Abstract
Background
Switching from nucleos(t)ide analogues to interferon (IFN) improves hepatitis B surface antigen (HBsAg) loss. We aimed to evaluate whether combining immunomodulators such as interleukin-2 (IL-2) and therapeutic vaccine with IFN enhances HBsAg loss in entecavir (ETV)-suppressed patients.
Methods
Ninety-four patients exhibiting virological suppression and hepatitis B e antigen (HBeAg) loss following ETV treatment were randomized 1:1:1 to receive ETV (group I) or IFN (group II) for 48 weeks, or IFN and vaccine for 48 weeks plus IL-2 for 12 weeks (group III). The primary endpoint was HBsAg loss at week 48. Peripheral natural killer (NK) cells and regulatory T cells (Treg) were measured as immune checkpoint indicators.
Results
Mean HBsAg decline at week 48 was significantly greater in group III (0.85 log 10 IU/mL) and group II (0.74 log 10 IU/mL), than in group I (0.13 log 10 IU/mL). At week 48, 9.38%, 3.03%, and 3.70% of subjects in group III, II, and I, respectively, achieved HBsAg loss. Among patients with baseline HBsAg titers ranging from 100 to 1500 IU/mL, HBsAg loss rate was 27.3, 7.1, and 0% in group III, II, and I, respectively. Responders in group III showed a significantly higher increase in CD56bright CD16−NK cells from week 24 to 36, and a significant decline in Treg from week 12 to 24 than non-responders.
Conclusion
For ETV-suppressed patients, particularly those with low baseline HBsAg levels, combination therapy with IFN and other immunomodulators may enhance HBsAg loss, while successful response correlates with partial restoration of NK cells and Tregs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite significant improvements in the efficacy of nucleos(t)ide analogues (NA) in treating chronic hepatitis B virus (HBV) infection, the majority of NA-treated patients rarely achieve sustained immune control, and may often require lifelong therapy [1]. A functional cure for chronic HBV infection characterized by sustained off-treatment clearance of serum hepatitis B surface antigen (HBsAg), is associated with improved long-term clinical outcomes including lower rates of HBV reactivation, as well as decreased risks of cirrhosis and hepatocellular carcinoma (HCC) [2,3,4]. Therefore, treatment guidelines have adopted HBsAg loss with or without development of hepatitis B surface antibody (anti-HBs) as an ideal endpoint for anti-HBV therapy [5,6,7]. However, this endpoint is rarely achieved with current antiviral therapies [8,9,10,11].
The barriers to elimination or inactivation of HBV covalently-closed-circular DNA (cccDNA) within infected hepatocyte nuclei, and to breaking the immune tolerance against HBV, constitute major obstacles to eradicating chronic hepatitis B (CHB). NA and interferon (IFN) when used alone are not endowed with both adequate antiviral and immunomodulatory activities. Combining these two agents with different mechanisms of action may help overcome obstacles, thereby facilitating successful discontinuation of long-term NA therapy and improving the chance of clinical cure [12]. Over the past decade, multiple attempts have been made to optimize the combination strategies, taking into consideration the choice of drugs and timing of their administrations [13,14,15,16]. Our previous OSST study [14] suggested that for HBeAg-positive patients with virological suppression by ETV, switching to a finite course of Peg-IFN significantly increased HBeAg seroconversion and HBsAg loss rates than continuing ETV.
The virus-mediated immune dysfunction contributes to the pathogenesis of chronic HBV infection [17]. Immune restoration is a crucial step toward functional cure. NA treatment recovers exhausted HBV-specific T cells transiently but not substantially [18], while IFN therapy leads to depletion of CD8+ T cells albeit boosting natural killer (NK) cells [19]. However, combination of both partially restores innate and adaptive immune responses [20]. Dynamic immune assays based on the OSST study indicated that successful response to IFN correlated with early significant restoration of immune responses, as manifested by increased NKG2C+ NK cells and attenuated regulatory T cell (Treg) function [21]. A recent meta-analysis suggested that the “switch-to” strategy was more likely to achieve HBsAg loss than the “add-on” or “de-novo combination” strategy [22]. It is conceivable that NA treatment would first suppress viral replication, thereby assisting the recovery of HBV-specific CD8+ T cells, sequential IFN will subsequently augment the innate immune response and enhance HBV clearance [12, 23, 24]. These data provide direct evidence that combining full viral suppression with immune modulation will likely lead to a cure for HBV infection [25]. However, the combination of potential drugs for HBV cure needs to be optimized and the respective mechanism of immune restoration is yet to be determined.
In light of the hypothetical benefit from immunomodulator therapy, attempts were made to boost the host immune response against HBV by therapeutic vaccine or recombinant human interleukin-2 (IL-2) [26, 27]. A pilot study showed that HBsAg vaccine induced HBV-specific CD4+T cell proliferative responses, which could be important in controlling viremia in chronic HBV carriers [28]. A previous study demonstrated that HBsAg vaccine in combination with lamivudine (LAM) and IL-2 induced antiviral immune responses, which might lead to consequent viral elimination [29]. However, this combined regimen was only tested in a small number of patients and did not include IFN or potent NA. We postulate that these immunomodulators could be of considerable potential to complement “switch-to” strategy and further enhance the immune response. In this proof of concept study, we aimed to investigate the clinical and immunological efficacy of sequential combination therapy with IFN, HBsAg vaccine, and IL-2 in ETV-suppressed patients with chronic hepatitis B.
