Abstract
Purpose
Our purpose was to conduct a meta-analysis to compare the effectiveness of vasopressin/terlipressin and somatostatin/octreotide on variceal re-bleeding within and after 5 days of initial control bleeding.
Methods
A search was conducted of PubMed, the Cochrane database, and Google Scholar until June 31, 2014 using combinations of the search terms: esophageal varices, variceal re-bleeding, recurrent variceal hemorrhage, early re-bleeding, vasopressin, somatostatin, terlipressin, octreotide. Inclusion criteria were: (1) randomized controlled trials, (2) patients with esophageal or esophageal and gastric varices confirmed by endoscopy, (3) re-bleeding control was evaluated, (4) treatment with somatostatin/vasopressin. Outcome measures were the re-bleeding rates within 5 days (≤5 days) or after 5 days (>5 days) after initial treatment.
Results
Six studies were included in the analysis. Five studies had complete data of re-bleeding rate within 5 days after initial treatment, and the combined odds ratio (OR) of 0.87 [95 % confidence interval (CI) 0.51, 1.50] indicated that there was no difference in the re-bleeding rate between patients treated with vasopressin/terlipressin or somatostatin/octreotide. Two studies had complete data of the re-bleeding rate 5 days after initial treatment, and the combined OR of 1.12 (95 % CI 0.64, 1.95) indicated there was no difference in the re-bleeding rate between patients who were treated with vasopressin/terlipressin or somatostatin/octreotide.
Conclusion
There is no difference between vasopressin/terlipressin and somatostatin/octreotide in prevention of re-bleeding after the initial treatment of bleeding esophageal varices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bleeding from esophageal varices occurs in approximately 50 % of patients with cirrhosis, and is one of the major complications of portal hypertension. Acute esophageal variceal bleeding is defined as active bleeding from esophageal varices at the time of endoscopy and blood in the esophagus/stomach with no other source of bleeding [1]. The prevalence of variceal hemorrhage in patients with cirrhosis is approximately 5–15 % yearly, and the mortality associated with variceal hemorrhage ranges from 7 to 20 % [2–5]. Recurrent esophageal variceal bleeding is defined as re-bleeding after 24 h following no sign of bleeding and occurs in 30–40 % of cases within the first 6 weeks after a bleeding episode [2].
The aim of medical therapy for acute bleeding from esophageal varices is to reduce splanchnic blood flow and portal pressure. Therapies used in the management of esophageal variceal hemorrhage include pharmacologic treatments (vasoactive agents and nonselective β-blockers), endoscopic therapies, transjugular intrahepatic portosystemic shunts (TIPS), and shunt surgery [1, 6]. The Baveno V consensus (2010) suggest that vasoactive drugs should be used in combination with endoscopic therapy and continued for up to 5 days, and that usage duration is very important [7].
The most common vasoactive agents used for the control of bleeding and prevention of variceal re-bleeding include vasopressin, terlipressin, somatostatin, or octreotide [2, 6, 8, 9]. Studies show that vasopressin is effective in controlling bleeding but does not affect mortality [10, 11]. Terlipressin is a synthetic analogue of vasopressin with a longer half-life and less adverse effects, and can achieve control of bleeding in approximately 75 and 67 % of patients at 48 h and at 5 days, respectively, after treatment [12, 13]. Continued administration of terlipressin is also effective at preventing early re-bleeding [13, 14]. Terlipressin is more effective in controlling variceal bleeding than vasopressin alone [15, 16], but has similar effectiveness as vasopressin plus nitrates [17] or somatostatin [18, 19]. Somatostatin and its synthetic analogue octreotide are both effective for the control of variceal bleeding, and have desirable safety profiles [20, 21]. Octreotide has a longer half-life compared with somatostatin [20, 21]. Guidelines recommend that somatostatin or octreotide therapy should be maintained for 5 days to prevent re-bleeding.
A number of meta-analyses have examined endoscopic therapies, β-blockers, and transjugular intrahepatic portosystemic shunts with respect to control of variceal bleeding and re-bleeding [22–25]. However, few meta-analyses have compared vasoactive medications used for the management of acute variceal bleeding [12, 26–28], and to our knowledge none have evaluated the efficacy of vasoactive drugs on preventing re-bleeding. Despite the many options for management, 10–20 % of patients may still have bleeding or early re-bleeding within the first 5 days following treatment [20]. The risk of re-bleeding is highest during the first 5 days after the initial bleed is treated, and this risk decreases over the following 6 weeks with the rate becoming similar to the index bleeding rate after 6 weeks [29].
