Abstract
Objective
This study aimed to evaluate the efficacy of Peg-interferon (Peg-IFN)–nucleoside analog (NA) sequential optimization therapy (SOT) in HBeAg-positive patients with chronic hepatitis B (CHB).
Methods
In this prospective two-center study, 132 CHB patients were assigned to receive Peg-IFN standard therapy for 48 weeks (65 patients) or Peg-IFN monotherapy for 12–24 weeks and NA add-on for those without early virological response (EVR) (67 patients). Both patient groups were monitored and followed for 24 weeks after treatments stop.
Results
At week 24 after treatments stop, the Peg-IFN–NA SOT group achieved more HBsAg levels drop (− 1.35 vs − 0.67 log10 IU/mL, p = 0.016), higher HBsAg ≤ 100 IU/mL (32.8% vs 9.2%, p = 0.001), HBV DNA undetectable (79.1% vs 49.2%, p < 0.001), and ALT normalization (80.6% vs 38.5%, p < 0.001) rates compared with Peg-IFN monotherapy. At week 24 after treatments stop, no significant difference was found in HBeAg seroconversion (35.8% vs 27.7%, p = 0.316), HBsAg loss (8.9% vs 4.6%, p = 0.323) and HBsAg seroconversion rates (4.5% vs 1.5%, p = 0.325) between Peg-IFN monotherapy group and Peg-IFN–NA SOT group.
Conclusion
Starting with Peg-IFN followed by addition of NA achieved more HBsAg levels drop, and higher HBsAg ≤ 100 IU/mL, HBV DNA undetectable, and ALT normalization rates compared with Peg-IFN monotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infection with hepatitis B virus (HBV) remains a global health problem affecting over 350 million people [1]. The number of HBV-related deaths due to liver cirrhosis and/or hepatocellular carcinoma increased between 1990 and 2013 by 33%, relating to 686,000 cases in 2013 worldwide [2]. According to the Asian-Pacific clinical guidelines on the management of hepatitis B, the ideal endpoint in both HBeAg-positive and HBeAg-negative patients is sustained off-therapy HBsAg loss, with or without seroconversion to anti-HBs [3]. This endpoint, however, is infrequently achievable with the currently available anti-HBV agents. With 1 year of Peg-interferon (Peg-IFN) therapy, the data revealed that HBsAg loss was reported in 4% at 6 months post-therapy [4]. Five-year continuous nucleoside analog (NA) therapy only achieved HBsAg loss in 10% patients [3]. Therefore, Peg-IFN–NA combination therapy could be considered to facilitate HBsAg loss in patients with CHB.
There are three approaches for administering Peg-IFN–NA combination therapy [5]: NA followed by addition of Peg-IFN and continuation of NA; starting with Peg-IFN followed by addition of NA; or simultaneous administration of NA and Peg-IFN. For patients with CHB, there is no robust evidence that a de novo combination of Peg-IFN and NA is superior compared to Peg-IFN or NA alone [6]. Previous studies with LAM or ADV combined with Peg-IFN failed to show an advantage of the de novo combination therapy [4, 7]. In CHB patients under long-term effective virological remission by NA treatment, Peg-IFN can be used as a ‘switch to’ or ‘add-on’ strategy. In the OSST trial, HBeAg-positive CHB patients who had received ETV for 9–36 months, with HBeAg < 100 PEIU/mL and HBV DNA < 1000 copies/mL, were randomized 1:1 to receive Peg-IFN or ETV for 48 weeks [8]. Patients who switched to Peg-IFN achieved higher HBeAg seroconversion rates vs those who continued ETV (14.9% vs 6.1%; p = 0.047) [8]. In the PEGON trial, patients who were treated with ETV/TDF for > 12 months and had an HBV DNA load of < 2000 IU/mL were randomly assigned in a 1:1 ratio to 48 weeks of Peg-IFN add-on therapy or continued NA monotherapy [9]. Among 58 Peg-IFN-naive patients, add-on therapy led to a greater frequency of HBeAg seroconversion (30% vs 7%; p = 0.034) compared with monotherapy [9]. In the New Switch Study, HBeAg-positive patients who achieved HBeAg loss and HBV DNA < 200 IU/mL with previous NA treatment were randomized 1:1 to receive Peg-IFN for 48 or 96 weeks [10]. At the end of 48 and 96 weeks' treatment, 14.4% and 20.7% of patients, respectively, cleared HBsAg [10].