Patients and methods
Study participants
This study was a phase IV, open-label, randomized controlled trial conducted at 12 hepatology centers in China between June 2013 and April 2017. Patients aged 18–65 years, who were HBeAg-positive prior to ETV treatment initiation, had received ETV treatment for 1–5 years, had been positive for HBsAg for ≥ 6 months prior to enrolment, and had serum HBV DNA ≤ 1000 copies/mL and HBeAg loss with or without HBeAg seroconversion were eligible. Patient exclusion criteria were as follows: history of drug resistance to ETV; receiving IFN or systemic antiviral therapy within the previous 6 months; coinfection with the human immunodeficiency virus, hepatitis C virus, or hepatitis D virus; presence of decompensated liver disease (defined as a Child–Pugh Score B or C), pregnancy or lactation; and having any other contraindication for IFN therapy described in a previous study [14]. To increase the number of eligible patients, the protocol was amended to include patients with an ETV treatment duration of ≥ 1 year, and history of other NA treatment before ETV without any drug resistance.
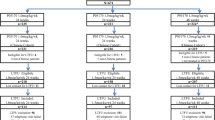
Patients were randomly and equally (1:1:1) assigned to one of three groups. Group I received continuous ETV (0.5 mg once daily, Fujian Cosunter Pharmaceutical Co Ltd.) for 48 weeks. Group II received IFN alfa-2b (IFNa2b) (6 million international unit (MIU) subcutaneously (SC) every other day, Beijing Kawin Technology Share-Holding Co., Ltd.) for 48 weeks. Group III received IFNa2b and hepatitis B vaccine (Hep B) (60 μg intramuscularly every 4 weeks, Shenzhen Kangtai Biological Products Co., Ltd.) for 48 weeks plus rhIL-2 (0.25 MIU SC every other day, Beijing Yuance Pharmaceutical Co., Ltd.) for the first 12 weeks (Fig. 1). Patients assigned to Group II and Group III continued ETV for the first 8 weeks to reduce the risk of alanine aminotransferase (ALT) flares during the switching period. All the patients who completed 48 weeks of treatment were followed up for 24 weeks. Patients assigned to Group I continued ETV treatment during follow-up period. The randomization schedule was generated by SAS PROC PLAN with a block size of 6 and were communicated to the sites in sealed envelopes prepared by the statistician based on the randomization sequence. All the authors had access to the study data and had reviewed and approved the final manuscript.
Laboratory measurements
Laboratory tests were performed at baseline and week 12, 24, 36, 48, and 72. HBsAg was measured at a central laboratory using the Roche Elecsys HBsAg II Quant assay (Roche Diagnostics, Penzberg, Germany; dynamic range 0.05–52,000 IU/mL). HBeAg, anti-HBs, antibody to hepatitis B e antigen (anti-HBe), and antibody to hepatitis B core antigen (anti-HBc) were determined by chemiluminescent assay (Kemei Biological Technology Co., Ltd., Beijing, China). HBV DNA level was quantified by qPCR assay (Kehua Bio-engineering Co. Ltd., Shanghai, China; the lower limit of detection was 500 copies/mL). All patients were assessed for blood sugar, routine blood tests, thyroid function, and autoimmune antibodies before and during treatment. Laboratory personnel were unaware of treatment assignments.
Adverse events (AEs) and laboratory test results were recorded according to the International Conference on Harmonisation Guideline Clinical Safety Data Management: Definitions and Standards for Expedited Reporting.
Proportions analysis of peripheral blood immunological indicators
The dynamics of CD56bright CD16−NK cells and Treg proportions were studied in patients from Tongji Hospital. APC-CD16/APC-Cy7-CD56/FITC-CD3, and FITC-CD4/PE-CD25/ALEXA647-CD127 multiple-color anti-human monoclonal antibodies (mAbs) combination reagents and matched isotype controls were used to determine the peripheral NK cell subsets and overall Tregs among lymphocytes at baseline, week 4, 12, 24, 36, and 48. Peripheral blood mononuclear cells (PBMCs) were isolated immediately from fresh blood and subjected to antibody staining followed by flow cytometry. All reagents were purchased from Becton, Dickinson, and Company (BD, Franklin Lakes, USA). Cell surface antigen staining for flow cytometry was conducted according to the standard procedure of the BD Pharmingen protocol. All samples were detected by BD FACS Canto II Flow Cytometry System and analyzed with the BD FACS Diva Software.
Statistical analysis
The primary endpoint was HBsAg loss at week 48. Secondary endpoints included rates of HBsAg seroconversion, HBsAg decline, HBeAg seroconversion, ALT normalization [ALT level < 1 × the upper limit of normal (ULN)] and HBV DNA levels < 1000 copies/mL at week 48.
All patients who received at least one dose of study drug were included in the intention-to-treat (ITT) and safety populations. In addition, a modified ITT (mITT) analysis was conducted, excluding one patient in group II who was HBeAg-positive at baseline prior to randomization.
Response rates for primary and secondary endpoints were assessed by calculating percentages and 95% confidence intervals (CIs) for the ITT and mITT populations. Patients with missing categorical data at week 48 were classified as non-responders. Categorical variables were analyzed using the v2 test or Fisher’s exact test, and continuous variables were analyzed by t test or Wilcoxon test as appropriate. Logarithmic transformation was performed in the case of skewed data. Statistical analyses were conducted using SAS version 9.2.
