Abstract
Large volume of new data on the natural history and treatment of chronic hepatitis B virus (HBV) infection have become available since 2008. These include further studies in asymptomatic subjects with chronic HBV infection and community-based cohorts, the role of HBV genotype/naturally occurring HBV mutations, the application of non-invasive assessment of hepatic fibrosis and quantitation of HBV surface antigen and new drug or new strategies towards more effective therapy. To update HBV management guidelines, relevant new data were reviewed and assessed by experts from the region, and the significance of the reported findings was discussed and debated. The earlier “Asian-Pacific consensus statement on the management of chronic hepatitis B” was revised accordingly. The key terms used in the statement were also defined. The new guidelines include general management, indications for fibrosis assessment, time to start or stop drug therapy, choice of drug to initiate therapy, when and how to monitor the patients during and after stopping drug therapy. Recommendations on the therapy of patients in special circumstances, including women in childbearing age, patients with antiviral drug resistance, concurrent viral infection, hepatic decompensation, patients receiving immune suppression or chemotherapy and patients in the setting of liver transplantation and hepatocellular carcinoma, are also included.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the 4th version of the “Asian-Pacific consensus statement on the management of chronic hepatitis B” was published in September 2008 [1], tenofovir has been approved globally and several updated guidelines on chronic hepatitis B virus (HBV) infection have been published [2–4]. Large amounts of new data on the natural history and treatment of chronic HBV infection have also become available or are emerging. These include further studies in asymptomatic subjects with chronic HBV infection and community-based cohorts, the role of HBV genotype and naturally occurring HBV mutations, the application of non-invasive methods in the assessment of hepatic fibrosis, the clinical utility of quantitative hepatitis B surface antigen (HBsAg), and newer drugs or new strategies towards more effective management. We have closely followed the progress in the field and invited experts from the Asian-Pacific region to review and assess relevant new data. The significance of the reported findings was discussed and debated during an expert meeting in Taipei, Taiwan on October 22–23, 2011. The 2008 update of the “Asian-Pacific consensus statement on the management of chronic hepatitis B” [1] was revised accordingly. The key terms defined in the 2008 statement were also revised (Table 1). Then, the revised version was circulated for further comments, and it was refined through electronic communications among the experts. The revised contents were presented and discussed at the Asian-Pacific Association for the Study of the Liver meeting in Taipei, Taiwan in February 2012. The following is the final version of the updated consensus and recommendations on the management of chronic hepatitis B.
Conceptual background
HBV, pathogenesis, and natural course
Chronic HBV infection is a serious clinical problem because of its worldwide distribution and potential adverse outcomes, including cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC). HBV infection is particularly important in the Asian-Pacific region, where it is endemic, with the majority of infections being acquired perinatally or in early childhood; some patients may be superinfected with other viruses later in life, an event that may adversely affect clinical outcomes. In addition, the countries in this region mostly have low to intermediate gross national income per capita [5].
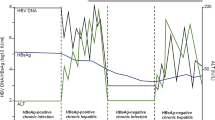
