and 23 (28.7%) in EVL-group had MAP ≤ 82 mmHg at baseline (p = 0.86). However, a higher proportion of patients in the PPL group developed MAP < 82 mmHg after achieving target heart rate than in the EVL group [46 (57.5%) vs. 25 (31.3%), p < 0.01]. Esophageal varices were eradicated in 63 (78.7%) patients with a median of two endoscopies (range 1–7) in the EVL-group, and recurrence was observed following obliteration in 17 (21.2%) patients.
Primary endpoint
The 12-month TFS was lower in PPL-group vs. EVL-group according to ITT (p = 0.02) and per-protocol analysis (p = 0.02) (Fig. 1; Table 2). The cause of death was V.H. in 1 (5.8%), sepsis in 7 (41.1%), liver failure in 3 (17.6%), and SBP in 4 (23.5%) patients in PPL-group, whereas it was sepsis in 2 (28.5%), liver failure in 2 (28.5%) and SBP in 2 (28.5%) patients in EVL-group. Two patients (11.7%) in the PPL group and one (14.2%) in EVL-group died of unknown causes. The causes of death were not significantly different between the groups (p < 0.05). Two patients (2.5%) in the PPL-group underwent LT with indications of RA-diuretic intractable (n = 1), and SBP (n = 1).
In the PPL-group, non survivors required a lower dose of PPL (mg/day) (42.3 ± 12 vs. 49.5 ± 12.3; p = 0.03) and had a higher CTP (9.8 ± 1.8 vs. 8.7 ± 1.4; p = < 0.01) and MELD-sodium (19 ± 5.6 vs.15 ± 4.1, p = < 0.01) at baseline, as compared with survivors. The TFS was similar between patients with baseline C.O. < 5 L/min vs. C.O. > 5 L/min (p = 0.91) in the PPL group. Patients with MAP < 82 mmHg at baseline in the PPL group had lower survival than MAP > 82 mmHg p = 0.02, respectively (Figure S2). Patients who developed MAP ≤ 82 mmHg on treatment had higher mortality in the PPL group than the EVL-group [14/46 vs. 2/25, p = 0.03]. Patients with hyponatremia after dose titration in the PPL group died more often than those without hyponatremia (8/15 vs. 9/65, p < 0.01). Univariable predictors of mortality (Table 3) were PPL treatment, past AKI, MAP ≤ 82 mmHg, CTP, and MELD-sodium. However, MAP ≤ 82 mmHg and MELD-sodium were the only independent predictors of mortality on multivariable analysis.
Secondary endpoints
Variceal hemorrhage
Six patients (7.5%) bled in the PPL-group as compared with 2 (2.5%) in the EVL-group (Table 2, Figure S3). There were no significant differences in V.H. and VH-related mortality between the groups (p = 0.13 and 0.56, respectively). Among six patients who bled from esophageal varices in the PPL group, three belonged to CTP-C and three to CTP-B. Etiology in patients with V.H. in the PPL group was alcohol (n = 3), hepatitis B (n = 1), and cryptogenic (n = 2) cirrhosis. Two patients who bled from esophageal varices in EVL-group were in CTP-B and C, and the etiology was alcohol. None of the patients bled from gastric varices in either group.
Control of ascites
A higher proportion of patients in the PPL group had worsening ascites, need for continued diuretics, R.A., and relapse of ascites compared to EVL-group (Table 4). No patient underwent transjugular intrahepatic portosystemic shunt.
AKI
The incidence of AKI, especially stage 2, was higher in the PPL group than the EVL-group (Table 2, Figure S4). These patients were treated with diuretic cessation with intravenous albumin and vasoconstrictors as appropriate. The diuretic was restarted in lower doses with careful monitoring of renal function. Fourteen (66.6%) patients with MAP ≤ 82 mmHg in PPL-group developed AKI compared to 5 (21.7%) in the EVL-group (p = 0.01). There was significantly higher mortality in patients with AKI after PPL titration than those without AKI (63.6% vs.14.5%, p < 0.01). Mortality was higher in those with AKI and MAP ≤ 82 mmHg in PPL as compared to EVL-group (64.2% vs.40%, p = 0.02).
Hospital admissions
Admission rates were similar in PPL-group and EVL-group (43.7% vs. 31.2%, p = 0.11). Both groups had similar rates of infections (Table S2). Three patients developed HCC, one in PPL-group, and two in EVL-group, and were treated with loco-regional therapies. There was a trend towards the higher occurrence of HE in the PPL-group as compared to EVL-group (25% vs. 13.7%; p = 0.07).