Results
Baseline characteristics and patient disposition
Of the 94 patients randomized, 93 patients received ≥ 1 dose of the study drug (group I, n = 27; group II, n = 34; group III, n = 32). One patient who was HBeAg-positive at baseline in group II was excluded from the mITT population (group I, n = 27; group II, n = 33; group III, n = 32) (Fig. 2). Most baseline demographic and disease characteristics were similar among the three groups, except that ALT levels were higher in group II, and lower in group III, while all levels were within the normal range. 48.1% (13/27), 42.4% (14/33), and 43.8% (14/32) in group I, II, and III respectively, developed HBeAg seroconversion prior to randomization (Table 1).
Efficacy
The results of mITT analysis for the primary endpoint of HBsAg loss at week 48 showed a trend towards being higher in patients treated with IFN, IL-2, and vaccine (9.38%), compared to those treated with IFN (3.03%) or ETV (3.70%) alone (Table 2), although the p values for the comparison were not statistically significant due to the small sample sizes. HBsAg seroconversion at week 48 occurred only in patients in group III (9.38%), and group II (3.70%), but none in group I (0%).
More patients receiving combination treatment had low levels of HBsAg at the end of treatment than those receiving IFN, or continuing ETV; 59.38% (19/32), 54.55% (18/33), and 44.44% (12/27) in group III, II, and I, respectively (p > 0.05) had HBsAg levels < 1000 IU/mL, while 15.63% (5/32), 12.12% (4/33), and 7.41% (2/27) in group III, II, and I, respectively had HBsAg levels < 10 IU/mL at week 48 (Table 2).
Mean HBsAg decline from baseline to week 48 was significantly greater in group III (0.85 log10 IU/mL) and group II (0.74 log10 IU/mL) than in group I (0.13 log10 IU/mL, p < 0.05 for all comparisons vs group I) (Fig. 3a).
On-treatment HBsAg decline from baseline to week 48. a On-treatment HBsAg decline from baseline to week 48 in mITT population. Data shown are mean ± SEM. Group I vs group II, p < 0.05; group I vs group III, p < 0.05; group II vs III, p > 0.05. b On-treatment HBsAg decline from baseline to week 48 in patients with qHBsAg 100–1500 IU/mL. Data shown are mean ± SEM. Group I vs group II, p > 0.05; group I vs group III, p < 0.05; group II vs III, p > 0.05
All individuals who lost HBsAg had a quantitative HBsAg titer below 1500 IU/mL at baseline. The only patient in group I who lost HBsAg had a very low baseline HBsAg level of 0.61 IU/mL. Among the patients with HBsAg titer ranging from 100 to 1500 IU/mL at baseline, HBsAg loss was 27.3% (3/11), 7.1% (1/14), and 0% (0/13) in group III, II, and I, respectively. Mean HBsAg decline from baseline to week 48 in group III (1.65 log10 IU/mL) was significantly greater than in group I (0.04 log10 IU/mL, p < 0.05), and tended to be greater than in group II (0.61 log10 IU/mL, p = 0.066) (Fig. 3b). ROC analyses identified a baseline HBsAg level < 560 IU/mL as the optimal cut-off to predict HBsAg loss in patients receiving combination treatment. Three out of five patients with baseline HBsAg level < 560 IU/mL lost HBsAg, while none of the patients with baseline HBsAg level > 560 IU/mL had HBsAg loss.
Among 65 patients receiving IFN-based therapy, the duration of prior ETV treatment in 4 patients achieving HBsAg loss was 5.75 ± 2.31 years, which was significantly longer than that in the remaining 61 patients who did not achieve initial response (3.35 ± 1.87 years, p < 0.05).
HBV DNA level < 1000 copies/mL was maintained at week 48 in 100.0%, 72.7%, and 84.4% of patients in group I, II, and III, respectively. During treatment, one patient in group I experienced a transient HBV DNA rebound (HBV DNA > 1000 copies/mL), while six patients in group II, and three patients in group III experienced HBV DNA rebound. Among them, two patients achieved HBeAg seroconversion, whereas none achieved HBsAg loss. One patient in group II with HBV DNA rebound was retreated with ETV after switching to IFN. At week 48, 96.3%, 75.8%, and 78.1% of patients in group I, II, and III, respectively, had normal ALT levels (< 1 × ULN). During treatment, ALT elevation did not appear to be associated with viral rebound or HBsAg loss. Of the four patients receiving IFN-based therapy who lost HBsAg (one in group II, three in group III), two had maximum ALT levels in the range of 1–2 × ULN. Of the patients who were anti-HBe negative at baseline, 7.1% (1/14), 57.9% (11/19), and 27.8% (5/18) in group I, II, and III, respectively, had HBeAg seroconversion at week 48 (Table 2).
Post-treatment response rates
Amongst 84 patients who completed 48 weeks of treatment (group I, n = 27; group II, n = 29; group III, n = 28), one patient in group I, one patient in group II and two out of three patients in group III who lost HBsAg at the end of treatment (EOT) maintained this response during 24 weeks of follow-up, of these, one patient in group II and two patients in group III maintained HBsAg seroconversion. One patient in group II with an EOT HBsAg level of 22.39 IU/mL achieved late HBsAg loss but failed to achieve HBsAg seroconversion at 24 weeks of untreated follow-up. The rate of HBeAg seroconversion was 21.4% (3/14), 66.7% (12/18), and 37.5% (6/16) in group I, II, and III, respectively, 24 weeks post-treatment. Sustained HBeAg seroconversion was achieved in 63.6% (7/11) of patients in group II, and 60% (3/5) in group III (Fig. 4). HBV DNA level < 1000 copies/mL was achieved in 100.0, 41.4, and 50% of patients in group I, II III, respectively, 24 weeks post-treatment. Seven patients in group II and eight patients in group III experienced virological relapse after cessation of IFN treatment. ETV therapy was reintroduced in these patients. At 24 weeks post-treatment, 96.3% (26/27), 86.2% (25/29), and 75% (21/28) of patients in group I, II, and III, respectively, had normal ALT levels (< 1 × ULN).