The purpose of this study was to conduct a systematic review and a meta-analysis to compare the effectiveness of vasopressin/terlipressin and somatostatin/octreotide on variceal re-bleeding within (≤5 days) and after 5 days (>5 days) after the control of the initial bleeding event in patients with esophageal varices.
Methods
Literature search strategy
A search was conducted on PubMed, the Cochrane database, and Google Scholar (until June 31, 2014) for clinical trials that investigated the rate of esophageal and gastric variceal re-bleeding ≤5 and >5 days following control of a variceal bleeding event with vasopressin/terlipressin and somatostatin/octreotide therapy. The search was performed using the following terms: esophageal varices, variceal re-bleeding, recurrent variceal hemorrhage, early re-bleeding, vasopressin, somatostatin, terlipressin, and octreotide. All potential articles were screened for inclusion by two independent reviewers and a third reviewer adjudicated any disagreement.
Selection criteria
Included studies were randomized controlled trials (RCTs) whose patient population had endoscopy confirmed esophageal varices or esophageal and gastric varices that were treated with somatostatin/vasopressin-based medications, and that reported the rate of re-bleeding. Studies were excluded if they were not RCTs, only evaluated patients with gastric varices, or included patients who received endoscopic therapy for esophageal varices over the previous month.
Data extraction and quality assessment
Two independent reviewers extracted the data from eligible studies. A third reviewer was consulted for resolution of any disagreement. Data extracted included the first author, year of publication, study type, drug used and treatment regimen, number of patients, gender distribution, age, Child-Pugh class, control of bleeding rate, re-bleeding ≤5 and >5 days after initial treatment, mortality, length of hospital stay, and blood transfusion requirements. The quality of the included studies was evaluated using the Delphi list [30].
Data analysis
The primary endpoint of the meta-analysis was the re-bleeding rate ≤5 or >5 days after treatment in patients who received vasopressin/terlipressin compared with those who received somatostatin/octreotide. The re-bleeding rates were summarized as percentage for each of the studies, and the estimated odds ratio (OR) with corresponding 95 % confidence interval (CI) was calculated for each individual study. Heterogeneity among the studies was assessed by calculating the Cochran Q and the I 2 statistic. For the Cochran Q statistic, p < 0.10 was considered to indicate statistically significant heterogeneity. The I 2 statistic indicates the percentage of the observed between-study variability caused by heterogeneity. If either the Q statistic (p < 0.1) or I 2 statistic (>50 %) indicated heterogeneity among the studies, the random-effects model (DerSimonian–Laird method) was used. Otherwise, the fixed-effects model was used (Mantel–Haenszel method). Combined ORs and 95 % CIs were calculated for overall studies, and a 2-sided p value <0.05 indicated statistical significance. Publication bias was assessed if more than five studies were included in the meta-analysis [31]. All analyses were performed using comprehensive meta-analysis statistical software, version 2.0 (Biostat, Englewood, NJ, USA).
Results
Literature search and study characteristics
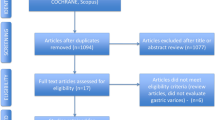
A flow diagram of study selection is shown in Fig. 1. The initial search identified 283 potential studies, of which 225 were excluded for not being relevant to the aim of the meta-analysis. The remaining 58 articles were assessed for eligibility and 49 were excluded (Fig. 1); thus nine studies were included in the qualitative synthesis [18, 32–39].
The number of patients in the studies ranged from 22 to 261 in the vasopressin group and from 20 to 519 in the somatostatin group (Table 1). The mean patient age in all studies was approximately 55 years, and approximately 75 % or more of the patients in all studies were male (Table 1). Re-bleeding within ≤5 days after initial treatment ranged from 3.4 to 21.7 %, and re-bleeding more than 5 days after initial treatment ranged from 4.8 to 30 % (Table 2). The 5-day mortality rate ranged from 8.0 to 10.4 %, in-hospital mortality ranged from 16 to 36 %, and 6-week mortality ranged from 13.7 to 16 % (Table 2).
