Abstract
Background
Liver resection for hepatocellular carcinoma (HCC) patients with portal vein tumor thrombus (PVTT) offers a chance of cure, although survival is often limited. The actual 3-year survival and its associated prognostic factors have not been reported.
Methods
A nationwide database of HCC patients with PVTT who underwent liver resection with ‘curative’ intent was analyzed. The clinicopathologic characteristics, the perioperative, and survival outcomes for the actual long-term survivors were compared with the non-long-term survivors (patients who died within 3 years of surgery). Univariable and multivariable regression analyses were performed to identify predictive factors associated with long-term survival outcomes.
Results
The study included 1590 patients with an actuarial 3-year survival of 16.6%, while the actual 3-year survival rate was 11.7%. There were 171 patients who survived for at least 3 years after surgery and 1290 who died within 3 years of surgery. Multivariable regression analysis revealed that total bilirubin > 17.1 μmol/l, AFP > 400 ng/ml, types of hepatectomy, extent of PVTT, intraoperative blood loss > 400 ml, tumor diameter > 5 cm, tumor encapsulation, R0 resection, liver cirrhosis, adjuvant TACE, postoperative early recurrence (< 1 year), and recurrence treatments were independent prognostic factors associated with actual long-term survival.
Conclusion
One in nine HCC patients with PVTT reached the long-term survival milestone of 3 years after resection. Major hepatectomy, controlling intraoperative blood loss, R0 resection, adjuvant TACE, and ‘curative’ treatment for initial recurrence should be considered for patients to achieve better long-term survival outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world [1]. HCC often invades into portal vein branches. The incidence of portal vein tumor thrombus (PVTT) has been shown to be 44–62.2% in HCC patients. PVTT represents a significant poor prognostic factor for HCC patients [2, 3]. The Barcelona Clinic Liver Cancer staging system classifies these patients to be at an advanced stage and recommends sorafenib as the only treatment option [4]. A phase III, randomized, controlled trial showed the median survival time (MST) of patients with advanced HCC treated with sorafenib was only 6.5 months [5]. Advances in surgical techniques and perioperative management have resulted in long-term survival outcomes in selected HCC patients with PVTT who underwent aggressive surgical treatment [6,7,8].
The reported actuarial 3-year survival and MST after liver resection (LR) ranged widely from 0 to 60.4% and from 8 to 22 months, respectively [9,10,11]. The ‘3-year survival’ was defined as ‘long-term survival’ in this study because of the relatively poor prognosis in HCC patients with PVTT. The great variability in the long-term survival outcomes can be attributed to differences in patient selection, surgical techniques, and whether multidisciplinary treatments were used. The survival outcomes presented in all the studies were actuarial survival based on the Kaplan–Meier method which often overestimates the actual long-term survival. Moreover, most studies put little emphasis to find out the prognostic factors for long-term survival.
The actual 3-year survival and its associated factors for long-term survival have not been reported. This is a nationwide study based on the multicenter data of the Chinese Liver Cancer with Portal Vein Thrombus Consortium on HCC patients with PVTT who underwent liver resection with ‘curative intent.’ The preoperative, intraoperative, and postoperative clinicopathological variables which were associated with long-term survival outcomes were analyzed. The aim was to establish prognostic factors with the goal to achieve better long-term survival outcomes for HCC patients with PVTT using liver resection as the mainstay of treatment.
Materials and method
Diagnostic criteria for PVTT
All HCC patients with PVTT were diagnosed based on typical preoperative radiological features on imaging studies, which included ultrasound, Doppler ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI), and finally confirmed by intraoperative and postoperative histopathology examinations. The Cheng’s classification [6, 12] classifies PVTT into four types according to the extent of PVTT in the portal vein: type I, tumor thrombus involving the segmental branches of the portal vein or above; type II, tumor thrombus involving the right/left portal vein; type III, tumor thrombus involving the main portal vein; and type IV, tumor thrombus extending to involve the superior mesenteric vein.