HBsAg loss, HBeAg seroconversion, and HBV DNA repression 24 weeks post-treatment. a Sustained and late HBsAg loss, HBeAg seroconversion, and HBV DNA repression in group I. b Sustained and late HBsAg loss, HBeAg seroconversion, and HBV DNA repression in group II. c Sustained and late HBsAg loss, HBeAg seroconversion, and HBV DNA repression in group III. aOnly patients who completed 48 weeks of allocated treatment were included in calculations. Seven patients with missing data 24 weeks post-treatment were classified as non-responders. bOnly patients who completed 48 weeks of allocated treatment were included in calculations. Six patients with missing data 24 weeks post-treatment were classified as non-responders. cOnly patients who were HBeAb-negative at the start of treatment were included in calculations
Safety
Combination of IFN, IL-2, and vaccine therapy was generally well-tolerated. AEs were more frequently observed in group II, and group III than in group I. No AEs were deemed related to the combination of IFN and immunomodulator therapy, except that post-treatment ALT flares (ALT > 5 ULN) seemed more common in patients treated with combination therapy, though not significantly (Table 3). Three patients in group II and 1 patient in group III discontinued treatment for safety reasons compared with none in group I. None of the patients in any of the three groups experienced an ALT flare during 48 weeks of treatment. After cessation of IFN therapy, post-treatment ALT flares which occurred in 5 out of 32 patients in group III, and 1 out of 34 patients in group II (p > 0.05), resolved after re-treatment with ETV. Of 6 patients experiencing post-treatment ALT flare, all were HBeAb negative at baseline, none of them achieved HBeAg seroconversion or HBsAg loss during treatment, while only one patient achieved post-treatment HBeAg seroconversion. A large proportion of patients in group II and group III experienced decreases in platelet, neutrophil, and white blood cell counts during treatment; however, the majority of such episodes (> 93%) were mild and cell counts returned to normal by week 48 in 91.2% of patients in group II, and 90.6% of patients in group III.
Dynamic changes in CD56bright CD16−NK cell and Treg proportions during treatment
CD56bright CD16−NK cells were defined as CD3−CD56brightCD16− lymphocytes (Fig. 5a). The frequency of CD56bright CD16−NK cells in group III not only showed a rising trend during treatment (Fig. 5b), but also was significantly higher than in group I or group II at week 4 and week 36 (week 4: 5.44 ± 4.00% vs. 1.72 ± 1.03%, and 2.98 ± 2.43%; week 36: 6.92 ± 2.84% vs. 2.18 ± 1.39%, and 3.97 ± 2.74%, p < 0.05 for all comparisons vs group III, Fig. 5c). Response to treatment was defined as HBsAg loss and/or HBeAg seroconversion. Responders in group III showed a significantly higher increase in the proportions of CD56bright CD16−NK cells from week 24 to 36 compared with non-responders (1.87 ± 0.74 vs. 0.06 ± 0.13, p < 0.05, Fig. 5d, e). Tregs were defined as CD4+CD25+CD127low/− lymphocytes (Fig. 5f). Proportions of Treg from week 12 to 24 decreased in responders in group II and group III, but increased in group I (Fig. 5g). Responders in group III showed a significantly greater decline in Tregs proportions from week 12 to 24 compared with responders in group II (0.55 ± 0.08 vs. 0.18 ± 0.08, p < 0.05, Fig. 5h) or non-responders in group III (0.55 ± 0.08 vs. − 0.89 ± 0.35, p < 0.05, Fig. 5i, j).