Meta-analysis of re-bleeding rates
Of the nine studies included in the qualitative synthesis, three were excluded from the meta-analysis—the study by Adarsh et al. [33] was available in abstract form only, the full text of the study by Walker et al. [19] was not available, and in the 1992 study by Walker et al. [39] data were calculated by bleeding episode, which was difficult to interpret. Thus, six studies were included in the meta-analysis [32, 34–38]. All six studies were RCTs, and quality assessment indicated the different treatment groups had similar baseline characteristics, and all reported point estimates for the primary outcome (Table 3). Inclusion criteria were reported in only three of the six studies, and only one study reported that the care provider and patient were blinded to treatment. None of the six studies reported that the analysis included an intent-to-treat (ITT) population.
Five studies [32, 34–36, 38] reported the rate of re-bleeding ≤5 days (Fig. 2a). There was no evidence of heterogeneity across the studies (Q statistic = 3.256, I 2 = 0 %, p = 0.516); hence, a fixed-effects model of analysis was used. The combined OR of 0.87 (95 % CI 0.51–1.50) indicated that there was no difference in the re-bleeding rate ≤5 days after initial treatment between patients treated with vasopressin or somatostatin (Z statistic = −0.492, p = 0.623).
Meta-analysis of re-bleeding rate with vasopressin versus somatostatin. a Re-bleeding within 5 days after treatment. The combined OR of 0.87 (95 % CI 0.51–1.50) indicated that there was no difference in the re-bleeding rate ≤5 days after initial treatment. b Re-bleeding after 5 days post treatment. The combined OR 1.12 (95 % CI 0.64–1.95) indicated there was no difference in the re-bleeding rate >5 days after initial treatment. CI confidence interval, LB lower bound of 95 % CI, OR odds ratio, UB upper bound of 95 % CI
Two studies reported data for the rate of re-bleeding >5 days after initial treatment [34, 37] (Fig. 2b). There was no evidence of heterogeneity between the studies (Q statistic = 0.030, I 2 = 0 %, p = 0.862); hence, a fixed-effects model of analysis was used. Similar to the re-bleeding rate ≤5 days after treatment, the combined OR 1.12 (95 % CI 0.64–1.95) indicated there was no difference in the re-bleeding rate >5 days after initial treatment between patients who were treated with vasopressin or somatostatin (Z statistic = 0.399, p = 0.690).
Sensitivity analysis
Sensitivity analysis using leave-one-out approach for both the meta-analysis for re-bleeding in ≤5 days (Fig. 3) after the initial treatment found that the direction and magnitude of pooled estimates did not have a large variation (Z statistic = −0.492, p = 0.623). These findings indicate that no one study dominated the results, which support the findings of the meta-analysis.
Sensitivity analysis of re-bleeding within 5 days after treatment with vasopressin versus somatostatin. The direction and magnitude of pooled estimates did not have a large variation (Z statistic = −0.492, p = 0.623) indicating that no one study dominated the results. CI confidence interval, LB lower bound of 95 % CI, OR odds ratio, UB upper bound of 95 % CI
Publication bias analysis
Publication bias was not performed because more than five studies are required to detect funnel plot asymmetry [31]. Thus, it is not possible to comment on publication bias.
Discussion
Bleeding from esophageal and gastric varices is a cause of morbidity and mortality in patients with cirrhosis. Vasoactive medications such as vasopressin, somatostatin and their analogues, terlipressin and octreotide, are often used to treat acute variceal bleeding. However, few meta-analyses have compared these medications in managing acute variceal bleeding following the intervention for the initial bleeding event. This analysis compared the effectiveness of vasopressin/terlipressin and somatostatin/octreotide on variceal re-bleeding ≤5 and >5 days post control of the initial bleeding event. The results showed there was no difference in the effectiveness of vasopressin/terlipressin and somatostatin/octreotide on the prevention of re-bleeding within and after 5 days after the initial control of bleeding.
Other meta-analyses have examined the use of vasoactive medications for the treatment of bleeding esophageal varices. In 2012, Wells et al. [26] conducted a meta-analysis including 30 trials and 3,111 patients to examine the use of vasoactive medications in the initial treatment of bleeding esophageal varices and concluded that vasoactive agents were associated with a lower risk of all-cause mortality and transfusion requirements, improved control of bleeding, and shorter hospital stay. A limitation of the Wells’ analysis was that the time points to assess the different outcomes were heterogeneous across the included studies, which may have confounded the findings. In an attempt to reduce this variability, this study focused on the control of rebleeding at two time points, ≤5 and >5 days following intervention for the initial bleeding event.