Study population
This study included patients who underwent liver resection with ‘curative-intent’ for HCC patients with PVTT between January 2003 and December 2012 at the Eastern Hepatobiliary Surgery Hospital, the Sun Yat-sen University Cancer Centre, the Affiliated Tumor Hospital of Guangxi Medical University, the West China Hospital, the Affiliated Provincial Hospital of Anhui Medical University, Fujian Provincial Cancer Hospital, Xinqiao Hospital, the Affiliated Southern Hospital of Southern Medical University, the Foshan First People’s Hospital, the Inner Mongolia People’s Hospital, the No. 940 Hospital of Joint Logistics Support Force, the Shanghai Public Health Center, the Sichuan Provincial People’s Hospital, the First People’s Hospital of Xuzhou, the Wenzhou People’s Hospital, the General Hospital of Northern War Zone, the Second Affiliated Hospital of Wenzhou Medical University, the Affiliated Hospital of Binzhou Medical College, and the LongYan First Hospital Affiliated to Fujian Medical University. This study was censored on December 30, 2018. This study was approved by the institutional ethics committee of all the included hospitals. Written informed consent was obtained from all the patients for their data to be used for research purposes. The patients were divided into two groups: the actual ‘long-term survivor group’ included only patients who survived for ≥ 3 years after liver resection, and the ‘non-long-term survivor group’ comprised of those who died within 3 years of surgery. The terms ‘3-year survivors’ and ‘long-term survivors’ were used interchangeably.
The inclusion criteria were: (1) type I, II, and III PVTT; (2) PVTT after LR with Child–Pugh class A or selected B liver function (score ≤ 7); (3) absence of macroscopic hepatic vein tumor thrombus and bile duct tumor thrombus, extrahepatic spread and distant metastases; (4) no previous anticancer treatment before LR. Patients with less than 3 years of follow-up but were alive at the last encounter were excluded from the study.
Surgical procedures
The surgical procedures have been described in previous reports [13]. Liver resection was performed through a right subcostal incision with a midline extension. Intraoperative ultrasound was routinely used. The abdominal cavity was carefully searched for extent of local disease, extrahepatic metastases, and peritoneal seedings. The blood inflow of the liver was occluded using the Pringle’s maneuver using clamp/unclamp cycles of 15/5 min. Liver resection was carried out by the clamp crushing method.
Thrombectomy was performed according to the location and extent of PVTT. For patients with PVTT located within the resected region, the PVTT was resected en bloc with the tumor. For patients with PVTT protruded into the main portal vein beyond the resection plane, the PVTT was extracted from the opened stump of the portal vein. For patients with PVTT extended into the main portal vein and both its primary branches, the main portal vein was exposed and was clamped distal to the PVTT. The portal vein was incised at the bifurcation of the right and left portal veins, and the PVTT was extracted. After flushing with normal saline and confirming that no PVTT remained, the stump was closed by a continuous suture.
Postoperative adjuvant TACE procedures
One month after the operation, all patients underwent a comprehensive evaluation of liver function. After excluding patients who were not suitable for adjuvant TACE, remaining patients were recommended TACE due to preoperative PVTT. Whether the patients followed the physician’s recommendation mainly depended on their socioeconomic status and compliance with the doctors.
TACE was performed using the Seldinger method for the entire remnant liver. Hepatic angiography, computed tomography angiography, or both were performed to detect any obvious tumor stains in the remnant liver. If no tumor stain was found, an emulsion of doxorubicin hydrochloride (10 mg), pirarubicin (THP) or pharmorubicin (20–40 mg), and lipiodol (5–10 ml) was infused through the right and left hepatic arteries. The dosage of lipiodol and doxorubicin was determined by body surface area and the underlying liver function.