Dynamic fluctuations of proportions of CD56bright CD16−NK cells and Tregs during 48 weeks of treatment. a Flow cytometry staining of CD56bright CD16− natural killer (NK) cells from a representative patient. Gate P1 and P2 illustrating the cell gating strategy for the selection of lymphocytes from peripheral blood mononuclear cells (PBMCs) and CD3− lymphocytes from lymphocytes, respectively. The cells in the lower right quadrant of the right dot plot (gate P3) are CD56bright CD16− NK cells. (B&C) A series of comparisons among three groups. Data are expressed as mean ± SEM. b CD56bright CD16− NK cell proportions during treatment. c CD56bright CD16− NK cell proportions at week 4. Significant p-value was evaluated by independent-sample t-test. d, e A series of comparisons between responders and non-responders in group III. Data are expressed as mean ± SEM. d CD56bright CD16− NK cell proportions during treatment. e Increase in CD56bright CD16− NK cell proportions from week 24 to week 36. Significant p-value was evaluated by independent-sample t-test. Increase in CD56bright CD16− NK cell proportions from week 24 to week 36 = CD56bright CD16− NK cell proportions (week 36–week 24)/week 24. f Flow cytometry staining experiment of regulatory T-cells (Tregs) from a representative patient. Gate P1 and P2 illustrating the cell gating strategy for the selection of lymphocytes from PBMCs and CD4+ cells from lymphocytes, respectively. The cells in the lower right quadrant of the right dot plot (gate P3) are Tregs. g, h A series of comparisons among responders in three groups. Data are expressed as mean ± SEM. g Treg proportions during treatment. h Decline in Treg proportions from week 12 to week 24. i, j A series of comparisons between responders and non-responders in group III. Data are expressed as mean ± SEM. i Treg proportions during treatment. j Decline in Treg proportions from week 12 to week 24. Significant p-value was evaluated by Mann–Whitney U test. Decline in Treg proportions from week 12 to week 24 = Treg proportions (week 12–week 24)/week 12. APC allophycocyanin, FITC fluorescein isothiocyanate, FSC forward scatter, Ig immunoglobulin, PE phycoerythrin, SSC side scatter. *p < 0.05
Discussion
In this proof-of-concept pilot study, higher rates of HBsAg loss were observed in patients treated with combination therapy consisting of IFN, IL-2, and a therapeutic vaccine than those treated with IFN or ETV alone, despite the absence of statistically significant difference due to the relatively small sample size. HBsAg seroconversion was only observed in patients receiving IFN-based treatment. The rate of HBsAg loss with sequential IFN therapy in group II (3.03%) was lower than that previously reported in the OSST study in which Peg-IFN was used as a “switch-to” strategy (8.5%) [14]. This may be ascribed to the fact that IFN is not more efficient than Peg-IFN. When IFN was combined with other immunomodulators, HBsAg loss rate reached 9.38% higher than that by switching to Peg-IFN alone. The use of Peg-IFN instead of IFN in this innovative multi-targeted therapeutic approach may improve the chance of cure in ETV-suppressed patients, and warrants further investigation.
A marked decline in serum HBsAg levels during IFN therapy may reflect improved host immune control of HBV infection, and thus is associated with a sustained response [30,31,32]. In the present study, combination therapy led to a more HBsAg decline than did IFN alone, particularly in those with low baseline HBsAg levels. HBsAg loss was durable and HBeAg seroconversion tended to increase after completion of a finite course of IFN-based treatment. Furthermore, 14 patients receiving IFN-based therapy had HBsAg level < 100 IU/mL at EOT, while one patient with an EOT HBsAg level of 22.36 IU/mL achieved late HBsAg loss 24 weeks post-treatment. Increase of HBsAg titers after treatment cessation was observed in 5 patients. Extended Peg-IFN treatment enables more NA-experienced patients to achieve HBsAg loss [15, 33, 34], which indicates the possible need for extending the combination therapy duration in the subgroup of patients with low EOT HBsAg levels to ensure a durable response, or at least to reduce the risk of relapse.
Consistent with previous reports of IFN therapy, including OSST study in chronic hepatitis B [14, 15, 35], the present pilot study revealed that favorable responses were more common in individuals with low initial levels of HBsAg (< 1500 IU/mL). This feature may help to predict which patients are more likely to achieve clinical cure during IFN-based combination therapy. However, for the patients with much lower HBsAg level (< 100 IU/mL), continuing ETV treatment may possibly lead to HBsAg loss. The only patient in group I who lost HBsAg had a very low baseline HBsAg titer of 0.61 IU/mL, and had been treated with ETV for 7.79 years until HBsAg loss. The remaining patients continuing ETV treatment showed slow HBsAg decline (0.04 log10 IU/mL) and minimal HBeAg seroconversion rate (7.6%). Although long-term potent NA therapy may induce HBsAg loss, in accordance with a previous report, the corresponding calculated median duration that NA needed to clear HBsAg was 52.2 years [36], thus, lifetime therapy may be required in the vast majority of CHB patients. At present, alternative strategies are needed to achieve early HBsAg loss or successful NA withdrawal.
NA treatment efficiently inhibits HBV replication and yields superior virologic response rates [10, 37], and may at least in part favor restoration of HBV-specific T cell reactivity [18], but it does not directly target cccDNA. In contrast, IFN exerts both immune-modulatory and direct antiviral effects, promotes partial cccDNA degradation, and mediates epigenetic repression of cccDNA transcriptional activity, which directly contributes to the decline of viral antigens (HBeAg, HBsAg) amounts [38, 39]. Besides, prior long-term ETV treatment significantly reduce viral loads and viral antigen levels, which might be beneficial to sequential IFN-induced immune activation. Therefore, switching to IFN from a stable NA regimen constitutes an encouraging strategy to optimize treatment efficacy through harnessing potent antiviral activity and immune-modulatory effects [13,14,15,16, 21, 40,41,42]. However, under this circumstance, restoration of adaptive immunity essential for viral clearance is still inadequate, additional strategies such as immunotherapeutic agents, might theoretically complement IFN-based therapy aiming at provoking more efficient adaptive immune responses.