Studies have revealed that covalently closed circular DNA (cccDNA) plays a key role in the maintenance of chronic HBV infection. As HBV is not cytopathogenic by itself, chronic HBV infection is a dynamic state of interactions among the virus, hepatocytes, and the host immune system. The natural course of chronic HBV infection in this geographic region can be divided into (1) immune tolerant phase, (2) immune clearance phase, and (3) residual or inactive phase. Patients in the immune tolerant phase are usually young, hepatitis B e antigen (HBeAg) seropositive with high HBV DNA levels (>2 × 106 to 2 × 107 IU/mL) but have normal serum alanine aminotransferase (ALT) and no or minimal histological changes [6]. The results of a 5-year follow-up study confirm that adults in the immune tolerant phase show no or minimal disease progression as long as their serum ALT levels remain normal [7]. However, HBeAg-positive subjects older than 40 years with persistently ‘high normal’ ALT levels may have significant hepatic necroinflammation or fibrosis [8]. During the immune clearance phase, HBeAg-positive hepatitis with ALT elevation and even acute flares with serum ALT levels increased over 5 times the upper limit of normal (ULN) may occur, sometimes complicated with hepatic decompensation. These ALT elevations and hepatitis flares are considered to be the results of host immune responses against HBV-infected hepatocytes, namely, HLA-class I antigen-restricted cytotoxic T lymphocyte (CTL)-mediated response against HBV antigen(s) expressed on hepatocytes with resultant apoptosis and necrosis. Higher ALT levels, therefore, usually reflect more extensive hepatocyte damage due to more vigorous immune response against HBV [9]. These events may eventually be followed by HBeAg seroconversion to its antibody (anti-HBe) and/or decreasing serum HBV DNA. The estimated annual incidence of spontaneous HBeAg seroconversion is 2–15 %, depending on factors such as age, ALT levels, and HBV genotype [9, 10]. Some patients may experience only transient and mild elevation of serum ALT levels before HBeAg seroconversion [11]. HBeAg seroconversion is followed by sustained clinical remission (inactive carrier state) in the majority of patients. However, relapse may occur due to HBeAg sero-reversion or the occurrence of HBeAg-negative hepatitis. The estimated annual incidence of hepatitis relapse is about 2–3 % [11, 12], being higher in males, those with genotype C infection, and those who have HBeAg seroconversion after 40 years of age [13, 14]. These findings suggest that earlier HBeAg seroconversion or shorter HBeAg-positive phase is associated with a higher chance of sustained remission. Asymptomatic HBeAg-negative subjects, especially those with HBV DNA >2,000 IU/mL, may also experience hepatitis flares and disease progression as HBeAg-positive patients do [11–16]. Since the immunopathogenesis of HBeAg-negative hepatitis is similar to that of HBeAg-positive hepatitis, this phase can be viewed as a variant form of the immune clearance phase [6].
Development of liver cirrhosis or HCC may occur during the natural course. A prospective study involving 684 patients with chronic hepatitis B showed that cirrhosis developed at an estimated annual incidence of 2.1 %, and that age; extent, severity, and frequency of flares; duration of hepatic lobular alterations were factors for disease outcomes [17]. Patients with persistent HBeAg seropositivity have an even higher incidence (3.5 % per year) of cirrhosis [18]. Age at HBeAg seroconversion is also an important factor, as demonstrated in a study involving 483 patients, in which the 15-year cumulative incidences of cirrhosis and HCC were lowest in those who had undergone HBeAg seroconversion before age 30 (3.7 and 2.1 %, respectively), and highest in those who had not undergone HBeAg seroconversion until after age 40 (42.9 and 7.7 %, respectively) [14]. Another 11.5-year follow-up study in a cohort of 1,965 incidentally detected HBeAg-negative carriers with normal ALT also showed that the risk of cirrhosis correlated significantly with advanced age at entry (p = 0.004) and hepatitis B reactivation (p < 0.0001) [15]. A long-term follow-up study (mean = 120 months) involving 188 Korean patients (52 HBeAg-negative patients) showed that age and persistent ALT elevation are independent factors for the development of cirrhosis, decompensation, and HCC [19]. Large studies in asymptomatic HBsAg carriers have also demonstrated that frequent ALT elevation during long-term follow-up is a factor for the development of cirrhosis and HCC [20, 21]. HCC develops at an annual incidence of 3–6 % in patients with cirrhosis and far less frequently in non-cirrhotic patients [17, 22, 23]. The community-based REVEAL-HBV study demonstrated that seropositivity for HBeAg and/or HBV DNA >104 copies/mL (>2,000 IU/mL) in adult HBV carriers at study entry are significant risk factors for cirrhosis and HCC development over time in a dose-dependent manner [24–26]. In addition, recent studies suggest that obesity and metabolic derangement may also increase the risk of HBV-related HCC in these carriers [27, 28].