Data collection
The standard demographics, preoperative, operative, pathologic characteristics, and postoperative data were retrospectively reviewed. The demographic data included age, sex, anti-viral treatment, and comorbidity. The operative details included the types of hepatectomy (major vs. minor hepatectomy, and major hepatectomy was defined as ≥ 3 Couinaud segments), anatomical resection, lymph node invasion, and intraoperative blood transfusion and blood loss. Anatomical resections were defined using the Brisbane 2000 Nomenclature of Liver Anatomy and Resections [14]. The pathological data included tumor diameter, tumor number, satellite nodules, differentiation, cirrhosis, and resection margin status. The presence of microscopic tumor cells at the resection margin was considered as R1 resection. The postoperative data included major complications and adjuvant TACE. Postoperative major complications were evaluated using the modified Clavien–Dindo classification of surgical complications [15]. The site of recurrence, time to recurrence, and treatment of recurrence were also documented. The treatment options after recurrence included potentially curative treatments, such as a second liver resection, and non-curative treatments included transarterial chemoembolization (TACE), sorafenib, radiotherapy, and supportive treatment.
Statistical analysis
The overall survival (OS) was calculated from the date of surgery to the date of death or last follow-up. The recurrence-free survival (RFS) was calculated from the date of surgery to the date of recurrence, death, or last follow-up. The post-recurrence survival (PFS) was defined to be between the date of recurrence to the date of death or last follow-up. Continuous variables were reported as medians with interquartile range (IQR) and compared using the Student’s t test or the Mann–Whitney test. Categorical data were presented as frequencies (%) and compared using the Chi-square test or Fisher’s exact test. Univariable and multivariable logistic regression analyses were performed to identify the associations between potentially important clinical factors and the actual 3-year survival after liver resection. Kaplan–Meier curves were generated for OS, RFS, and PFS. Differences were considered statistically significant if the p value was lower than 0.05. Statistical analyses were performed using the SPSS software version 25.0 (SPSS, Chicago, IL, USA).
Results
Study population
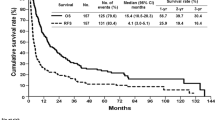
Of the 1590 patients who were included in the study, 129 patients were excluded because they had less than 3 years of follow-up but were still alive at the last encounter after liver resection. There were 171 patients who survived for ≥ 3 years (11.7%, the long-term survivor group) and 1290 who died within 3 years of surgery (88.3%, the non-long-term survivor group). The actuarial 3-year OS for the entire cohort was 16.6% (Fig. 1a), while the actual 3-year survival rate was 11.7%. The actuarial 3-year disease-free survival for the entire cohort was 12.5% (Fig. 1b).
Clinical demographics and preoperative data
The patient demographics and comorbidities were comparable between the two groups (Table 1). The incidence of anti-viral treatment was similar between the long-term survivor and non-long-term survivor groups (7.6% vs. 8.4%, p = 0.731). The percentages of HBeAg positivity and TBIL > 17.1 μmol/l were significantly higher in the non-long-term survivor group than the long-term survivor group (28.4% vs. 19.9%, p = 0.019; 36.4% vs. 26.3%, p = 0.009, respectively). In addition, there was a significant difference in the level of AFP between the two groups of patients (p = 0.001). No significant differences were found in the levels of HBV-DNA, ALB, ALT, AST, blood glucose, platelet count, and esophageal and gastric varices between the two groups of patients.
Operative and pathologic data
The data on the operative management are shown in Table 1. The percentages of anatomical resection, ascites, lymph node invasion, intraoperative chemotherapy, and intraoperative blood transfusion were comparable between the two groups. Significantly more of the long-term survivors underwent major hepatectomy (59.6% vs. 46.0%, p = 0.001). There were higher rates of type I and II PVTT in the long-term survivors group than the non-long-term survivor group (p = 0.001). Intraoperative blood loss > 400 ml was more commonly encountered in the non-long-term survivor group than the long-term survivors group (34.0% vs. 28.1%, p = 0.003).
The pathologic characteristics of the actual long-term survivors and non-long-term survivors are shown in Table 1. Significantly more of the long-term survivors underwent R0 liver resection (91.2% vs. 77.9%, p < 0.001). Poor prognostic factors, including tumor diameter > 5 cm, satellite nodules, absence of tumor encapsulation, and liver cirrhosis, were more commonly encountered in the non-long-term survival group than the long-term survivor group. There were no significant differences in tumor numbers and tumor differentiation between the two groups.