Early studies showed that vaccine containing HBV-related proteins exhibit considerable immune-modulatory capacity [26, 29]. The boosting of specific immune response induced by HBsAg vaccine reduced HBV replication in about 50% of CHB patients [28]. Combination therapy with LAM plus vaccine was better than LAM alone in CHB patients in terms of HBV DNA negativity and HBeAg seroconversion [26]. It has been demonstrated in several studies that IL-2 therapy may amplify the immune response by regulating T lymphocytes and NK cells as well as inducing tumor necrosis factor alpha (TNF-a) and IFN-γ production [43, 44]. A pilot study in Japan showed that HBeAg levels did not change during IL-2 treatment, while HBeAg loss occurred after discontinuation of therapy [27]. Nevertheless, some studies reported that IL-2 used alone or in combination with IFN showed limited clinical efficacy in Caucasian HBV DNA and HBeAg positive patients [45, 46]. Patient characteristics including race as well as dose and schedule of IL-2 administration varied from study to study, providing possible explanations for the discrepancies regarding the efficacy. A therapeutic HBsAg vaccine in combination with IL-2 and LAM was efficient in strongly inducing HBsAg-specific T cells and cytotoxic lymphocytes (CTL) instrumental in improving viral clearance [29]. Thus, together with previously reported findings, the present data suggest that following complete viral suppression by prior NA treatment, both HBsAg vaccine and IL-2 may have the potential to complement IFN therapy for the synergistic induction of HBsAg loss.
CD56bright CD16−NK cells, constituting 70% of NK cells in peripheral lymphoid organs, secrete various cytokines, such as IFN-γ and TNF [47]. Micco et al. reported that Peg-IFN is capable of potently and cumulatively driving proliferation and potential antiviral activity of CD56bright NK cells [19]. The OSST parallel immunologic study showed that in ETV-treated patients switching to IFN, the percentage of CD56bright NK cells was significantly elevated, more importantly, these cells contributed to HBsAg and cccDNA clearance through TRAIL-induced cytolysis and TNF-α/IFNγ-mediated noncytolytic pathways [48]. The present study showed that at week 4 after initiating therapy, patients receiving combination therapy had significantly higher frequency of CD56bright CD16−NK cells than those receiving ETV or IFN alone, moreover, responders to combination therapy showed a sustained increase in CD56bright CD16−NK cells proportions until week 36. A previous study reported that an expanded population of activated, functional NK cells induced by Peg-IFN could be maintained for at least 36 weeks [49]. On the other hand, IFN-based combination therapy can induce HBV-specific T cell restoration [20]. Treg can significantly inhibit the HBV-specific T cell response [50]. Here we observed that Tregs proportions markedly decreased from week 12 to 24 in responders to IFN-based therapy, with the most significant decline in responders to combination therapy, but did not in ETV responders or non-responders to combination therapy. These data supported the notion that adding immunomodulators to IFN may better restore or augment both innate and adaptive arms of the immune response, thereby potentially enhancing HBsAg decline and loss.
Considering safety, either IFN alone, or in combination with IL-2 and vaccine was generally well tolerated without observable unexpected AEs. A previous study revealed that 0.3 million units IL-2 (twice per week for 3 months) was biologically active but non-toxic dose, while 1.0 million units was a toxic dose [51]. For safety reason, the dose of IL-2 that we used is 1–10% of the maximum tolerated dose reported in studies of IL-2 therapy in patients with metastatic cancer. Besides, because there were only reports on the treatment with IL-2 combined with IFN rather than Peg-IFN in CHB patients [46], we chose IFN in this combination regimen to reduce potential side effects. ALT flares were more common in patients receiving combination therapy, though not significantly, the overall AE incidence rates were comparable between IFN and combination groups, but higher than in the ETV group, indicating that most AEs may be associated with IFN, while combining low-dose IL-2 and HBsAg vaccine did not significantly increase the risk of side effects.
This study has certain limitations. It was difficult to determine the HBV genotype due to the undetectable HBV DNA levels at baseline, thus the impact of genotype on HBsAg loss was not ascertained. Although the data on combination therapy with ETV, IFN, IL-2, and vaccine is promising, it would be premature to draw a firm conclusion regarding the efficacy of the combination therapy from this pilot study because only a limited number of patients were enrolled. Nonetheless, this study provides the proof of concept supporting further studies to assess the efficacy and long-term benefit of the combination of Peg-IFN with immunomodulators in NA-experienced patients, and may help to develop novel therapeutic strategies to reconstitute antiviral functions enabling cure of hepatitis B. We are currently carrying out two multicenter real-world studies (COST and OCEAN studies) to confirm these preliminary findings.
In conclusion, in patients with HBeAg loss and sustained HBV DNA suppression by long-term ETV treatment, particularly in those with low baseline HBsAg levels, switching to combination therapy consisting of IFN, IL-2, and vaccine may enhance rates of HBsAg loss. Moreover, HBsAg loss is durable in the majority of patients, and HBeAg seroconversion rates tend to increase during 24 weeks of follow-up. The clinical efficacy may be closely associated with partial restoration of CD56bright CD16−NK cells and Tregs immune responses.