Adverse events
Adverse events were reported in 25 (31.2%) patients in the PPL group (Table 5). Symptomatic hypotension occurred in two patients needing drug discontinuation. The dose of PPL was reduced in five patients: due to worsening breathlessness in two and bradycardia in three patients. In the EVL-group, 22 (27.5%) patients reported side effects. One (1.25%) patient developed post EVL ulcer bleed, which was controlled with conservative management. In the majority, these side effects were mild to moderate.
Discussion
In this RCT, we demonstrated that as compared to EVL, the primary prophylaxis for variceal bleed with PPL led to a lower survival, poor control and higher relapse of ascites, higher incidence refractory ascites, and increased risk of AKI in cirrhosis patients with ≥ grade2 ascites. NSBBs reduce portal pressures in patients with clinically significant portal hypertension (CSPH) by reducing portal inflow and cardiac output [3, 19]. RCTs and observational studies have shown a reduced risk of first variceal bleed with beta-blockers, which led to their use in primary VH-prophylaxis [3, 19]. However, emerging evidence suggests beneficial effects of NSBB beyond prevention of V.H. PREDSCI trial showed improved survival, reduced decompensations in compensated patients with CSPH, and small varices [4]. Others showed an immunomodulatory role and a reduced risk of SBP with NSBB in cirrhosis [20]. However, such literature is generalizable to patients with compensated or early decompensated cirrhosis with seldom reporting of crucial information like hemodynamic parameters, NSBB dosing, and titration strategy [8,9,10,11, 13,14,15]. In this unique RCT, we reported harmful effects of NSBBs in decompensated cirrhosis with significant ascites. Our results, however, contradict with a meta-analysis, where NSBBs were not associated with increased mortality in patients with ascites (low certainty of the evidence) [15].
In our study, the development of MAP below the critical level in about 57% of patients in PPL-group and significant heart rate reduction due to PPL possibly led to reduced C.O., AKI, and increased mortality. A decrease in the cardiac compensatory reserve, impaired left ventricular stroke work index, ejection intraventricular pressure difference, and adaptive response are observed with the progression of ascites in cirrhosis [21,22,23]. The β1 adrenoreceptors are down-regulated and desensitized in advanced cirrhosis, mediating the adaptive stress response [24]. The maintenance of C.O. and MAP is dependent on the heart rate in patients with advanced cirrhosis and ascites [25]. Therefore, it is plausible that the harmful effects of NSBBs are caused by the blunting of β1-mediated increase in C.O., which is critical to maintaining systemic and renal perfusion in advanced cirrhosis. Others have also demonstrated a correlation between low cardiac index and blood pressures with poor survival and AKI in cirrhosis with ascites [17, 18, 26]. Kalambokis et al. reported reduced survival and increased HRS risk with PPL in cirrhosis patients with ascites. Importantly, HRS was evident 6 months after PPL treatment in CTP-C/MELD ≥ 18 patients and 2 years after in CTP-B cirrhosis [11].
We showed a higher incidence of AKI in the PPL group. This was likely due to a reduction in MAP in the PPL group as compared with EVL-group. Kim et al. also reported that PPL use was an independent predictor of AKI in LT-waitlisted patients with ascites even with a median PPL dose of PPL of 52.2 ± 26.8 mg/day [10]. Serste et al. observed NSBB was associated with a higher incidence of AKI and lower MAP in severe alcoholic hepatitis [27]. Mandorfer et al. showed that NSBB use was associated with increased AKI and poor survival among patients with SBP [12]. In our study, no benefit of PPL on the infections was observed. This is in contrast to meta-analysis showing a reduction in SBP incidence with NSBB use [20]. Our findings conflict with few other studies that found lower mortality among NSBB users with ascites [13, 28].The disparities would be attributed to prospective design, populations studied, and baseline characteristics. While our data are in concordance with the “Window hypothesis” [16], we demonstrated that the beneficial window of NSBBs closes in patients with significant ascites and low MAP. Our data validate the consensus recommendations by AASLD and Baveno VI as patients with low MAP, hyponatremia, and AKI had higher mortality with PPL even in non-RA patients.
There was no difference between PPL and EVL-group for V.H. prevention. EVL treatment was well tolerated. Therefore, EVL is an alternate option in cirrhosis patients with significant ascites requiring V.H. prophylaxis [3]. Guidelines recommend a lower dose of PPL for V.H. prophylaxis in cirrhosis patients with ascites [3, 9, 13]. We used a lower starting dose of PPL and followed weekly dose titration. The median dose was much lower compared to most of the previous trials [29]. However, even with a dose of 40 mg/day of PPL, we noticed harmful effects. We also demonstrated that the baseline severity of cirrhosis and MAP were independent predictors of mortality rather than the dose of PPL. The advanced stage of cirrhosis, sarcopenia, differential metabolism of PPL in Asians might have influenced the harmful effects due to the lower dose of PPL in our study.