Postoperative outcomes and recurrence characteristics
The rates of major complication were comparable between the two groups (Table 1). However, almost half of the long-term survivors underwent postoperative adjuvant TACE, which was significantly higher than the 32.8% of non-long-term survivors who underwent adjuvant therapy (p < 0.001).
The details of tumor recurrence in the two groups are shown in Table 2. The long-term survivors had significantly lower cumulative overall recurrence (56.7% vs. 70.5%, p < 0.001) and early recurrence (< 1 year) rates (26.3% vs. 41.9%, p < 0.001). The majority of recurrences occurred intrahepatically for both the long-term (n = 66, 38.6%) and the non-long-term survivor groups (n = 516, 40.0%). The long-term survivors had lower rates of extrahepatic metastases and synchronous intrahepatic and extrahepatic recurrences (p = 0.035 and p = 0.027). For treatments of the initial recurrence, the long-term survivors underwent more ‘curative’ treatments for recurrence (30.4% vs. 21.1%, p = 0.006), whereas the non-long-term survivors underwent more ‘non-curative’ treatments for recurrence (49.4% vs. 26.3%, p < 0.001).
Multivariable analyses for independent factors and survivals
On multivariate analysis (Table 3), factors which were independently associated with actual long-term survivors were TBIL > 17.1 μmol/l, AFP > 400 ng/ml, types of hepatectomy, types of PVTT, intraoperative blood loss > 400 ml, tumor diameter > 5 cm, tumor encapsulation, R0 resection, liver cirrhosis, adjuvant TACE, postoperative early recurrence (< 1 years), and recurrence treatments.
Kaplan–Meier curves were generated to compare the OS and RFS rates for the independent predictive factors. As shown in Fig. 2, patients with TBIL ≤ 17.1 μmol/l and AFP ≤ 400 ng/ml had significantly longer OS and RFS than their counterparts (all p < 0.001). Patients who underwent R0 resection and with intraoperative blood loss ≤ 400 ml had better OS and RFS (all p < 0.001, Fig. 3a–d). Patients who underwent major hepatectomy did not have significantly better OS (p = 0.0052, Fig. 3e). However, they had significantly better RFS (p = 0.005, Fig. 3f). Adjuvant TACE was a significant predictor of OS and RFS (all p < 0.001, Figure S1A-B). Patients with early recurrence within 1 year had significantly shorter PFS compared to those who developed recurrence after 1 year (p < 0.001, Figure S1C). For patients with tumor recurrence, ‘curative’ treatment for initial recurrence resulted in significantly better PFS than ‘non-curative’ treatment (p < 0.001, Figure S1D). In addition, OS and RFS were significantly worse for patients with tumor diameter > 5 cm, liver cirrhosis, and type III PVTT (Figure S2).
Discussions
Based on the nationwide study with a very large sample series, this first study was conducted to determine the actual long-term survival after liver resection for HCC patients with PVTT. In this study, 171 actual long-term survivors (11.7%) were identified. The percentage of patients who actually survived over 3 years (11.7%) differed significantly from the actuarial 3-year survival rate of 16.6% for the entire cohort of 1590 patients in the study. The Kaplan–Meier survival analyses tend to overestimate survival due to the impact of patients being lost to follow-up and were censored in the analysis. In contrast, the actual 3-year survival from our study provided more pragmatic and tangible information for these patients. Our study revealed that TBIL > 17.1 μmol/l, AFP > 400 ng/ml, types of hepatectomy, types of PVTT, intraoperative blood loss > 400 ml, tumor diameter > 5 cm, tumor encapsulation, R0 resection, liver cirrhosis, adjuvant TACE, postoperative early recurrence (< 1 year), and recurrence treatments were independent predictive factors associated with actual long-term survival.
