Abstract
Aim
The protective role of invariant natural killer T cells (iNKTs) against hepatitis B virus (HBV) infection remains controversial. We sought to clarify the role of peripheral iNKT cells during chronic HBV infection.
Methods
Sixty patients with chronic HBV infection were categorized into an immune tolerance phase (HBV-IT) (n = 16), an immune clearance phase (HBV-IC) (n = 19) and an inactive carrier phase (HBV-IA) (n = 25). Twenty healthy individuals were enrolled as healthy controls. Another 21 HBeAg-positive patients were administrated with entecavir (0.5 mg/day) for 6 months. The percentages of circulating iNKT cells and their IFN-γ and IL-4 expression levels were examined by flow cytometry. The relationships between serum HBV DNA, ALT levels, the percentages of iNKT cells, and their IFN-γ and IL-4 levels were analyzed.
Results
Compared to healthy controls, the percentage of iNKT cells decreased in HBV-IT, but increased in HBV-IC and HBV-IA. Circulating IFN-γ-producing iNKT cells gradually increased, whereas IL-4-producing iNKT cells gradually decreased from HBV-IT stage to HBV-IC and HBV-IA stages. The frequency of iNKT cells and their IFN-γ levels were reversely correlated with viral load. The levels of IL-4 expressed by iNKT cells were positively correlated to viral load and the serum ALT levels. After anti-virus therapy, the percentage of IFN-γ-producing iNKT cells increased while the percentage of IL-4-producing iNKT cells decreased.
Conclusions
During chronic HBV infection, the percentages of peripheral iNKT cells and its cytokines expressions of IFN-γ and IL-4 showed dynamic changes. The expression levels of IFN-γ and IL-4 were correlated with the clearance of HBV and liver injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic infection with hepatitis B virus (HBV) remains a major cause of liver disease worldwide. More than 350 million people are persistently infected with HBV [1, 2]. Virus-related immunopathogenesis is largely responsible for the necroinflammatory process of chronic HBV infection which consists of immune tolerance phase, immune clearance phase and inactive carrier phase [3–5]. Some patients even progress to a reactivation stage with advanced liver disease. Understanding the dynamic nature of chronic HBV infection is crucial in the management of HBV carriers and underscores the need for long-term monitoring [5].
Innate and adaptive host immune responses play an important role in the successful elimination of HBV. T cells orchestrate antiviral immunity principally through the production of cytokines that coordinate one another. In acute HBV infection, CD8 cytotoxic T and CD4 helper T-cell-mediated immune responses are activated and promote the clearance of HBV from hepatocytes [6]. In chronic HBV infection, however, CD8 and CD4 T-cell immunity are hyporesponsive in association with persistent HBV serum load [7], which suggests that high HBV load may impair T-cell immunity [8].
Natural killer T cells (NKT cells) are a unique T-lymphocyte sublineage that has been increasingly recognized as a major contributor in the development of inflammatory responses. NKT cells include the predominant type I NKT cells (also called invariant NKT; iNKT) and type II NKT cells [9]. Human iNKT cells express an invariant Vα24–JαQ TCRα chain paired preferentially with junctionally diverse Vβ11DβJβ regions [10, 11]. The key features of NKT cells include heavily biased T-cell receptor (TCR) gene usage, CD1d restriction and high levels of cytokine production, particularly interleukin 4 (IL-4) and interferon (IFN-γ). Activation of iNKTs is primarily mediated through cytokine receptors or TCR engagement by CD1d-restricted lipid antigen. Following activation, iNKTs rapidly secrete various cytokines including Th1 and Th2 cytokines within a few hours [12, 13]. Given the central role of NKT cells in both direct and indirect modulation of the immune system, NKT cells have been shown to be crucial in the defense against a variety of microbial pathogens [14].
Recent studies have shown that NKT cells may contribute to the control of HBV infection through sensing of HBV-induced modified self-lipids in mouse models [15], and blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to HBV [16]. NKT cells have also been shown to play an important role in regulating innate and adaptive immunity via the production of a variety of cytokines, including IFN-γ and IL-4 [17–19]. In the liver, iNKT cells play important roles in regulating liver fibrosis, inflammation, and regeneration [20–23]. A synthetic glycolipid mitogen α-galactosyl ceramide, a potent iNKT activator, has been shown to suppress the viral replication via the IFN-γ- and IFN-α/β-dependent mechanisms in a mouse model [24]. In chronic hepatitis B (CHB) patients, however, treatment with α-galactosyl ceramide did not affect HBV DNA and serum transaminase levels [25]. In addition, iNKTs are significantly enriched in chronically inflamed liver with a substantial modification in their effector potential which are associated with the cirrhotic states, suggesting that iNKTs may contribute to the pathogenesis of cirrhosis by expressing a set of cytokines involved in the progression of fibrosis [26].