Abbreviations
- ALT:
-
Alanine aminotransferase
- CHB:
-
Chronic hepatitis B
- cccDNA:
-
Covalently closed circular DNA
- ETV:
-
Entecavir
- HBeAg:
-
Hepatitis B e antigen
- HBsAg:
-
Hepatitis B surface antigen
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- ITT:
-
Intention-to-treat
- IFN:
-
Interferon
- IL-2:
-
Interleukin-2
- LAM:
-
Lamivudine
- mITT:
-
Modified intention-to-treat
- NK cell:
-
Natural killer cell
- NA:
-
Nucleos(t)ide analogue
- Peg-IFN:
-
Pegylated Interferon
- ROC:
-
Receiver-operating characteristic
- Treg:
-
Regulatory T cell
- ULN:
-
Upper limit of normal
References
Dandri M, Locarnini S. New insight in the pathobiology of hepatitis B virus infection. Gut 2012;61(Suppl 1):i6–17
Chen YC, Sheen IS, Chu CM, Liaw YF. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology 2002;123:1084–1089
Buster EH, Flink HJ, Cakaloglu Y, Simon K, Trojan J, Tabak F, et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology 2008;135:459–467
Moucari R, Korevaar A, Lada O, Martinot-Peignoux M, Boyer N, Mackiewicz V, et al. High rates of HBsAg seroconversion in HBeAg-positive chronic hepatitis B patients responding to interferon: a long-term follow-up study. J Hepatol 2009;50:1084–1092
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL. Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;2017(67):370–398
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1–98
Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH, American Association for the Study of Liver D. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261–283
Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–2695
Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206–1217
Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology 2010;51:422–430
Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008;359:2442–2455
Lin CL, Kao JH. Review article: novel therapies for hepatitis B virus cure—advances and perspectives. Aliment Pharmacol Ther 2016;44:213–222
Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak WY, Elkashab M, et al. Combination of tenofovir disoproxil fumarate and peginterferon alpha-2a increases loss of hepatitis b surface antigen in patients with chronic hepatitis B. Gastroenterology 2016;150(134–44):e10
Ning Q, Han M, Sun Y, Jiang J, Tan D, Hou J, et al. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). J Hepatol 2014;61:777–784
Hu P, Shang J, Zhang W, Gong G, Li Y, Chen X, et al. HBsAg loss with peg-interferon alfa-2a in hepatitis b patients with partial response to nucleos(t)ide analog: new switch study. J Clin Transl Hepatol 2018;6:25–34
Ouzan D, Penaranda G, Joly H, Khiri H, Pironti A, Halfon P. Add-on peg-interferon leads to loss of HBsAg in patients with HBeAg-negative chronic hepatitis and HBV DNA fully suppressed by long-term nucleotide analogs. J Clin Virol 2013;58:713–717
Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, et al. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology 2008;134:1938–1949
Boni C, Laccabue D, Lampertico P, Giuberti T, Vigano M, Schivazappa S, et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology 2012;143(963–73):e9
Micco L, Peppa D, Loggi E, Schurich A, Jefferson L, Cursaro C, et al. Differential boosting of innate and adaptive antiviral responses during pegylated-interferon-alpha therapy of chronic hepatitis B. J Hepatol 2013;58:225–233
de Niet A, Stelma F, Jansen L, Sinnige MJ, Remmerswaal EB, Takkenberg RB, et al. Restoration of T cell function in chronic hepatitis B patients upon treatment with interferon based combination therapy. J Hepatol 2016;64:539–546
Yan W, Wu D, Wang X, Chen T, Lai Q, Zheng Q, et al. Upregulation of NKG2C + natural killer cells, TLR-2 expression on monocytes and downregulation of regulatory T-cells influence PEG-IFN treatment efficacy in entecavir-suppressed patients with CHB. Antivir Ther 2015;20:591–602
Qiu K, Liu B, Li SY, Li H, Chen ZW, Luo AR, et al. Systematic review with meta-analysis: combination treatment of regimens based on pegylated interferon for chronic hepatitis B focusing on hepatitis B surface antigen clearance. Aliment Pharmacol Ther 2018;47:1340–1348
Wu D, Ning Q. Toward a Cure for Hepatitis B Virus Infection: combination Therapy Involving Viral Suppression and Immune Modulation and Long-term Outcome. J Infect Dis 2017;216:S771–S777
Thimme R, Dandri M. Dissecting the divergent effects of interferon-alpha on immune cells: time to rethink combination therapy in chronic hepatitis B? J Hepatol 2013;58:205–209
Wu D, Han M, Ning Q. An integration of deep viral suppression with sequential immune modulation (cocktail therapy) to restore antiviral capacity: the future of chronic hepatitis B? J Hepatol 2015;62:240–241
Horiike N, Fazle Akbar SM, Michitaka K, Joukou K, Yamamoto K, Kojima N, et al. In vivo immunization by vaccine therapy following virus suppression by lamivudine: a novel approach for treating patients with chronic hepatitis B. J Clin Virol 2005;32:156–161
Kakumu S, Fuji A, Yoshioka K, Tahara H, Ohtani Y, Hirofuji H, et al. Pilot study of recombinant human interleukin 2 for chronic type B hepatitis. Hepatology 1988;8:487–492
Couillin I, Pol S, Mancini M, Driss F, Brechot C, Tiollais P, et al. Specific vaccine therapy in chronic hepatitis B: induction of T cell proliferative responses specific for envelope antigens. J Infect Dis 1999;180:15–26
Dahmen A, Herzog-Hauff S, Bocher WO, Galle PR, Lohr HF. Clinical and immunological efficacy of intradermal vaccine plus lamivudine with or without interleukin-2 in patients with chronic hepatitis B. J Med Virol 2002;66:452–460
Wursthorn K, Lutgehetmann M, Dandri M, Volz T, Buggisch P, Zollner B, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology 2006;44:675–684