In conclusion, the main advantages of Peg-IFN are the potential for immune-mediated control of HBV infection with a chance of HBsAg loss. However, HBsAg loss is infrequently achievable with Peg-IFN monotherapy. According to the Asian-Pacific clinical guidelines, 1 year of Peg-IFN monotherapy resulted in HBsAg seroconversion was achieved in 3–5% of patients [3]. In recent years, most clinical investigators have evaluated the efficacy of Peg-IFN–NA combination therapy including NA followed by addition of Peg-IFN, and simultaneous administration of NA and Peg-IFN. However, the approach, starting with Peg-IFN followed by addition of NA, is rarely evaluated. Therefore, in this prospective two-center study, we evaluated the efficacy of Peg-IFN–NA sequential optimization therapy in HBeAg-positive patients with CHB.
Methods
Patients
A total of 132 HBeAg-positive patients with CHB from Shanghai Public Health Clinical Center and Huashan Hospital from June 1st, 2015 to April 28th, 2018 were prospectively enrolled. Shanghai Public Health Clinical Center and Huashan Hospital are tertiary teaching hospitals in Shanghai, China. Inclusion criteria: (1) HBsAg positive > 6 months; (2) HBeAg positive; (3) HBV DNA ≥ 5 log10 IU/mL; and (4) ALT > 2 upper limit of normal (ULN) (ULN = 40 IU/mL). Exclusion criteria: (1) alcohol consumption > 20 g/day for more than 5 years, (2) previous or current antiviral therapy, (3) hepatitis C virus, hepatitis D virus, or human immunodeficiency virus co-infection; (4) combined with autoimmune liver disease, non-alcoholic fatty liver disease, hereditary metabolic liver disease, and other chronic liver disease; and (5) pregnant female.
Definition
Early virological response (EVR): HBV DNA drop ≥ 2 log10 IU/mL at week 12, and HBsAg drop ≥ 1 log10 IU/mL or HBsAg ≤ 1500 IU/mL at week 24. Partial response at week 48 (48 W PR): 1.0 ≤ HBeAg ≤ 10 S/CO or HBeAg > 10 S/CO but HBsAg < 1000 IU/mL. The clinical outcomes included the rates for HBV DNA undetectable, ALT normalization, HBeAg seroconversion, and HBsAg ≤ 100 IU/mL.
Study design
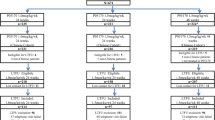
Patients were randomly assigned to two groups: Peg-IFN standard treatment (ST) group (n = 65) and Peg-IFN–NA sequential optimization therapy (SOT) group (n = 67). In Peg-IFN ST group, patients received Peg-IFN-α for 48 weeks. In Peg-IFN–NA SOT group, patients received Peg-IFN for 12–24 weeks and ETV or TDF add-on for patients without EVR. Similarly, in Peg-IFN–NA SOT group, Peg-IFN stop was based on the response to treatment (48 W PR). Patients without 48 W PR stopped Peg-IFN at week 48, but those with 48 W PR continued to receive therapy (Peg-IFN for patients with EVR; Peg-IFN + TDF/ETV for patients without EVR) for further 24 weeks. The flow chart of the study design is shown in Fig. 1. Both patient groups were monitored and followed for 24 weeks after treatment stop.