In light of these reports, we sought to characterize iNKTs in peripheral blood from chronic HBV-infected patients and to investigate the roles of iNKTs in the natural history of chronic HBV infection. Our study revealed that circulating iNKT cells may play opposite roles by producing both IFN-γ and IL-4 during chronic HBV infection. IFN-γ seems to promote HBV clearance while IL-4 may contribute to liver injury during chronic HBV infection.
Materials and methods
Subjects
Sixty patients with chronic HBV infection were categorized into an immune tolerance phase group (n = 16), an immune clearance phase group (n = 19) and an inactive carrier phase group (n = 25), and 20 healthy controls were enrolled as a healthy control group (Table 1). The definitions of phages of chronic HBV infection are shown in the supplementary data. In addition, another 21 HBeAg-positive patients were enrolled, and they were administrated with entecavir (0.5 mg/day) for 6 months (Table 2). All patients were positive for HBsAg and anti-HBc but negative for antibodies to HCV, delta virus, HIV-1 and -2, and other symptoms of chronic liver damage. The patients were observed for more than 72 weeks, and liver functions and serum DNA levels were tested every 12 weeks during this observation period. The healthy control group, matched for age, sex, and race, comprised 20 healthy volunteers who had no evidence of exposure to HBV [HBV surface antigen (HBsAg)-negative, anti-HBc-negative].
Serum viral load and alanine aminotransferase (ALT) determination
Serum HBV DNA load in patients with chronic HBV infection was measured by real-time fluorescent quantitative polymerase chain reaction method (PCR) using a light cycler PCR system (FQD-33A; BIOER) with a lower limit of approximately 1000 viral genome copies/ml. The handling procedures were performed in strict accordance with the instructions in the reagent kit (Shenzhen PG Biotech, China). The results were considered abnormal when HBV DNA was >1000 copies/ml. HBsAg, HBeAg, anti-HBs, anti-HBc, anti-HBe, and antibodies to HCV, HDV, HIV-1, and HIV-2 were detected by enzyme-linked immunosorbent assay with commercially available kits (Sino-American Biotechnology).
The serum ALT levels were assayed by DXC 800 Fully-auto Bio-Chemistry Analyzer, at the Department of Clinical Laboratory, Shanghai Shuguang Hospital. The results were considered abnormal when ALT was >60 U/L.
Intracellular cytokine staining and polychromatic flow cytometry
In this experiment, peripheral blood from 60 patients and 20 healthy volunteers (Table 1) was collected. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by standard density gradient centrifugation with Lympholyte-H (Cedarlane,Canada) according to manufacturer’s protocol. Intracellular cytokine production was detected by flow cytometry [27]. Briefly, PBMCs were activated in RPMI 1640 with 10 % FBS with PMA (10 ng/ml), Ionomycin (0.75 μg/ml) and Monensin (1.78 μg/ml) for 4 h at 37 °C. Cells were stained with CD3-PerCP-cy5.5 (BD Bioscience), TCRVá24-PE, TCR Vâ11-FITC (Beckman Coulter) for 15 min. Cells were fixed and permeated respectively with FIX & PERM A and B (Invitrogen). IL-4-PE-Cy7 (ebioscience) and IFN-γ- APC-Alexa Fluor 750 (Caltag) were added into cells for 15 min. After washing with PBS, the stained cells were analyzed on a FACSAria.
Anti-virus therapy
Twenty-one HBeAg-positive CHB patients (Table 2) were enrolled, and they were treated with entecavir 0.5 mg daily for 6 months, and the frequency of iNKT and expression of IFN-γ/IL-4 were measured before and after treatment by flow cytometry.
Statistical analysis
One-way ANOVA was used to compare multiple groups and Student’s t test was used to compare the two groups using SPSS software. Pearson correlation analysis was performed. Differences were considered statistically significant at p < 0.05. The test of significance was two-sided.