Thompson AJ, Nguyen T, Iser D, Ayres A, Jackson K, Littlejohn M, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology 2010;51:1933–1944
Sonneveld MJ, Rijckborst V, Boucher CA, Hansen BE, Janssen HL. Prediction of sustained response to peginterferon alfa-2b for hepatitis B e antigen-positive chronic hepatitis B using on-treatment hepatitis B surface antigen decline. Hepatology 2010;52:1251–1257
Lampertico P, Vigano M, Di Costanzo GG, Sagnelli E, Fasano M, Di Marco V, et al. Randomised study comparing 48 and 96 weeks peginterferon alpha-2a therapy in genotype D HBeAg-negative chronic hepatitis B. Gut 2013;62:290–298
Huang J, Zhang K, Chen W, Liao J, Luo X, Chen R. Switching to PegIFNalpha-2b leads to HBsAg loss in patients with low HBsAg levels and HBV DNA suppressed by NAs. Sci Rep 2017;7:13383
Honer Zu Siederdissen C, Cornberg M. The role of HBsAg levels in the current management of chronic HBV infection. Ann Gastroenterol 2014;27:105–112
Chevaliez S, Hezode C, Bahrami S, Grare M, Pawlotsky JM. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol 2013;58:676–683
Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006;354:1001–1010
Belloni L, Allweiss L, Guerrieri F, Pediconi N, Volz T, Pollicino T, et al. IFN-alpha inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest 2012;122:529–537
Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science 2014;343:1221–1228
Han M, Jiang J, Hou J, Tan D, Sun Y, Zhao M, et al. Sustained immune control in HBeAg-positive patients who switched from entecavir therapy to pegylated interferon-alpha2a: 1 year follow-up of the OSST study. Antivir Ther 2016;21:337–344
Boglione L, D’Avolio A, Cariti G, Milia MG, Simiele M, De Nicolo A, et al. Sequential therapy with entecavir and PEG-INF in patients affected by chronic hepatitis B and high levels of HBV-DNA with non-D genotypes. J Viral Hepat 2013;20:e11–e19
Moucari R, Boyer N, Ripault MP, Castelnau C, Mackiewicz V, Dauvergne A, et al. Sequential therapy with adefovir dipivoxil and pegylated interferon alfa-2a for HBeAg-negative patients. J Viral Hepat 2011;18:580–856
Nishioka M, Kagawa H, Shirai M, Terada S, Watanabe S. Effects of human recombinant interleukin 2 in patients with chronic hepatitis B: a preliminary report. Am J Gastroenterol 1987;82:438–442
Mizoguchi Y, Shin T, Sakagami Y, Seki S, Kuroki T, Kobayashi K, et al. Effects of recombinant interleukin 2 on immunological effector cells of the peripheral blood in patients with HBe antigen-positive chronic hepatitis. Gastroenterol Jpn 1988;23:147–152
Artillo S, Pastore G, Alberti A, Milella M, Santantonio T, Fattovich G, et al. Double-blind, randomized controlled trial of interleukin-2 treatment of chronic hepatitis B. J Med Virol 1998;54:167–172
Bruch HR, Korn A, Klein H, Markus R, Malmus K, Baumgarten R, et al. Treatment of chronic hepatitis B with interferon alpha-2b and interleukin-2. J Hepatol 1993;17(Suppl 3):S52–S55
Lunemann A, Lunemann JD, Munz C. Regulatory NK-cell functions in inflammation and autoimmunity. Mol Med 2009;15:352–358
Shi A, Zhang X, Xiao F, Zhu L, Yan W, Han M, et al. CD56(bright) natural killer cells induce HBsAg reduction via cytolysis and cccDNA decay in long-term entecavir-treated patients switching to peginterferon alfa-2a. J Viral Hepat 2018;25:1352–1362
Gill US, Peppa D, Micco L, Singh HD, Carey I, Foster GR, et al. Interferon alpha induces sustained changes in NK cell responsiveness to hepatitis B viral load suppression in vivo. PLoS Pathog 2016;12:e1005788
Kosinska AD, Pishraft-Sabet L, Wu W, Fang Z, Lenart M, Chen J, et al. Low hepatitis B virus-specific T-cell response in males correlates with high regulatory T-cell numbers in murine models. Hepatology 2017;66:69–83
Tilg H, Vogel W, Tratkiewicz J, Aulitzky WE, Herold M, Gruber M, et al. Pilot study of natural human interleukin-2 in patients with chronic hepatitis B immunomodulatory and antiviral effects. J Hepatol 1993;19:259–267
Acknowledgements
The authors would like to thank all the patients in this study, and the nurses who assisted in the patient management and the collection of serum samples. Data management and clinical trial monitoring were provided by Hangzhou Tigermed Consulting Co., Ltd.
Funding
This work was supported by grants from the Chinese National Twelfth Five Years Project in Science and Technology (2013ZX10002003), the Chinese National Thirteenth Five Years Project in Science and Technology (2017ZX10202201) and Hubei Provincial Natural Science Foundation of China (2018CFB206).
Author information
Authors and Affiliations
Contributions
QN, DW, and MH developed the concepts and designed the experiments. DW, and PW analyzed and interpreted the data. DW drafted the manuscript. YC, XC, QX, WY, CZ, QX, JJ, LW, DT, XD, YY, and JH were involved in patient recruitment, and data collection. QN, DW, XL, MH, and XW revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was performed in accordance with Good Clinical Practice and the Declaration of Helsinki principles for ethical research. The study protocol was approved by the independent central ethics committee of Tongji Medical College at Wuhan. Written informed consent was obtained from each participant. ClinicalTrials.gov Identifier: NCT02360592. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, D., Wang, P., Han, M. et al. Sequential combination therapy with interferon, interleukin-2 and therapeutic vaccine in entecavir-suppressed chronic hepatitis B patients: the Endeavor study. Hepatol Int 13, 573–586 (2019). https://doi.org/10.1007/s12072-019-09956-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-019-09956-1