Flow chart of the study design. 132 patients were randomly assigned to two groups: Peg-IFN standard treatment (ST) group (n = 65) and Peg-IFN–NA sequential optimization therapy (SOT) group (n = 67). For Peg-IFN ST group, patients received Peg-IFN-α for 48 weeks. In Peg-IFN–NA SOT group, patients received Peg-IFN for 12–24 weeks and ETV or TDF add-on for patients without EVR. Similarly, in Peg-IFN–NA SOT group, Peg-IFN stop was based on the response to treatment (48 W PR). Patients without 48 W PR stopped Peg-IFN at week 48, but those with 48 W PR continued to receive therapy (Peg-IFN for patients with EVR; Peg-IFN + TDF/ETV for patients without EVR) for further 24 weeks
According to the Asian-Pacific clinical guidelines, NA (ETV or TDF) represents the first-line treatment options for CHB patients [3]. However, TDF and ETV had some differences in drug prices, medical insurances, and adverse side effects. Therefore, in this study, TDF or ETV was selected based on patients’ will, comorbidities, medical insurances, and economic conditions.
Laboratory testing
Serum HBV DNA levels were quantified by the real-time polymerase chain reaction assay (DAAN Diagnostics, Guangzhou, China) with a lower detection limit of 500 IU/mL. The serological markers of HBV were detected with enzyme-linked immune-sorbent assay kits (ARCHITECT i2000 SR; Abbott Architect, USA). The biochemical parameters including liver function were measured by a biochemistry analyzer (7600 Series; Hitachi, Tokyo, Japan).
Statistical analysis
The normality test was performed for continuous variables using the Kolmogorov–Smirnov test. Normal distribution variables, non-normal distribution continuous variables, and categorical variables, were shown as means and standard deviations, medians and interquartile ranges (IQR), and counts and percentage, respectively. T tests, Mann–Whitney tests, and Chi-square tests were applied to normal distribution variables, non-normal distribution continuous variables, and categorical variables, respectively. The univariable and multivariable analyses were performed to identify the independent predictors of clinical outcomes. For all statistical tests, we consider a two-sided p value of less than 0.05 to be statistically significant. Statistical Package for Social Sciences (SPSS) 19.0 (SPSS Inc., Chicago, IL) and Graphpad Prism 8.0 (Inc., La Jolla, CA) were used for statistical analyses. Efficacy analyses were conducted using intent-to-treat (ITT) analysis.
Results
Baseline characteristics of patients
The baseline characteristics of patients are shown in Table 1. The median age of patients was 33 years, 76.5% were male, 44.7% were HBV genotype B, and 55.3% were HBV genotype C. The median HBV DNA, ALT, and HBeAg levels were 7.1 log10 IU/mL, 175 IU/L, and 2.9 log10 IU/mL, respectively. The mean HBsAg levels were 3.9 log10 IU/mL. There were no significant differences found in age, gender, HBsAg, HBeAg, HBV DNA, ALT, and HBV genotype between Peg-IFN ST group and Peg-IFN–NA SOT group (all p > 0.05).
Follow-up results of patients
In Peg-IFN ST group, 62 patients finished the treatment and follow-up; among them, 17 patients achieved EVR and 45 patients did not achieve EVR. In Peg-IFN–NA SOT group, 24 patients achieved EVR, and 43 patients did not achieve EVR. Among 24 patients with EVR in Peg-IFN–NA SOT group, 14 patients achieved 48 W PR, and 8 patients did not achieve 48 W PR. Among 43 patients without EVR in Peg-IFN–NA SOT group, 7 patients achieved 48 W PR, and 31 patients did not achieve 48 W PR.
Evaluation of efficacy for Peg-IFN ST and Peg-IFN–NA SOT
Evaluation of efficacy for Peg-IFN ST and Peg-IFN–NA SOT is shown in Table 2. At the end of treatment, the Peg-IFN–NA SOT group achieved more HBsAg levels drop (− 1.26 vs − 0.72 log10 IU/mL, p = 0.018), higher HBsAg ≤ 100 IU/mL (26.9% vs 9.2%, p = 0.009), HBV DNA undetectable (82.1% vs 53.9%, p < 0.001), and ALT normalization (79.1% vs 38.5%, p < 0.001) rates compared with the Peg-IFN ST group. No significant difference was found in HBeAg seroconversion (34.3% vs 24.6%, p = 0.221), HBsAg loss (7.5% vs 4.6%, p = 0.493) and HBsAg seroconversion rates (3.0% vs 1.5%, p = 0.586) between Peg-IFN ST group and Peg-IFN–NA SOT group.