Results
Correlation between the frequency of iNKTs and serum HBV DNA load or ALT levels
iNKTs are a subset of nonconventional T lymphocytes. Besides the immunoregulatory functions, iNKTs may play an important role in controlling microbial infections, liver inflammation and regeneration [20, 21, 23] [28]. We thus tried to explore the relationship between the frequency of iNKTs and serum viral load or ALT levels. As illustrated in Fig. 1a, b, a negative correlation was found between the serum HBV DNA level and the percentage of iNKTs, and there was no significant correlation between the percentage of iNKTs and serum ALT levels. In addition, the percentages of iNKTs in patients with various immune phases were analyzed. Representative dot plots illustrating iNKT cells (CD3+ Vá24+ Vâ11+) from PBMCs are shown in Fig. 1c. In patients with HBV-IT, the circulating iNKTs showed a decreased percentage as compared with those from NC. A possible explanation might be the down-regulation of TCRs or cell exhaustion as the result of continuous triggering during the persistent viral infection [29, 30]. Interestingly, the percentage of iNKTs increased kinetically in HBV-IC and HBV-IA (Fig. 1d). Significantly, the frequency of iNKTs in HBV-IA expanded dramatically compared with that from NC and patients of earlier phases (Fig. 1d).
Correlations between the frequency of iNKTs and serum HBV DNA load or ALT levels. a Correlation between serum HBVDNA (log) and the percentages of iNKTs during chronic HBV infection. b Correlation between serum ALT levels and the percentages of iNKTs during chronic HBV infection. c Representative dot plot illustrating iNKTs from healthy individuals and patients in HBV-IT, HBV-IC, and HBV-IA. d Analysis of the percentages of iNKTs in patients at different phases of chronic HBV infection
IFN-γ and/IL-4 cytokine profile of circulating iNKTs during chronic HBV infection
The causative relationship between iNKTs and the immunopathogenesis of chronic HBV infection is poorly understood. HBV infection may stimulate different profiles of cytokine production by iNKTs; on the other hand, the immune state during chronic HBV infection may modulate the regulatory roles of iNKTs. We thus tried to investigate the cytokine profile produced by the circulating iNKTs. As illustrated in Fig. 2a, b, the percentage of IFN-γ-producing iNKTs in the peripheral blood was negatively correlated with HBV viral load, but there was no significant correlation between the percentage of IFN-γ-producing iNKTs and serum ALT levels. Upon stimulation, the majority of iNKTs produced IFN-ã in all subjects (Fig. 2c). Interestingly, the IFN-γ+ iNKTs presented the lowest percentage in patients of HBV-IT, and were resumed in the HBV-IC and HBV-IA groups with a level similar to NC (Fig. 2d).
IFN-γ expression profile of circulating iNKTs in patients with chronic HBV infection. a Correlation between serum HBVDNA(log) and the percentages of IFN-γ+iNKTs during chronic HBV infection. b Correlation between serum ALT levels and the percentages of IFN-γ+iNKTs during chronic HBV infection. c Representative dot plot illustrating IFN-γ+iNKTs from healthy individuals and patients in HBV-IT, HBV-IC, and HBV-IA. d Analysis of the percentages of IFN-γ+iNKTs in patients at different phases of chronic HBV infection
The correlations between the percentage of IL-4+-producing iNKTs and serum HBV DNA load or ALT levels were also analyzed. As illustrated in Fig. 3a, b, the percentage of IL-4+-producing iNKTs had a positive correlation with serum HBV DNA load and serum ALT levels. In addition, representative dot plots in Fig. 3c also illustrate the IL-4+ iNKTs gated from iNKTs. IL-4+ iNKT cells consisted of about 37 % of total iNKT cells in NC. The percentage of IL-4+ iNKT cells was markedly elevated in patients with HBV-IT, but was gradually decreased in patients with HBV-IC and HBV-IA (Fig. 3c, d).
IL-4 expression profile of circulating iNKTs in patients with chronic HBV infection. a Correlation between serum HBVDNA (log) and the percentages of IL-4+iNKTs during chronic HBV infection. b Correlation between serum ALT levels and the percentages of IL-4+iNKTs during chronic HBV infection. c Representative dot plot illustrating IL-4+ iNKTs from healthy individuals and patients in HBV-IT, HBV-IC, and HBV-IA. d Analysis of the percentages of IL-4+iNKTs in patients at different phases of chronic HBV infection
Anti-viral treatment oppositely modulates IFN- and IL-4 cytokine profile of circulating iNKTs in patients with chronic HBV infection
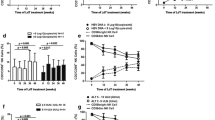
In order to explore the role of iNKTs against chronic HBV infection, 21 HBeAg-positive CHB patients were treated with antivirus therapy (0.5 mg entecavir daily) for 6 months. Such antiviral treatment effectively decreased serum ALT levels and HBV DNA load (Fig. 4a, b). Interestingly, the percentage of IFN-γ+NKT cells was markedly increased, while the percentage of IL-4+NKT cells was significantly decreased after anti-viral therapy compared to those before the therapy (Fig. 4c).