Inactive carrier state usually indicates favorable long-term clinical outcomes. A study including 3,673 HBeAg-negative carriers with normal baseline ALT showed that ALT became abnormal mostly in the first 3 years, while the 1,953 carriers who maintained persistently normal ALT (PNALT) had excellent long-term prognosis, with only two developing HCC and no deaths of liver disease during a mean follow-up period of 13.4 ± 5.2 years [29]. The REVEAL-HBV study has also demonstrated that those with PNALT over 13-year follow-up have a significantly lower risk of HCC [21]. Compared with well-matched patients with HBeAg-negative chronic hepatitis B, inactive carriers were more frequently female, had lower HBV DNA levels, lower prevalence of genotype C infection, and fewer basal core promoter A1762T/G1764A mutations [30]. It was demonstrated that 19 % of those with PNALT and no disease progression over 10 years had serum HBV DNA between 2,000 and 20,000 IU/mL, but rarely (4 %) >20,000 IU/mL [31]. A large nested case–control study involving 112 HCC and 1,031 non-HCC patients who underwent a median follow-up of 7.3 (0.6–15.0) and 13.4 (2.1–16.0) years, respectively, also showed that maintenance of HBV DNA <4.39 log10 copies/mL (<4,700 IU/mL) was associated with PNALT and decreased risk of HCC [20]. The REVEAL-HBV study also showed that HCC incidence increased from 0.073 % per year in those with serum HBV DNA <104 copies/mL (<2000 IU/mL) at entry to 0.185 % per year in those with serum HBV DNA persistence at 104–105 copies/mL (2,000–20,000 IU/mL), but was still much lower than the 0.381 ~ 1.481 % per year in those with serum HBV DNA maintained >105 copies/mL (>20,000 IU/mL) [21]. Thus, it seems reasonable to define ‘inactive carrier state’ as HBeAg-negative, HBsAg-positive subject with PNALT for at least 3 years and HBV DNA <20,000 IU/mL. Of note, the REVEAL-HBV study has shown that inactive carriers are still at risk of HCC development, as those with HBV DNA <4 log10 copies/mL (<2,000 IU/mL) had an adjusted hazard ratio (95 % confidence internal) of 4.6 (2.5–8.3) for HCC development as compared with the HBsAg-negative controls [32].
Spontaneous HBsAg seroclearance may occur during the inactive phase. An 11-year follow-up study in 1,965 asymptomatic anti-HBe-positive subjects of a median age of 34 (16–76) years at entry showed an annual HBsAg seroclearance rate of 1.2 %, and the cumulative HBsAg seroclearance rate was 8 % at 10 years, increasing disproportionately to 25 % at 20 years, and 45 % at 25 years of follow-up [33]. The REVEAL-HBV study has further revealed that undetectable serum HBV-DNA level (<300 copies/mL) at entry or during follow-up is a major predictor of spontaneous HBsAg seroclearance, and the cumulative incidence of HBsAg seroclearance at 60 and 100 months after serum HBV DNA level had decreased to undetectable levels was 25.8 and 51.3 %, respectively [34]. Although a pool of transcriptionally silent cccDNA molecules can be found in the liver and low serum viremia (almost all <200 IU/mL) may remain for >10 years after HBsAg seroclearance in 14 % of subjects [35], HBsAg seroclearance is considered was a state closest to a cure and usually confers an excellent prognosis. However, HCC may still occur albeit at a very low rate, especially if cirrhosis has already developed before HBsAg seroclearance, or if HBsAg seroclearance occurs at an old age (>50 years), or in the presence of concurrent hepatitis C or D viral infection [33].
New application of an old biomarker: quantitative HBsAg (qHBsAg)
HBsAg is produced by more than one pathway: from translation of transcriptionally active cccDNA molecules that serve as a template for replication and from translation of viral genes transcribed from integrated HBV DNA sequences in the host genome. In addition to being the envelope of the infectious HBV particle, HBsAg is also found as non-infectious spheres or filaments, exceeding infectious virions by 102–105 times. Studies have shown that serum HBsAg appears to correlate with cccDNA and is considered a surrogate marker of infected cells. Using recently available commercial quantitative assays, qHBsAg has been shown to be helpful in the understanding and management of chronic hepatitis B [36, 37].
Studies have consistently shown that HBsAg level is highest in the immune tolerant phase (4.5–5.0 log10 IU/mL), starts to decline during the immune clearance phase (3.0–4.5 log10 IU/mL), and decreases slowly and progressively after HBeAg seroconversion. HBsAg level is lowest (1.5–3.0 log10 IU/mL) in those who maintain PNALT, but higher (2.5–4.0 log10 IU/mL) in those who develop HBeAg-negative hepatitis [38–40]. Longitudinal studies have further shown that HBsAg level remained stable in HBeAg-positive patients and tended to reduce slowly in HBeAg-negative patients, and a reduction of HBsAg by >1 log IU/mL could reflect improved immune control [40]. It was shown that combined single-point quantification of HBsAg <1,000 IU/mL and HBV DNA ≤2,000 IU/mL identified inactive carriers with up to 90 % positive predictive value (PPV) and 97 % negative predictive value (NPV) in genotype D HBeAg-negative subjects [36]. In genotype B and C HBeAg-negative carriers with normal ALT, the lower the serum HBsAg level <1,000 IU/mL, the higher the chance of spontaneous HBsAg seroclearance, and an HBsAg level of ≤100 IU/mL is an appropriate cut-off for predicting HBsAg loss over time [41–43]. In particular, an HBsAg level <200 IU/mL may predict HBsAg seroclearance in 3 years [41, 44], especially if combined with a ≥1 log10 IU/mL decline in the preceeding 2 years [44].