A Cochrane database systematic review in 2003 by Ioannou et al. [12] that analyzed the use of terlipressin for acute esophageal bleeding found that terlipressin was safe and effective and was the only pharmacologic agent to reduce mortality [34 % relative risk (RR) reduction] as compared to placebo. However, the number of studies comparing terlipressin to other pharmacologic agents and endoscopic treatments was limited and no differences in major outcomes were found. A 2001 meta-analysis by Corely et al. [27] examining octreotide for acute esophageal variceal bleeding found that it provided better control of bleeding than vasopressin/terlipressin.
In a 1995 meta-analysis comparing somatostatin and vasopressin in the management of acute esophageal variceal bleeding, Imperiale et al. [28] reported that in trials that examined sustained control of bleeding somatostatin was more effective in controlling acute hemorrhage and was associated with a lower risk of adverse effects than vasopressin. A 2008 Cochrane systematic database review [40] of somatostatin analogues for the treatment of acute bleeding esophageal varices that included 21 trials and 2,588 patients reported that the number of patients with re-bleeding was not significantly reduced for the trials with a low risk of bias, RR 0.84 (0.52–1.37), while it was substantially reduced in the other trials (RR 0.36; 0.19–0.68), and that the drugs did not result in a significant reduction in mortality.
Techniques for treating an initial variceal hemorrhage have changed over the years. In 2001 McCormick and O’Keefe [41] reviewed the literature to determine if the prognosis for cirrhotic patients after a first variceal bleeding episode had improved over a 40-year period. The review included 28 studies from 1969 to 1999, and the authors found that there was a significant reduction in bleeding related mortality over the 40-year period. Studies have shown that endoscopic variceal ligation is better than endoscopic sclerotherapy in controlling esophageal variceal bleeding. It would have been ideal in this analysis to take into account the different types of endoscopic therapy with respect to the medications used. For example, if endoscopy was performed in a timely fashion and banding was used, this would be expected to reduce re-bleeding compared to other types of interventions, while on the other hand if endoscopy was not performed, or another mode of treatment was performed, this would affect the re-bleeding rates differently. However, only three studies clearly described the endoscopic treatment used and the endoscopic procedures differed among the studies. In addition, the studies included Child-Pugh C patients and an early TIPS procedure is now generally performed for Child-Pugh C cirrhotic patients with variceal bleeding, making obsolete the >5 days re-bleeding end point.
There are a number of limitations of this analysis that should be considered. There is a risk of bias across studies as the doses and duration of administration of the medications differed among the studies. The number of studies and the sample size were relatively small, the included studies were not designed to specifically assess variceal re-bleeding, and the management of variceal bleeding has changed over the time period of the included studies. Endoscopy was not a criterion for study inclusion, and endoscopic treatments differed among the studies. Endoscopic treatment is a first-line intervention for treating patients with bleeding esophageal varices, and each mode of endoscopic therapy is associated with its own risk of complications, including therapy-specific rates of re-bleeding [42], which may have affected the results. It was not possible to perform an adequate sensitivity analysis (e.g., low versus high quality studies). The studies did not include, as Cochrane suggests, a sequence generation of randomization nor allocation concealment, and the lack of an ITT analysis in any of the studies calls their validity into question.
Conclusions
The results of this meta-analysis indicate that there is no difference between vasopressin/terlipressin and somatostatin/octreotide in the prevention of re-bleeding after the initial treatment of bleeding esophageal varices. However, the limitations of this analysis suggest caution in the interpretation of the results, and highlight the need for future high-quality studies to determine the effectiveness of medical therapies for preventing re-bleeding from esophageal and gastric varices.