Variation of the cytokines profile of circulating iNKTs after anti-viral therapy. a Serum ALT levels of patients before and after entecavir treatment. b Serum HBV DNA levels before and after entecavir treatment. c The percentages of IFN-γ+iNKTs in peripheral blood of patients before and after entecavir treatment. d The percentages of IL-4+iNKTs in peripheral blood of patients before and after entecavir treatment
Discussion
NKT cells have been shown to play complex roles in controlling immune responses as their activation in some cases can polarize the immune response in Th1 direction, while in other cases a Th2 response may generate. Activation of iNKTs may provide rapid help for a cell-mediated immune response (IFN-γ) or an antibody-mediated response (IL-4) than the slow responses generated by conventional T-helper cells [13], thereby representing a regulatory link between innate and adaptive immunity during the early/acute phase of viral infections. In the context of HBV infection, T cell responses have been shown to play an important role in controlling HBV clearance; but the roles of iNKT cells remain largely unknown. In the current paper, we have demonstrated that circulating IFN-γ-producing iNKT cells gradually increased, whereas IL-4-producing iNKT cells gradually decreased from the HBV-IT stage to the HBV-IC and HBV-IA stages. The frequency of iNKT cells and their IFN-γ levels were reversely correlated to viral load. The levels of IL-4 expressed by iNKT cells were positively correlated to viral load and the serum ALT levels (Supplementary data, Figure 1).
The number of iNKT cells shows dynamic change during chronic HBV infection
The study of Jiang et al. [31] showed that there was a low frequency of peripheral iNKT cells in CHB patients, which increases to normal levels with viral control. However, we found that only the frequency of iNKT in patients in HBV-IT was decreased, and the frequencies of iNKT in patients with HBV-IC and HBV-IA were increased. The reason for different results may be that the ALT levels of patients with HBV-IC in our study were lower than that in the study of Jiang. Our study showed that the frequency of iNKT in patients with HBV-IT was lowest during HBV infection. In addition, our study showed that anti-virus therapy contributed to the increase of IFN-γ -producing iNKT cells and the decrease of IL-4-producing iNKT cells in CHB patients.
iNKT cells promote HBV clearance by producing IFN-γ
Our study revealed that both the frequency and the cytokine secretion pattern of circulating iNKTs kinetically varied at the different stages of chronic HBV infection. Upon PMA/inomycin activation, a gradual increase was seen in IFN-γ-producing iNKTs in the recovering stage of HBV-IA, while IL-4 production declined reciprocally. De Lalla et al. [26] previously showed that conventional T cells displayed an elevated number of IL-4-producing cells in cirrhotic patients, suggesting that progression toward cirrhosis was accompanied by the up-regulation of Th2 cytokines in both conventional T cells and iNKTs. In addition, we focused on the kinetic variations of iNKTs in a more longitudinal natural course of HBV chronic infection. The number of circulating IFN-γ-producing iNKTs was found to be reversely associated with HBV DNA levels. High viral load (e.g., patients in the HBV-IT phase) may drive functionally impaired circulating iNKTs as occurred for conventional T effectors during persistent viral infection. Although patients in the HBV-IA phase largely displayed building pathogenesis of liver fibrosis, our study exhibited up-regulated IFN-γ production by circulating iNKTs.