Taking these lines of evidence together, serum HBsAg level could be used together with, but not as a substitute for, HBV-DNA in clinical practice.
Clinical significance of HBV genotype and naturally occurring mutations
Hepatitis B virus has been classified into at least 10 genotypes on the basis of an intergroup divergence of 8 % or more in the complete genome nucleotide sequence. Subtypes are identified within some genotypes. Each genotype has its distinct geographical and ethnic distribution worldwide and within the Asian-Pacific region. HBV genotypes B and C are prevalent in East and South-East Asia, the Pacific Islands, and Pakistan, whereas HBV genotypes D and A are prevalent in India and genotype A in the Philippines. HBV genotype D is also found in the Pacific Islands. HBV genotypes B and C are prevalent in highly endemic areas where perinatal or vertical transmission plays an important role in spreading the virus, whereas genotypes A, D, E, F, and G are frequently found in areas where the main mode of transmission is horizontal (Table 2). The clinical significance and virologic characteristics of HBV genotypes have only been reliably compared between genotypes B and C, and between genotypes A and D. In general, genotype B is associated with less progressive liver disease than genotype C, and genotype D has a less favorable prognosis than genotype A [10]. A study in 1,536 Alaskan natives with chronic HBV infection has shown that the median age for HBeAg clearance was less than 20 years for genotypes A, B, D, and F, but more than 40 years for genotype C, and that patients with genotypes C and F have significantly more frequent HBeAg reversion and higher risk of HCC [45]. Several studies have shown that genotype B is associated with spontaneous HBeAg seroconversion at a younger age, less active liver disease, slower progression to cirrhosis, and less frequent development of HCC than genotype C [10, 45, 46]. A study from India indicated that genotype D is more often associated with HBeAg-negative chronic HBV infection and more severe diseases, and may predict the occurrence of HCC in young patients [47]. It has also been shown that recombinant genotypes lead to more severe disease [10].
In addition, HBV subgenotypes, mixed HBV genotype infections, and recombination of HBV genotype are not rare clinically. Subgenotypes of HBV have been identified on the basis of 4–8 % heterogeneity of the entire HBV genome. Similar to HBV genotype, these HBV subgenotypes may have different clinical and virologic characteristics. For example, in a case–control study including 172 patients from Japan and 156 patients from Hong Kong infected by HBV genotype C, HBV subgenotype Ce (vs. Cs) was found to be an independent risk factor of HCC in addition to male sex, older age, and positive HBeAg status [48]. The study was limited by the potential confounding effect of the enrolled patient ethnicity. Moreover, the status of liver cirrhosis, which may be the most important risk factor for HCC, was not evaluated. In a subsequent study involving 1,006 patients with a median follow-up of 7.7 years, 86 patients (8.5 %) developed HCC, and high HBV DNA level and HBV genotype C, particularly subgenotype Ce, increased the risk of HCC [49]. Furthermore, in an epidemiologic study of acute hepatitis B, Chinese patients with subgenotype C2 developed chronic infection more often than those infected with subgenotype B2, and subgenotype C2 was an independent factor for the chronicity of HBV infection [50]. The clinical significance of subgenotype and mixed genotype infections needs further examinations to draw definite conclusions.
Due to the spontaneous error rate of viral reverse transcription, naturally occurring HBV mutations arise during the course of infection under the pressure of host immunity or specific therapy. Several HBV strains including mutations in precore, core promoter, and deletion mutation in pre-S/S genes have been reported to be associated with the pathogenesis of fulminant or progressive liver disease, including cirrhosis and HCC [10]. Patients harboring HBV genotype C have a higher HBV-DNA level, higher frequency of pre-S deletions, higher prevalence of core promoter A1762T and/or G1768A mutations and A1762T/G1764A double mutations, and a significantly higher chance of developing HCC than patients infected with HBV genotype B [10, 46, 51, 52]. A study revealed that a complex mutation pattern rather than a single mutation was associated with disease progression [51]. Viral quasispecies evolution has recently been shown to play an important role in the natural history and pathogenesis of chronic hepatitis B. In particular, the evolution of viral quasispecies may play an important role in the pathogenesis of HBeAg seroconversion [53] and the immune clearance phase [54].