At week 24 after treatments end, the Peg-IFN–NA SOT group achieved more HBsAg levels drop (− 1.35 vs − 0.67 log10 IU/mL, p = 0.016), higher HBsAg ≤ 100 IU/mL (32.8% vs 9.2%, p = 0.001), HBV DNA undetectable (79.1% vs 49.2%, p < 0.001), and ALT normalization (80.6% vs 38.5%, p < 0.001) rates compared with the Peg-IFN ST group. From week 48 to week 72, the HBsAg levels drop was more significant in Peg-IFN–NA SOT group compared with Peg-IFN ST group (p < 0.05) (Fig. 2). No significant difference was found in HBeAg seroconversion (35.8% vs 27.7%, p = 0.316), HBsAg loss (8.9% vs 4.6%, p = 0.323) and HBsAg seroconversion (4.5% vs 1.5%, p = 0.325) rates between Peg-IFN ST group and Peg-IFN–NA SOT group.
Evaluation of efficacy for Peg-IFN ST by EVR
Evaluation of efficacy for Peg-IFN ST by EVR is shown in Table 3. At the end of treatment, patients with EVR achieved more HBsAg levels drop (− 1.66 vs − 0.44 log10 IU/mL, p < 0.001), higher HBsAg ≤ 100 IU/mL (20% vs 4%, p = 0.046) compared with those without EVR. No significant difference was found in HBsAg loss, HBsAg seroconversion, HBeAg loss, HBeAg seroconversion, HBV DNA undetectable, and ALT normalization rates between EVR group and non-EVR group (all p > 0.05).
At week 24 after treatments end, patients with EVR achieved more HBsAg levels drop (− 1.39 vs − 0.40 log10 IU/mL, p = 0.006), higher HBsAg ≤ 100 IU/mL (25% vs 2%, p = 0.003), HBeAg loss (55% vs 22%, p = 0.009), and HBeAg seroconversion (45% vs 20%, p = 0.038) rates compared with those without EVR (Table 3). No significant difference was found in HBsAg loss, HBsAg seroconversion, HBV DNA undetectable, and ALT normalization rates between EVR group and non-EVR group (all p > 0.05) (Table 3).
Evaluation of efficacy for Peg-IFN–NA SOT by EVR
Evaluation of efficacy for Peg-IFN–NA SOT by EVR is shown in Table 4. At the end of treatment, patients with EVR achieved more HBsAg levels drop (− 1.87 vs − 0.78 log10 IU/mL, p < 0.001), higher HBeAg loss (58% vs 28%, p = 0.014), and HBeAg seroconversion (50% vs 26%, p = 0.044) rates compared with those without EVR. No significant difference was found in HBsAg ≤ 100 IU/mL, HBsAg loss, HBsAg seroconversion, HBV DNA undetectable, and ALT normalization rates between EVR group and non-EVR group (all p > 0.05).
At week 24 after treatments end, patients with EVR achieved more HBsAg levels drop (− 1.92 vs − 1.05 log10 IU/mL, p = 0.017), higher HBsAg ≤ 100 IU/mL (50% vs 23%, p = 0.025), HBeAg loss (63% vs 28%, p = 0.006), and HBeAg seroconversion (58% vs 23%, p = 0.004) rates compared with those without EVR (Table 4). No significant difference was found in HBsAg loss, HBsAg seroconversion, and HBV DNA undetectable rates between EVR group and non-EVR group (all p > 0.05) (Table 4).
The independent predictors of clinical outcome
The independent predictors of clinical outcome are shown in Table 5. Univariate analysis showed that age, HBV DNA (≤ 8 vs > 8 log10 IU/mL), HBsAg (≤ 25,000 vs > 25,000 IU/mL), ALT (2–5 ULN vs > 5ULN), type of treatment (ST vs SOT), and type of analogs (ETV vs TDF) were associated with HBsAg ≤ 100 IU/mL at week 24 after treatments end (all p < 0.05). Multivariable analysis identified HBV DNA [OR (95% CI) 2.40 (1.27–4.78); p = 0.004], HBsAg [OR (95% CI) 6.88 (2.33–9.10); p < 0.001], ALT [OR (95% CI) 1.71 (1.37–3.02); p < 0.001], and type of treatment [OR (95% CI) 4.60 (2.36–11.23); p < 0.001] as the independent predictors of HBsAg ≤ 100 IU/mL at week 24 after treatments end.