Currently, many tertiary liver referral centers consider liver resection to be a potentially curative treatment which provides an acceptable long-term survival outcome for carefully selected HCC patients with PVTT [7, 12, 16, 17]. The reported surgical mortality rates in HCC patients with PVTT who underwent liver resection ranged from 0 to 10.0% [10]. Our group has also established an accurate and convenient scoring system to select appropriate HCC patients with PVTT limited to a first-order branch of the main portal vein or above for LR [8]. As liver resection has evolved to benefit selected HCC patients with PVTT, this study aimed to identify predictive factors for long-term survivors to help to even better select these patients for liver resection.
For preoperative characteristics, some high-risk variables such as TBIL > 17.1 μmol/l and AFP > 400 ng/ml were found to associate with worse long-term survival outcomes after LR. These findings are in agreement with other reported studies [18, 19]. Although these risk factors do not preclude long-term survival in some of our patients, recommending liver resection for HCC patients with PVTT having high levels of both AFP and total bilirubin should be done very carefully and should only be done when complete tumor resection with an adequate future liver remnant can both be achieved.
For intraoperative and pathologic characteristics, the unfavorable predictive factors of long-term survival were minor hepatectomy, type of PVTT, intraoperative blood loss > 400 ml, tumor diameter > 5 cm, absence of tumor encapsulation, R1 resection, and liver cirrhosis. Among these predictors of survival outcomes, some factors which are modifiable include type of hepatectomy, intraoperative blood loss, and resection margin status. Major hepatectomy has been reported to yield acceptable perioperative and long-term outcomes in carefully selected patients with advanced HCC [20]. Most patients with PVTT are accompanied with large tumors. Major hepatectomy may provide a better chance to clear all the adjacent marco and microvascular invasion foci. Increased intraoperative blood loss has been reported to be an independent prognostic factor of tumor recurrence and in-hospital mortality for patients with HCC [21, 22]. Good surgeons using refined surgical techniques can reduce intraoperative blood loss. Several studies have also demonstrated that the resection margin status is an important prognostic factor of long-term survival for HCC patients [23, 24]. Surgeons should aim at achieving R0 liver resection for this group of patients.
For postoperative outcomes and treatment of HCC recurrence, postoperative adjuvant TACE has been shown to be effective in achieving better long-term survival. A series of studies showed that adjuvant TACE significantly reduced tumor recurrence and prolonged long-term survival outcomes for HCC patients with microvascular invasion and macrovascular invasion [25,26,27]. The non-long-term survivors in our study had a high early recurrence (< 1 year) rate, as early recurrence is likely to be secondary to metastases from the primary tumors through microvascular invasion which is often associated with large tumor size, multiple tumors, and satellites nodules [28]. Our team has established a nomogram model to predict early recurrence for HCC patients with PVTT after R0 resection [29]. This model can help clinicians to select appropriate patients with high early recurrence risks for postoperative adjuvant TACE, or for close postoperative monitoring to detect early HCC recurrence. Curative treatment such as liver re-resection can then be used for the recurrence. Currently, some new postoperative treatments like immunotherapy have proven promising effects in management of HCC recurrence after surgery [30]. However, the role of adjuvant immunotherapy was not analyzed in this study because this modality was not commonly applied in the clinical practice during the time period in this study.
The current study has several limitations. First, this is a retrospective study with its inherent defects and selection biases; second, variability and lack of standardization in the operative and perioperative management were unavoidable among the multiple institutions. Third, this study was conducted in China where the prevailing etiology of HCC is HBV infection. These data require validation from other groups with HCC of different etiologies.
In conclusion, although the management of HCC patients with PVTT is still challenging, one in nine patients in our study actually survived over 3 years after liver resection. The long-term survival outcomes of these patients can further be improved with better selection of patients for surgery, better surgical techniques, postoperative adjuvant TACE for patients identified by the nomogram to have high risks of early HCC recurrence, and early detection of recurrent HCC followed by further curative treatments.
References
European Association for the Study of the Liver. Electronic address eee, European Association for the study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
Bruix J, Sherman M. American Association for the study of liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2.
Schoniger-Hekele M, Muller C, Kutilek M, Oesterreicher C, Ferenci P, Gangl A. Hepatocellular carcinoma in Central Europe: prognostic features and survival. Gut. 2001;48(1):103–9.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90.