Nevertheless, it is not known whether high viral load is the cause or just the consequence of iNKTs activation. iNKTs can play an effector role in recognition and protective response to microbial infections through secreting IFN-γ which is one of the most important mediators in the immune system and is associated with inhibition of viral replication. However, the role of iNKTs in the anti-HBV immunity remains controversial. iNKTs were enriched in chronically inflamed liver with a substantial modification in their effector potential [26]. In a recent clinical trial, α-galactosyl ceramide treatment of chronically infected patients with HBV did not affect HBV DNA and serum ALT level, which raises the possibility that iNKTs are not directly involved in the protective immunity against HBV infection.
iNKT cells contribute to hepatocellular damage via the production of IL-4
iNKT cells contribute to liver fibrosis during chronic viral hepatitis via production of IL-4 and IL-13 [26, 32–34], which supports the idea of iNKT functional modification toward a pathogenic cytokine secretion profile. According to our study, IFN-γ-producing iNKTs did not significantly associate with serum ALT levels, which may reflect an indirect or passively regulated role of iNKTs during the recurrent hepatic inflammation. A positive correlation was actually shown between serum ALT levels and the percentage of IL-4-producing iNKTs in patients with chronic HBV infection, which suggests that iNKTs may contribute to the liver injury.
It should be noted that HBV antigens are generally nonlipid in nature and thus CD1d-independent. There is no evidence so far showing that HBV antigens could directly act on iNKT cells. On the other hand, iNKTs are uniquely equipped for immediate, cytokine-driven activation. Jin et al. [34] recently showed that innate and cytokine signals, rather than microbial antigens, dominate in iNTKs activation during microbial infection. Minami et al. [35] also reported that the recognition of Th1 or Th2 cytokines by dendritic cells acted as a negative feedback loop to maintain Th1/Th2 cytokine balance via NKT cell functions. Wang et al. [20] showed that activated iNKT cells rapidly release IL-4, which promotes neutrophil survival and hepatitis but also sequentially produces IFN-γ, which acts in a negative feedback loop to ameliorate iNKT hepatitis by inducing neutrophil apoptosis in the model of α-Galcer-induced hepatitis. So, IFN-γ and IL-4 expressed by iNKT cells played different roles when the liver function is injuried.
T cell immunity plays a critical role in determining the outcome of HBV infection. Immune responses with virus-specific T cells play key effector and regulatory roles in both liver pathogenesis and viral clearance. In our study, NKT cell reactions were perceptibly biased toward IL-4 over IFN-γ production in HBV-IT, and generally deflected to IFN-γ-preferential pattern in patients of HBV-IA. The long course of viral persistent infection may lead to a decrease in the size of the naïve T cell repertoire but to an increase in the number of effector T cells functionally affected, which could be a possible explanation for the general skewing of the IFN-γ/IL-4 balance during chronic HBV infection. Our study also displayed a coordinated IFN-γ/IL-4 cytokine profile of iNTKs that highly associated with the clearance of HBV along chronic HBV infection. Interestingly, antiviral treatments could effectively assume an IFN-γ/IL-4 balance in iNKTs in the peripheral blood of CHB patients.
Conclusions
Circulating iNKT cells exhibit a function skewing and play dual immunoregulatory roles during chronic HBV infection. On the one hand, iNKT cells contribute to the clearance of HBV by expressing IFN-γ, and on the other hand, iNKT cells induce the liver injury by expressing IL-4.
References
Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006;354:1001–10.
Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2006;354:1011–20.
Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007;45:507–39.
Pan CQ, Zhang JX. Natural history and clinical consequences of hepatitis B virus infection. Int J Med Sci 2005;2:36–40.
Shi YH, Shi CH. Molecular characteristics and stages of chronic hepatitis B virus infection. World J Gastroenterol 2009;15:3099–105.
Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003;77:68–76.
Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol 2007;81:4215–25.
Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 2005;5:215–29.
Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol 1997;15:535–62.
Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4-8- T cells. J Exp Med 1994;180:1171–6.
Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med 1994;180:1097–106.
Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today 2000;21:573–83.
Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol 2002;2:557–68.
Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol 2007;5:405–17.
Zeissig S, Murata K, Sweet L, Publicover J, Hu Z, Kaser A et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat Med 2012;18:1060–8.
Vilarinho S, Ogasawara K, Nishimura S, Lanier LL, Baron JL. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc Natl Acad Sci USA 2007;104:18187–92.
Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol 2005;23:877–900.
Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. J Exp Med 2005;202:1623–6.
Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol 2007;25:297–336.
Wang H, Feng D, Park O, Yin S, Gao B. Invariant NKT cell activation induces neutrophil accumulation and hepatitis: opposite regulation by IL-4 and IFN-gamma. Hepatology 2014;58:1474–85.
Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol 2009;86:513–28.
Yin S, Wang H, Bertola A, Feng D, Xu MJ, Wang Y et al. Activation of invariant natural killer T cells impedes liver regeneration by way of both IFN-gamma- and IL-4-dependent mechanisms. Hepatology 2014;60:1356–66.
Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME et al. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology 2009;49:1683–94.
Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med 2000;192:921–30.
Woltman AM, Ter Borg MJ, Binda RS, Sprengers D, von Blomberg BM, Scheper RJ et al. Alpha-galactosylceramide in chronic hepatitis B infection: results from a randomized placebo-controlled Phase I/II trial. Antivir Ther 2009;14:809–18.
de Lalla C, Galli G, Aldrighetti L, Romeo R, Mariani M, Monno A et al. Production of profibrotic cytokines by invariant NKT cells characterizes cirrhosis progression in chronic viral hepatitis. J Immunol 2004;173:1417–25.
Hoffmann F, Albert MH, Arenz S, Bidlingmaier C, Berkowicz N, Sedlaczek S et al. Intracellular T-cell cytokine levels are age-dependent in healthy children and adults. Eur Cytokine Netw 2005;16:283–8.
Kinjo Y, Kronenberg M. V alpha14 i NKT cells are innate lymphocytes that participate in the immune response to diverse microbes. J Clin Immunol 2005;25:522–33.
Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 1993;362:758–61.
Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med 1998;187:1383–93.
Jiang X, Zhang M, Lai Q, Huang X, Li Y, Sun J et al. Restored circulating invariant NKT cells are associated with viral control in patients with chronic hepatitis B. PLoS One 2011;6:e28871.
Wang H, Park O, Gao B NKT cells in liver fibrosis: controversies or complexities. J Hepatol 2011;55:1166; author reply 1166–1167.
Ishikawa S, Ikejima K, Yamagata H, Aoyama T, Kon K, Arai K et al. CD1d-restricted natural killer T cells contribute to hepatic inflammation and fibrogenesis in mice. J Hepatol 2011;54:1195–204.
Jin Z, Sun R, Wei H, Gao X, Chen Y, Tian Z. Accelerated liver fibrosis in hepatitis B virus transgenic mice: involvement of natural killer T cells. Hepatology 2011;53:219–29.
Minami K, Yanagawa Y, Iwabuchi K, Shinohara N, Harabayashi T, Nonomura K et al.. Negative feedback regulation of T helper type 1 (Th1)/Th2 cytokine balance via dendritic cell and natural killer T cell interactions. Blood 2005;106:1685–93.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [81202662 and 81473477], and Science Research Project of Twelve Five-year Plan [2012ZX10005004-002], and Doctoral Fund of Ministry of Education of China [20123107110003], and Construction project of TCM clinical research base of State Administration of TCM China [JDZX2012058], and Shanghai Rising-Star Program [13QA1403500], and Three-year action plan of development of TCM in Shanghai [ZYSNXD-CC-ZDYJ015 and ZY3-CCCX-3-3027], and Training plan of outstanding young medical talents, Shanghai Municipal Health Bureau [XYQ2013093].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors concur with the submission and the authors have no potential conflicting interests.
Ethical standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). This study was approved by the local ethics committee, and all patients provided their written informed consent according to a protocol reviewed and approved by the Institutional Review Board of Shuguang Hospital affiliated to Shanghai University of Traditional Chinese Medicine. All of these patients and normal controls were Chinese. Our study was approved by the local ethics committee, and all patients provided their written informed consent according to a protocol reviewed and approved by the Institutional Review Board of Shuguang Hospital affiliated to Shanghai University of Traditional Chinese Medicine.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Zhen-Hua Zhou has contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12072_2015_9650_MOESM2_ESM.tif
Figure 1 Dynamic changes of peripheral iNKTs and its cytokines expressions during chronic HBV infection. Circulating IFN-γ-producing iNKT cells gradually increased, whereas IL-4-producing iNKT cells gradually decreased from HBV- IT stage to HBV- IC and HBV -IA stages. The frequency of iNKT cells and their IFN-γ levels were reversely correlated to viral load. The levels of IL-4 expressed by iNKT cells were positively correlated to viral load and the serum ALT levels. Supplementary material 2 (TIFF 28 kb)
Rights and permissions
About this article
Cite this article
Li, M., Zhou, ZH., Sun, XH. et al. The dynamic changes of circulating invariant natural killer T cells during chronic hepatitis B virus infection. Hepatol Int 10, 594–601 (2016). https://doi.org/10.1007/s12072-015-9650-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-015-9650-0