Discussion
In this study, at week 24 after treatment ends, the Peg-IFN–NA SOT group achieved more HBsAg ≤ 100 IU/mL (32.8% vs 9.2%, p = 0.001) compared with Peg-IFN ST group (Table 2). From week 48 to week 72, the HBsAg levels drop was more significant in Peg-IFN–NA SOT group compared with Peg-IFN ST group (Fig. 2). The results were consistent with a recent open-label study, a total of 740 CHB patients were randomly assigned to receive TDF + Peg-IFN for 48 weeks, TDF + Peg-IFN for 16 weeks, continuing on TDF alone through week 48, TDF monotherapy for 120 weeks or Peg-IFN monotherapy for 48 weeks [11]. At the end of treatment, HBsAg levels declined most in the 48-week TDF + Peg-IFN arm (− 1.1 log10), followed by Peg-IFN monotherapy (− 0.8 log10), the 16-week TDF + Peg-IFN regimen (− 0.5 log10) and TDF monotherapy (− 0.3 log10) [11]. Our study suggested starting with Peg-IFN followed by addition of NA may lead to significantly more decline in HBsAg compared to patients treated with Peg-IFN monotherapy.
Marcellin et al. reported that a significantly greater proportion of patients receiving TDF plus Peg-IFN had HBsAg loss than those receiving TDF or Peg-IFN alone [11]. The ARES study reported that Peg-IFN add-on may lead to significantly more decline in HBsAg compared with patients treated with ETV monotherapy [12]. Although previous reports showed that Peg-IFN–NA combination therapy can have a benefit for achieving HBsAg loss, this study has some innovation. First, simultaneous administration of NA and Peg-IFN was used in the study by Marcellin et al. [11]; NA followed by addition of Peg-IFN and continuation of NA was used in the ARES study [12]; but starting with Peg-IFN followed by addition of NA was used in this study. Second, in this study, response-guided therapy (RGT) was used for Peg-IFN–NA SOT. TDF/ETV add-on was based on the response to Peg-IFN (EVR). Peg-IFN stop was also based on the response to treatment (48 W PR). Patients without 48 W PR stopped Peg-IFN at week 48, but those with 48 W PR continued to receive therapy for further 24 weeks. As a results, in Peg-IFN–NA SOT group, patients with EVR achieved more HBeAg seroconversion (58% vs 23%, p = 0.004) and HBsAg ≤ 100 IU/mL (50% vs 23%, p = 0.025) compared with those without EVR at week 24 after treatments end (Table 4).
Previous studies showed that HBV genotype, qHBsAg, and HBV DNA levels may be useful for selecting patients to be treated with Peg-IFN offering prognostic information for the probability of response to Peg-IFN therapy. In HBeAg-positive CHB patients, a decline of HBsAg levels below 1500 IU/mL at 12 weeks is a reasonable predictor of HBeAg seroconversion, while HBsAg levels > 20,000 IU/mL for HBV genotype B and C or no decline of HBsAg levels for HBV genotype A and D are associated with a very low probability of subsequent HBeAg seroconversion [13]. In HBeAg-negative CHB patients, a combination of a lack of decrease in HBsAg levels and < 2 log10 IU/mL decline in HBV DNA at 12 weeks of Peg-IFN predicts a no response in genotype D patients [14, 15]. For Peg-IFN–NA combination therapy, HBV genotype is also an important predictor of response to treatment HBeAg loss: genotype A, 47%; B, 44%; C, 28%; and D, 25% [16]. In this study, all patients were infected with HBV genotype B (59 patients, 44.7%) or C (73 patients, 55.3%). There was no significant difference in HBV genotype (B, 47.7% vs 41.8%, p = 0.495; C, 52.3% vs 58.2%, p = 0.495) between Peg-IFN ST group and Peg-IFN–NA SOT group (Table 1). Univariate analysis indicated the HBV genotype is not of relevance for treatment outcomes in this study (OR 0.16, 95% CI 0.02–1.22, p = 0.077) (Table 5). The relatively small sample size (65 in ST group, 67 in SOT group) and relatively low response rates (9.2% in ST group, 32.8% in SOT group) might lead to no statistically significant differences between HBV genotype B and C (Table 2).