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34.
Shi J, Lai E, Li N, Guo W, Xue J, Lau W, et al. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci. 2011;18(1):74–80.
Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65(5):938–43.
Zhang XP, Gao YZ, Chen ZH, Chen MS, Li LQ, Wen TF, et al. An eastern hepatobiliary surgery hospital/portal vein tumor thrombus scoring system as an aid to decision making on hepatectomy for hepatocellular carcinoma patients with portal vein tumor thrombus: a multicenter study. Hepatology. 2019;69(5):2076–90.
Kamiyama T, Kakisaka T, Orimo T, Wakayama K. Hepatectomy for hepatocellular carcinoma with portal vein tumor thrombus. World J Hepatol. 2017;9(36):1296–304.
Sakamoto K, Nagano H. Surgical treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus. Hepatol Res. 2017;47(10):957–62.
Chen XP, Qiu FZ, Wu ZD, Zhang ZW, Huang ZY, Chen YF, et al. Effects of location and extension of portal vein tumor thrombus on long-term outcomes of surgical treatment for hepatocellular carcinoma. Ann Surg Oncol. 2006;13(7):940–6.
Shi J, Lai E, Li N, Guo W, Xue J, Lau W, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17(8):2073–80.
Peng ZW, Guo RP, Zhang YJ, Lin XJ, Chen MS, Lau WY. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2012;118(19):4725–36.
Strasberg SM, Phillips C. Use and dissemination of the brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg. 2013;257(3):377–82.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96.
Wang K, Guo WX, Chen MS, Mao YL, Sun BC, Shi J, et al. Multimodality treatment for hepatocellular carcinoma with portal vein tumor thrombus. Medicine. 2016;95(11):e3015.
Liu PH, Lee YH, Hsia CY, Hsu CY, Huang YH, Chiou YY, et al. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Ann Surg Oncol. 2014;21(6):1825–33.
Zheng N, Wei X, Zhang D, Chai W, Che M, Wang J, et al. Hepatic resection or transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus. Medicine (Baltimore). 2016;95(26):e3959.
Hiraoka A, Michitaka K, Kumada T, Izumi N, Kadoya M, Kokudo N, et al. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in japan: the need for a more detailed evaluation of hepatic function. Liver Cancer. 2017;6(4):325–36.
Pesi B, Giudici F, Moraldi L, Montesi G, Romagnoli S, Pinelli F, et al. Hepatocellular carcinoma on cirrhosis complicated with tumoral thrombi extended to the right atrium: results in three cases treated with major hepatectomy and thrombectomy under hypothermic cardiocirculatory arrest and literature review. World J Surg Oncol. 2016;14:83.
Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, et al. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249(4):617–23.
Zhang XP, Gao YZ, Chen ZH, Wang K, Cheng YQ, Guo WX, et al. In-hospital mortality after surgical resection in hepatocellular carcinoma patients with portal vein tumor thrombus. J Cancer. 2019;10(1):72–80.
Yoshida Y, Kanematsu T, Matsumata T, Takenaka K, Sugimachi K. Surgical margin and recurrence after resection of hepatocellular carcinoma in patients with cirrhosis. Further evaluation of limited hepatic resection. Ann Surg. 1989;209(3):297–301.
Goh BK, Chow PK, Teo JY, Wong JS, Chan CY, Cheow PC, et al. Number of nodules, child-pugh status, margin positivity, and microvascular invasion, but not tumor size, are prognostic factors of survival after liver resection for multifocal hepatocellular carcinoma. J Gastrointest Surg. 2014;18(8):1477–85.
Sun JJ, Wang K, Zhang CZ, Guo WX, Shi J, Cong WM, et al. Postoperative adjuvant transcatheter arterial chemoembolization after R0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann Surg Oncol. 2016;23(4):1344–51.