Role of non-invasive examinations in evaluating severity of hepatic fibrosis
In the management of patients with chronic hepatitis B, assessment of hepatic fibrosis is of paramount importance. The severity of liver fibrosis is a strong prognostic factor by itself, and it helps to identify patients who will benefit from antiviral therapy, assess response to antiviral therapy, determine the optimal time to start surveillance, and stratify the risk of HCC and hepatic decompensation [1, 55]. To date, liver biopsy is the best standard for assessing liver fibrosis. Although it is generally accepted to be a safe procedure, it can cause discomfort and carries an occasional risk of serious complications. Furthermore, liver biopsy is subject to sampling error and interobserver variability. In addition, it is not practical to use liver biopsy repeatedly in monitoring patients undergoing antiviral therapy because of its limitations and invasive nature.
Recently, non-invasive examinations to evaluate the severity of hepatic fibrosis have been introduced and are known to assess the severity of liver fibrosis accurately. There are several reports that non-invasive methods such as liver stiffness measurement (LSM) by transient elastography (TE, FibroScan) and other serologic tests could be used as an alternative to liver biopsy in patients with chronic hepatitis B [56, 57]. LSM was found to have the best performance in diagnosing advanced fibrosis (METAVIR ≥ 3) than any other serum test formulae, with an NPV of 92 % for a cut-off of ≤6.0 kPa for patients with normal ALT and ≤7.5 kPa for those with elevated ALT and a PPV of 98 % for a cut-off of ≥9 kPa for patients with normal ALT and ≥12 kPa for patients with elevated ALT [56]. However, neither biomarkers nor biopsy alone are sufficient to make a definitive decision in a given patient, and all the clinical and biological data must be taken into account. These noninvasive tests cannot replace liver biopsy completely and should be used as a complementary tool in the management of patients with chronic hepatitis B.
Clinical significance of outcome calculators
To help practicing physicians, HCC risk calculators were created based on the data obtained from long-term follow-up cohorts [58–60]. For example, the REVEAL-HBV study team has developed easy-to-use nomograms for predicting HCC risk in patients with chronic HBV infection, based on sex, age, family history of HCC, alcohol consumption, serum ALT level, HBeAg serostatus, serum HBV DNA level, and HBV genotype [60]. The clinical application of the scoring system has been validated by the REACH-B Working Group [61]. This scoring system accurately estimates the risk of developing HCC at 3, 5, and 10 years in adult chronic hepatitis B patients. Clinicians may use this scoring system to assess HCC risk in chronic hepatitis B patients and make evidence-based decisions to manage their patients.
Concurrent infection with other virus(es)
Hepatitis B virus, hepatitis C virus (HCV), hepatitis delta virus (HDV), and human immunodeficiency virus (HIV) share similar transmission routes. Therefore, concurrent infection with these virus(es) may occur and complicate the natural course of chronic HBV infection. In general, concurrent infection with these viruses usually results in more severe and progressive liver disease and thus needs treatment [62].
Goals of treatment for chronic HBV infection
It is now clear that active HBV replication is the key driver of liver injury and disease progression, thus sustained viral suppression is of paramount importance. Therefore, the primary aim of chronic hepatitis B treatment is to permanently suppress HBV replication. This will decrease the infectivity and pathogenicity of the virus, thereby reducing hepatic necroinflammation. Clinically, the short-term goal of treatment is to achieve ‘initial response’ in terms of HBeAg seroconversion and/or HBV-DNA suppression, ALT normalization, and prevention of hepatic decompensation, and to ensure ‘maintained/sustained response’ to reduce hepatic necroinflammation and fibrosis during/after therapy. The ultimate long-term goal of therapy is to prevent hepatic decompensation, reduce or prevent progression to cirrhosis and/or HCC, and prolong survival [1].
Currently available treatments
Currently, interferon-alfa (IFN-α), lamivudine (LAM), adefovir, entecavir, telbivudine, tenofovir, and pegylated IFN (Peg-IFN)-α2a have been licensed globally. Peg-IFN-α2b has been approved for the treatment of chronic HBV infection in a few countries. Thymosin-α1 has also been approved in some countries in Asia. Clevudine has been approved only in Korea and the Philippines.