Previous evidences are available about the role of sequential therapy only in patients with higher viremia [17]. The opinion is consistent with the results in this study. In this study, the baseline levels of HBV DNA were high both in Peg-IFN ST group and Peg-IFN–NA SOT group (median, 7.0 log10 IU/mL and 7.2 log10 IU/mL, respectively). According to the Asian-Pacific clinical guidelines, compared with the baseline levels of HBV DNA, using HBV DNA reduction would be more appropriate to guide Peg-IFN optimization strategies [3]. Therefore, in this study, adding TDF/ETV depended on whether patients achieved EVR (including HBV DNA reduction ≥ 2 log10 IU/mL at week 12), rather than the baseline levels of HBV DNA.
In this study, the clinical outcomes were not completely referred at clinical guidelines, in which, serological responses for HBsAg are HBsAg loss and HBsAg seroconversion [3]. However, in this study, HBsAg < 100 IU/mL was used as one of the clinical outcomes. First, HBsAg loss and HBsAg seroconversion were only achieved in rare patients (3–9%) using Peg-IFN/NA monotherapy or combination therapy. In an open-label, active-controlled study of 740 patients with CHB, by 72 weeks, the rate of HBsAg loss rose to 9.0% in the 48-week TDF plus Peg-IFN group [11]. Considering the small sample size (65 in ST group, 67 in SOT group) and consequently rare HBsAg loss (3 patients in ST group, 6 patients in SOT group), the p values might be untrusted if we used HBsAg loss and HBsAg seroconversion as the clinical outcomes. For example, at week 24 after treatments end, no significant difference was found in HBsAg loss (8.9% vs 4.6%, p = 0.323) and HBsAg seroconversion (4.5% vs 1.5%, p = 0.325) between Peg-IFN–NA SOT group and Peg-IFN ST group (Table 2). Second, a systematic review reported that HBsAg level < 100 IU/mL at the end of treatment seems to be a useful marker for deciding when to discontinue NA therapy [18]. When the HBsAg levels were < 100 IU/mL and > 100 IU/mL, the respective off-therapy virological relapse rates were 9.1%–19.6% and 31.4%–86.8% at > / = 12 months off-therapy, regardless of HBeAg status [18]. Based on these two points, we used HBsAg < 100 IU/mL as an alternative endpoint of HBsAg loss or seroconversion in this study.
This study has some limitations. First, using HBsAg ≤ 100 IU/mL as the clinical outcome may be suboptimal. Second, compared with Peg-IFN ST group (3 patients lost, 4.6%), the dropout rate is relatively higher in Peg-IFN–NA SOT group (3 patients lost, 10.4%). Third, this study had relatively small sample sizes.
Despite its limitations, the study provides clinical evidence for starting with Peg-IFN followed by addition of NA. We found that Peg-IFN–NA sequential optimization therapy achieved more HBsAg levels drop, and higher HBsAg ≤ 100 IU/mL, HBV DNA undetectable, and ALT normalization rates compared with Peg-IFN monotherapy. Patients with EVR achieved higher HBeAg seroconversion and HBsAg ≤ 100 IU/mL compared with those without EVR in Peg-IFN–NA sequential optimization therapy group. Author contributions.
Study concept and design: Liang Chen and Jiming Zhang. Data collection: Wei Xu, Qiang Li, Chenglu Huang, Qiankun Hu, Xun qi and Yuxian Huang. Analysis and interpretation of data: Wei Xu, Qiang Li, Chenglu Huang, and Liang Chen. Drafting of the manuscript: Wei Xu, Qiang Li, and Chenglu Huang. Critical revision of the manuscript: Liang Chen.