References
Jalan R, Hayes PC. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. British Society of Gastroenterology. Gut 2000;46(Suppl 3–4):III1–III15
D’Amico G, Luca A. Natural history. Clinical-haemodynamic correlations. Prediction of the risk of bleeding. Ballieres Clin Gastroenterol 1997;11:243–256
Abraldes JG, Villanueva C, Bañares R, Aracil C, Catalina MV, Garcia-Pagán JC, et al. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol 2008;48:229–236
Augustin S, Altamirano J, González A, Dot J, Abu-Suboh M, Armengol JR, et al. Effectiveness of combined pharmacologic and ligation therapy in high-risk patients with acute esophageal variceal bleeding. Am J Gastroenterol 2011;106:1787–1795
Villanueva C, Piqueras M, Aracil C, Gómez C, López-Balaguer JM, Gonzalez B, et al. A randomized controlled trial comparing ligation and sclerotherapy as emergency endoscopic treatment added to somatostatin in acute variceal bleeding. J Hepatol 2006;45:560–567
Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010;362:823–832
De Franchis R. Baveno V consensus faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762–768
Rockey DC. Pharmacologic therapy for gastrointestinal bleeding due to portal hypertension and esophageal varices. Curr Gastroenterol Rep 2006;8:7–13
de Franchis R. Somatostatin, somatostatin analogues and other vasoactive drugs in the treatment of bleeding oesophageal varices. Dig Liver Dis 2004;36(Suppl 1):S93–S100
Fogel MR, Knauer CM, Andres LL, Mahal AS, Stein DE, Kemeny MJ, et al. Continuous intravenous vasopressin in active upper gastrointestinal bleeding. Ann Intern Med 1982;96:565–569
Mallory A, Schaefer JW, Cohen JR, Holt SA, Norton LW. Selective intra-arterial vasopressin in fusion for upper gastrointestinal tract hemorrhage: a controlled trial. Arch Surg 1980;115:30–32
Ioannou G, Doust J, Rockey DC. Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database Syst Rev 2003 1:CD002147
Escorsell A, Ruiz del Arbol L, Planas R, Albillos A, Bañares R, Calès P, et al. Multicenter randomized controlled trial of terlipressin versus sclerotherapy in the treatment of acute variceal bleeding: the TEST study. Hepatology 2000;32:471–476
Escorsell A, Bandi JC, Moitinho E, Feu F, García-Pagan JC, Bosch J, et al. Time profile of the haemodynamic effects of terlipressin in portal hypertension. J Hepatol 1997;26:621–627
Chiu KW, Sheen IS, Liaw YF. A controlled study of glypressin versus vasopressin in the control of bleeding from oesophageal varices. J Gastroenterol Hepatol 1990;5:549–553
Freeman JG, Cobden I, Record CO. Placebo-controlled trial of terlipressin (glypressin) in the management of acute variceal bleeding. J Clin Gastroenterol 1989;11:58–60
D’Amico G, Traina M, Vizzini G, Tinè F, Politi F, Montalbano L, et al. Terlipressinor vasopressin plus transdermal nitroglycerin in a treatment strategy for digestive bleeding in cirrhosis. A randomized clinical trial. Liver Study Group of V. Cervello Hospital. J Hepatol 1994;20:206–212
Silvain C, Carpentier S, Sautereau D, Czernichow B, Métreau JM, Fort E, et al. Terlipressin plus transdermal nitroglycerin vs. octreotide in the control of acute bleeding from esophageal varices: a multicenter randomized trial. Hepatology 1993;18:61–65
Walker S, Kreichgauer HP, Bode JC. Terlipressin (glypressin) versus somatostatin in the treatment of bleeding esophageal varices—final report of a placebo-controlled, double-blind study. Z Gastroenterol 1996;34:692–698
García-Pagán JC, Reverter E, Abraldes JG, Bosch J. Acute variceal bleeding. Semin Respir Crit Care Med 2012;33:46–54
Abraldes JG, Bosch J. Somatostatin and analogues in portal hypertension. Hepatology 2002;35:1305–1312
Qi X, Liu L, Bai M, Chen H, Wang J, Yang Z, et al. Transjugular intrahepatic portosystemic shunt in combination with or without variceal embolization for the prevention of variceal rebleeding: a meta-analysis. J Gastroenterol Hepatol 2014;29:688–696
Zheng M, Chen Y, Bai J, Zeng Q, You J, Jin R, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic therapy in the secondary prophylaxis of variceal rebleeding in cirrhotic patients: meta-analysis update. J Clin Gastroenterol 2008;42:507–516