Zhang XP, Liu YC, Chen ZH, Sun JX, Wang K, Chai ZT, et al. Postoperative adjuvant transarterial chemoembolization improves outcomes of hepatocellular carcinoma associated with hepatic vein invasion: a propensity score matching analysis. Ann Surg Oncol. 2019;26(5):1465–73.
Chen ZH, Zhang XP, Zhou TF, Wang K, Wang H, Chai ZT, et al. Adjuvant transarterial chemoembolization improves survival outcomes in hepatocellular carcinoma with microvascular invasion: a systematic review and meta-analysis. Eur J Surg Oncol. 2019;45(11):2188–96.
Poon RT. Differentiating early and late recurrences after resection of HCC in cirrhotic patients: implications on surveillance, prevention, and treatment strategies. Ann Surg Oncol. 2009;16(4):792–4.
Zhang XP, Chen ZH, Zhou TF, Li LQ, Chen MS, Wen TF, et al. A nomogram to predict early postoperative recurrence of hepatocellular carcinoma with portal vein tumour thrombus after R0 liver resection: a large-scale, multicenter study. Eur J Surg Oncol. 2019;45(9):1644–51.
Brown ZJ, Greten TF, Heinrich B. Adjuvant treatment of hepatocellular carcinoma: prospect of immunotherapy. Hepatology. 2019;70(4):1437–42.
Funding
This study was supported by the Key Project of Natural Science Foundation of China (No: 81730097); the National Key Basic Research Programme ‘973 project’ (No: 2015CB554000); the National Natural Science Foundation of China (No: 81602523); the Shanghai Municipal Health Bureau (No: SHDC12015106); and the Shanghai Science and Technology Committee (No: 134119a0200).
Author information
Authors and Affiliations
Contributions
Shu-Qun Cheng, Wan Yee Lau, Zhen-Hua Chen, Xiu-Ping Zhang, Yu-Gang Lu, and Meng-Chao Wu contributed to conception and design, Shu-Qun Cheng contributed to financial support, Le-Qun Li, Min-Shan Chen,Tian-Fu Wen, Wei-Dong Jia, Dong Zhou, Jing Li, Ding-Hua Yang, Zuo-Jun Zhen, Yi-Jun Xia, Rui-Fang Fan, Yang-Qing Huang, Yu Zhang, Xiao-Jing Wu, Yi-Ren Hu, Yu-Fu Tang, Jian-Hua Lin, Fan Zhang, Cheng-Qian Zhong, Wei-Xing Guo, and Jie Shi contributed to provision of study materials or patients, Zhen-Hua Chen, Xiu-Ping Zhang, and Yu-Gang Lu contributed to collection and assembly of data, Zhen-Hua Chen, Xiu-Ping Zhang, and Yu-Gang Lu contributed to data analysis and interpretation, Zhen-Hua Chen, and Wan Yee Lau wrote the manuscript, all authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Ethical approval
This study was approved by the Institutional Ethics Committee of the Eastern Hepatobiliary Surgery Hospital, the Sun Yat-sen University Cancer Centre, the Affiliated Tumor Hospital of Guangxi Medical University, the West China Hospital, the Affiliated Provincial Hospital of Anhui Medical University, Fujian Provincial Cancer Hospital, Xinqiao Hospital, the Affiliated Southern Hospital of Southern Medical University, the Foshan First People’s Hospital, the Inner Mongolia People’s Hospital, the No.940 Hospital of Joint Logistics Support Force, the Shanghai Public Health Center, the Sichuan Provincial People’s Hospital, the First People’s Hospital of Xuzhou, the Wenzhou People’s Hospital, the General Hospital of Northern War Zone, the Second Affiliated Hospital of Wenzhou Medical University, the Affiliated Hospital of Binzhou Medical College, and the LongYan First Hospital Affiliated to Fujian Medical University.
Informed consent
Informed consent was obtained from the patients for their data to be used for research purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, ZH., Zhang, XP., Lu, YG. et al. Actual long-term survival in HCC patients with portal vein tumor thrombus after liver resection: a nationwide study. Hepatol Int 14, 754–764 (2020). https://doi.org/10.1007/s12072-020-10032-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-020-10032-2