Few studies on NSBBs in cirrhosis with ascites have shown reduced natriuresis when used in combination with diuretics than alone [30]. We showed that a greater number of patients in the PPL group had worsening of ascites, required diuretics for a longer duration, and had a relapse of ascites. Fall in MAP in the PPL group might have caused sodium retention and higher relapse of ascites with the stoppage of diuretics. There was a frequent occurrence of diuretic intractable ascites in the PPL group, which may be due to the combined effect of a decrease in C.O., MAP, and volume depletion by the diuretics and NSBBs. This would have led to activation of the renin-angiotensin system, sympathetic nervous system, and higher AKI and R.A.in our study.
Limitations include single-center, open-label design, the small sample size to detect a difference in risk of V.H. between PPL and EVL-groups, and the results are not generalizable to other NSBBs. Frequent monitoring of patients in the PPL group could have led to biased care, but even with that, the survival was poor in the PPL group. Hepatic venous pressure gradient, serial C.O. or hormonal evaluation, and invasive hemodynamic assessments were not done, which would have substantiated the hypothesis proposed above.
In conclusion, although PPL and EVL were equally effective in preventing variceal bleed, PPL was associated with lower survival, poor control of ascites, and increased risk of AKI in cirrhosis patients with ≥ grade 2 ascites. Patients with ≥ grade 2 ascites needing VH prophylaxis can be subjected to EVL program till the control of ascites with subsequent introduction of NSBBs. Else, simultaneous low-dose diuretics and NSBBs can be initiated with delayed up-titration of NSBB dose after the control of ascites.
Data availability
Due to privacy and ethical concerns, neither the data nor the source of data can be made available.
Abbreviations
- V.H.:
-
Variceal hemorrhage
- EVL:
-
Endoscopic variceal ligation
- NSBB:
-
Non-selective beta blockers
- R.A.:
-
Refractory ascites
- SBP:
-
Spontaneous bacterial peritonitis
- HRS:
-
Hepatorenal syndrome
- L.T.:
-
Liver transplantation
- PPL:
-
Propanolol
- RCT:
-
Randomized controlled trial
- CTP:
-
Child-Turcotte-Pugh
- HE:
-
Hepatic encephalopathy
- HCC:
-
Hepatocellular carcinoma
- CO:
-
Cardiac output
- MAP:
-
Mean arterial pressure
- SyBP:
-
Systolic blood pressure
- LVP:
-
Large volume paracentesis
- AKI:
-
Acute kidney injury
References
Planas R, Montoliu S, Ballesté B, Rivera M, Miquel M, Masnou H, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4:1385–1394
North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319(15):983–9. https://doi.org/10.1056/NEJM198810133191505. PMID: 3262200
Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–335
Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, et al. β Blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597–1608
Rodrigues SG, Mendoza YP, Bosch J. Beta-blockers in cirrhosis: evidence-based indications and limitations. JHEP Rep. 2020;2: 100063
Poynard T, Calès P, Pasta L, Ideo G, Pascal JP, Pagliaro L, et al. Beta-adrenergic-antagonist drugs in the prevention of gastrointestinal bleeding in patients with cirrhosis and esophageal varices. An analysis of data and prognostic factors in 589 patients from four randomized clinical trials. Franco-Italian Multicenter Study Group. N Engl J Med. 1991;324:1532–1538
D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19:475–505
Sersté T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017–1022
Madsen BS, Nielsen KF, Fialla AD, Krag A. Keep the sick from harm in spontaneous bacterial peritonitis: dose of beta blockers matters. J Hepatol. 2016;64:1455–1456
Kim SG, Larson JJ, Lee JS, Therneau TM, Kim WR. Beneficial and harmful effects of nonselective beta blockade on acute kidney injury in liver transplant candidates. Liver Transpl. 2017;23:733–740
Kalambokis GN, Christodoulou D, Baltayiannis G, Christou L. Propranolol use beyond 6 months increases mortality in patients with Child-Pugh C cirrhosis and ascites. Hepatology. 2016;64:1806–1808
Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M, et al. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:1680-1690.e1681
Bang UC, Benfield T, Hyldstrup L, Jensen JE, Bendtsen F. Effect of propranolol on survival in patients with decompensated cirrhosis: a nationwide study based Danish patient registers. Liver Int Off J Int Assoc Study Liver. 2016;36:1304–1312
Bossen L, Krag A, Vilstrup H, Watson H, Jepsen P. Nonselective β-blockers do not affect mortality in cirrhosis patients with ascites: post hoc analysis of three randomized controlled trials with 1198 patients. Hepatology. 2016;63:1968–1976