Thiele M, Krag A, Rohde U, Gluud LL. Meta-analysis: banding ligation and medical interventions for the prevention of rebleeding from oesophageal varices. Aliment Pharmacol Ther 2012;35:1155–1165
Funakoshi N, Ségalas-Largey F, Duny Y, Oberti F, Valats JC, Bismuth M, et al. Benefit of combination β-blocker and endoscopic treatment to prevent variceal rebleeding: a meta-analysis. World J Gastroenterol 2010;16:5982–5992
Wells M, Chande N, Adams P, Beaton M, Levstik M, Boyce E, et al. Meta-analysis: vasoactive medications for the management of acute variceal bleeds. Aliment Pharmacol Ther 2012;35:1267–1278
Corley DA, Cello JP, Adkisson W, Ko WF, Kerlikowske K. Octreotide for acute esophageal variceal bleeding: a meta-analysis. Gastroenterology 2001;120:946–954
Imperiale TF, Teran JC, McCullough AJ. A meta-analysis of somatostatin versus vasopressin in the management of acute esophageal variceal hemorrhage. Gastroenterology 1995;109:1289–1294
Clinical Practice Guideline “Management of Acute Variceal Bleeding” May 2007. Ministry of Health, Malaysia
Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998;51:1235–1241
Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000;320:1574–1577
Seo YS, Park SY, Kim MY, Kim JH, Park JY, Yim HJ, et al. Lack of difference among terlipressin, somatostatin, and octreotide in the control of acute gastroesophageal variceal hemorrhage. Hepatology 2014;60(3):954-63. doi:10.1002/hep.27006
Adarsh CK, Prasanna KS, Devarbhavi H, Karanth D, Patil M, Balaraju G, et al. Comparative study of terlipressin, somatostatin, and octreotide in acute esophageal variceal bleed: a prospective randomised study. Hepatology 2011;54:459A
Cho SB, Park KJ, Lee JS, Lee WS, Park CH, Joo YE, et al. Comparison of terlipressin and octreotide with variceal ligation for controlling acute esophageal variceal bleeding—a randomized prospective study. Korean J Hepatol 2006;12:385–393
Seo YS, Um SH, Hyun JJ, Kim YH, Park S, Keum BR, et al. A prospective study comparing the efficacy of early administration of terlipressin and somatostatin for the control of acute variceal bleeding in patients with cirrhosis. Korean J Hepatol 2006;12:373–384
Lee HY, Lee HJ, Lee SM, Kim JH, Kweon SW, Lee BS, et al. A prospective randomized controlled clinical trial comparing the effects of somatostatin and vasopressin for control of acute variceal bleeding in the patients with liver cirrhosis. Korean J Intern Med 2003;18:161–166
Feu F, Ruiz del Arbol L, Bañares R, Planas R, Bosch J. Double-blind randomized controlled trial comparing terlipressin and somatostatin for acute variceal hemorrhage. Variceal Bleeding Study Group. Gastroenterology 1996;111:1291–1299
Saari A, Klvilaakso E, Inberg M, Pääkkönen M, Lahtinen J, Höckerstedt K, et al. Comparison of somatostatin and vasopressin in bleeding esophageal varices. Am J Gastroenterol 1990;85:804–807
Walker S, Kreichgauer HP, Bode JC. Terlipressin vs. somatostatin in bleeding esophageal varices: a controlled, double-blind study. Hepatology 1992;15:1023–1030
Gøtzsche PC, Hróbjartsson A. Somatostatin analogues for acute bleeding oesophageal varices. Cochrane Database Syst Rev 2008:CD000193
McCormick PA, O’Keefe C. Improving prognosis following a first variceal haemorrhage over four decades. Gut 2001;49:682–685
Krige JE, Bornman PC, Shaw JM, Apostolou C. Complications of endoscopic variceal therapy. S Afr J Surg 2005;43:177–188
Acknowledgements
This study was supported by the Specialized Research Fund for the Doctoral Program of Higher Education of China (20120142120048) and Nature Science Foundation of Hubei Province (2012FFB02308).
Compliance with ethical requirements and Conflict of interest
This analysis has complied with all the ethical requirements with Tongji Medical College and Science and Technology of Huazhong University. Chao Wang, Juan Han, Liang Xiao, Chang-e Jin, Dong-jian Li, and Zhen Yang declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Han, J., Xiao, L. et al. Efficacy of vasopressin/terlipressin and somatostatin/octreotide for the prevention of early variceal rebleeding after the initial control of bleeding: a systematic review and meta-analysis. Hepatol Int 9, 120–129 (2015). https://doi.org/10.1007/s12072-014-9594-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-014-9594-9