Availability of data and materials
We declared that materials described in the manuscript, including all relevant raw data, would be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.
References
Liu J, Liang W, Jing W, et al. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ 2019;97:230–238
EASL. Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;2017(67):370–398
Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1–98
Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206–1217
Seto WK, Lo YR, Pawlotsky JM, et al. Chronic hepatitis B virus infection. Lancet 2018;392:2313–2324
Vigano M, Invernizzi F, Grossi G, et al. Review article: the potential of interferon and nucleos(t)ide analogue combination therapy in chronic hepatitis B infection. Aliment Pharmacol Ther 2016;44:653–661
Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–2695
Ning Q, Han M, Sun Y, et al. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). J Hepatol 2014;61:777–784
Chi H, Hansen BE, Guo S, et al. Pegylated interferon Alfa-2b Add-on treatment in hepatitis b virus envelope antigen-positive chronic hepatitis b patients treated with nucleos(t)ide analogue: a randomized, controlled trial (PEGON). J Infect Dis 2017;215:1085–1093
Hu P, Shang J, Zhang W, et al. HBsAg loss with peg-interferon Alfa-2a in hepatitis B patients with partial response to nucleos(t)ide Analog: new switch study. J Clin Transl Hepatol 2018;6:25–34
Marcellin P, Ahn SH, Ma X, et al. Combination of tenofovir disoproxil fumarate and peginterferon alpha-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology 2016;150:134–144
Brouwer WP, Xie Q, Sonneveld MJ, et al. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: a multicenter randomized trial (ARES study). Hepatology 2015;61:1512–1522
Brunetto MR, Marcellin P, Cherubini B, et al. Response to peginterferon alfa-2a (40KD) in HBeAg-negative CHB: on-treatment kinetics of HBsAg serum levels vary by HBV genotype. J Hepatol 2013;59:1153–1159
Rijckborst V, Hansen BE, Ferenci P, et al. Validation of a stopping rule at week 12 using HBsAg and HBV DNA for HBeAg-negative patients treated with peginterferon alfa-2a. J Hepatol 2012;56:1006–1011
Goulis I, Karatapanis S, Akriviadis E, et al. On-treatment prediction of sustained response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B patients. Liver Int 2015;35:1540–1548
Janssen HL, van Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 2005;365:123–129
Li GJ, Yu YQ, Chen SL, et al. Sequential combination therapy with pegylated interferon leads to loss of hepatitis B surface antigen and hepatitis B e antigen (HBeAg) seroconversion in HBeAg-positive chronic hepatitis B patients receiving long-term entecavir treatment. Antimicrob Agents Chemother 2015;59:4121–4128
Liu J, Li T, Zhang L, et al. The role of hepatitis B surface antigen in nucleos(t)ide analogues cessation among Asian patients with chronic hepatitis b: a systematic review. Hepatology 2019;70:1045–1055
Funding
This study was supported by Grant no. SHDC12015129 and No. SHDC12017125 from Shanghai Shen Kang Hospital Development Center; Grant no. 17411969700 and no. 18411966500 from Shanghai Association for Science and Technology; Grant no. 19YF1441200 from Shanghai Sailing Plan Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
Wei Xu, Qiang Li, Chenlu Huang, Qiankun Hu, Xun Qi, Yuxian Huang, Jiming Zhang, Liang Chen declare no competing financial and/or non-financial interests.
Consent for publication
All authors read and approved the manuscript.
Role of the Sponsor
The funding organizations are public institutions and had no role in the design and conduct of the study; collection, management, and analysis of the data; or preparation, review, and approval of the manuscript.
Ethics approval
The study protocol was approved by the ethics committees of Shanghai Public Health Clinical Center and Huashan Hospital. Written informed consents were obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, W., Li, Q., Huang, C. et al. Efficacy of peg-interferon–nucleoside analog sequential optimization therapy in HBeAg-positive patients with CHB. Hepatol Int 15, 51–59 (2021). https://doi.org/10.1007/s12072-020-10095-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-020-10095-1