Chirapongsathorn S, Valentin N, Alahdab F, Krittanawong C, Erwin PJ, Murad MH, et al. Nonselective β-blockers and survival in patients with cirrhosis and ascites: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1096-1104.e1099
Krag A, Wiest R, Albillos A, Gluud LL. The window hypothesis: haemodynamic and non-haemodynamic effects of β-blockers improve survival of patients with cirrhosis during a window in the disease. Gut. 2012;61:967–969
Tergast TL, Kimmann M, Laser H, Gerbel S, Manns MP, Cornberg M, et al. Systemic arterial blood pressure determines the therapeutic window of nonselective beta blockers in decompensated cirrhosis. Aliment Pharmacol Ther. 2019;50:696–706
Llach J, Ginès P, Arroyo V, Rimola A, Titó L, Badalamenti S, et al. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology. 1988;94:482–487
Garcia-Tsao G, Abraldes JG. Nonselective beta-blockers in compensated cirrhosis: preventing variceal hemorrhage or preventing decompensation? Gastroenterology. 2021;161:770–773
Senzolo M, Cholongitas E, Burra P, Leandro G, Thalheimer U, Patch D, et al. beta-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver Int Off J Int Assoc Study Liver. 2009;29:1189–1193
Ruiz-del-Arbol L, Urman J, Fernández J, González M, Navasa M, Monescillo A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210–1218
Téllez L, Ibáñez-Samaniego L, Pérez Del Villar C, Yotti R, Martínez J, Carrión L, et al. Non-selective beta-blockers impair global circulatory homeostasis and renal function in cirrhotic patients with refractory ascites. J Hepatol. 2020;73:1404–1414
Giannelli V, Roux O, Laouénan C, Manchon P, Ausloos F, Bachelet D, et al. impact of cardiac function, refractory ascites and beta blockers on the outcome of patients with cirrhosis listed for liver transplantation. J Hepatol. 2020;72:463–471
Krag A, Møller S, Burroughs AK, Bendtsen F. Betablockers induce cardiac chronotropic incompetence. J Hepatol. 2012;56:298–299
Bendtsen F, Henriksen JH, Sørensen TI. Propranolol and haemodynamic response in cirrhosis. J Hepatol. 1991;13:144–148
Krag A, Bendtsen F, Henriksen JH, Møller S. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut. 2010;59:105–110
Sersté T, Njimi H, Degré D, Deltenre P, Schreiber J, Lepida A, et al. The use of beta-blockers is associated with the occurrence of acute kidney injury in severe alcoholic hepatitis. Liver Int Off J Int Assoc Study Liver. 2015;35:1974–1982
Leithead JA, Rajoriya N, Tehami N, Hodson J, Gunson BK, Tripathi D, et al. Nonselective β-blockers are associated with improved survival in patients with ascites listed for liver transplantation. Gut. 2015;64:1111–1119
Gluud LL, Krag A. Banding ligation versus beta-blockers for primary prevention in oesophageal varices in adults. Cochrane Database Syst Rev. 2012;(8):Cd004544. https://doi.org/10.1002/14651858.CD004544.pub2. PMID: 22895942
Rector WG Jr, Reynolds TB. Propranolol in the treatment of cirrhotic ascites. Arch Intern Med. 1984;144:1761–1763
Acknowledgements
The paper was presented as a late-breaking abstract at The International Liver Congress held at Amsterdam, in April 2017.
Funding
Partially funded by Society for the study of liver diseases (SSLD).
Author information
Authors and Affiliations
Contributions
VS conceived and designed the study, was the study coordinator, analyzed data, interpreted data, wrote the draft of the manuscript, and approved the final manuscript submitted. PK designed the study, recruited patients, analyzed data, wrote the draft of the manuscript, did the statistical analysis, interpreted the data, revised the manuscript, and approved the final manuscript submitted. NV followed up the patients, analyzed the data, wrote the manuscript, interpreted the data, revised the manuscript and approved the final version. RV performed systemic vascular resistance. AS statistical analysis and manuscript writing, AB, were involved in analyzing data and recruiting patients.
Corresponding author
Ethics declarations
Conflict of interest
Virendra Singh, Pramod Kumar, Nipun Verma, Rajesh Vijayvergiya, Akash Singh, and Ashish Bhalla declare no competing interests.
Animal research (ethics)
Not applicable.
Consent to participate (ethics)
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki 1975, as revised in 2008. Institute Ethics Committee approved the protocol (INT/IEC/2015/742).
Consent to publish (ethics)
Written informed consent was taken.
Clinical trials registration
Trial was registered at clinicaltrials.gov NCT02649335.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, V., Kumar, P., Verma, N. et al. Propranolol vs. band ligation for primary prophylaxis of variceal hemorrhage in cirrhotic patients with ascites: a randomized controlled trial. Hepatol Int 16, 944–953 (2022). https://doi.org/10.1007/s12072-022-10361-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10361-4