Immunodulatory therapies
Immunomodulatory agents include conventional IFN-α, Peg-IFN, and thymosin α1. Immunomodulatory agents have dual actions: enhancing host immune system to mount a defense against HBV and modest antiviral action. IFN has been the mainstay of HBV treatment for two to three decades.
Conventional IFN
HBeAg-positive chronic hepatitis B: Meta-analyses of controlled trials in HBeAg-positive patients showed that treatment with IFN at a dose of 5 MU daily or 10 MU three times weekly for 4–6 months achieved higher HBeAg loss (33 vs. 12 %), HBV DNA suppression (37 vs. 17 %), and ALT normalization than untreated controls with a risk difference of around 25 % for each parameter. The rate of HBsAg seroclearance was also higher (7.8 vs. 1.8 %) in IFN-treated patients, with a risk difference of 5.6 %. Higher IFN doses and extended treatment duration appear to improve treatment response. However, increased adverse events and costs associated with higher doses and prolonged treatment warrant caution in recommending it in practice. A lower dosage of IFN 5–6 MU three times weekly has been used in Asian patients with similar efficacy. Asian patients with elevated baseline ALT responded to IFN treatment at rates similar to Caucasians. The efficacy of IFN treatment in children with elevated ALT was similar to that in adults. Re-treatment of patients who failed to respond to previous IFN therapy could achieve HBeAg loss in 20–40 % of cases [63]. A study of a tailored regimen of IFN [5 MU three times weekly until achieving virologic and biochemical endpoints or when HBV DNA was no longer decreasing; median 10 (6–24) months] in 247 HBeAg-positive patients showed better sustained response than fixed 6-month treatment duration (40.5 vs. 28.3 %, p = 0.013) [64]. HBeAg seroconversion is durable in over 90 % patients, and delayed HBeAg seroconversion could occur in 10–15 % patients at 1–2 years post-treatment, and a 15-year cumulative incidence of HBeAg seroconversion of up to 75 % (vs. 52 % in control). It also results in less cirrhosis development, better overall survival, and reduced incidence of HCC [18], especially among responders [63].
HBeAg-negative chronic hepatitis B: IFN treatment resulted in an end-of-treatment biochemical and virological response in 60–90 % in European patients; however, the sustained response rate was low: 10–15 % with 4–6 months of treatment and 22 % with 12 months of treatment. In Asian patients, a 6- to 10-month course of IFN therapy achieved a 6-month post-treatment response in 30 % patients versus 7 % in untreated patients [64]. The preferred IFN treatment duration for HBeAg-negative chronic hepatitis B is 12 months. A study of extended IFN treatment for 24 months reported a sustained response in 30 % patients and HBsAg clearance in 18 % patients at 6-year post-therapy [65]. IFN treatment improved long-term outcomes, including reduced HCC development, and prolonged survival and hepatic complication-free survival in patients with sustained response [66].
Patients with well-compensated cirrhosis treated with IFN had comparable or even better response and a similar side effect profile as those without cirrhosis, with a reduced risk of hepatic decompensation and HCC development, and prolonged survival in responders. However, IFN is contraindicated in patients with overt decompensated cirrhosis because it can precipitate hepatic decompensation, resulting in fatal complications [21].
Long-term follow-up studies showed that IFN treatment increased HBsAg seroclearance over time in patients with HBeAg loss. Collective data show that IFN therapy was associated with an increased likelihood of HBsAg seroclearance (3.16-fold in Western studies, 6.63-fold in Asian patients) as compared with untreated controls [32]. Two meta-analyses have confirmed these long-term benefits of IFN treatment in reducing liver disease progression to cirrhosis and HCC.
Peg-IFN-α
Pegylation of IFN-α improves its pharmacokinetics and prolongs its half-life, which allows weekly injections. A study of a 24-week course of weekly Peg-IFN-α2a in Asian patients showed a higher combined response rate (HBeAg loss, HBV DNA <500,000 copies/mL, and ALT normalization; 28 vs. 12 %, p = 0.036) and a higher HBeAg seroconversion rate than a 24-week course of IFN-α2a (33 vs. 25 %, p < 0.05) [67]. The superior HBeAg loss over conventional IFN was confirmed by a study of Peg-IFN-α2b treatment for 24 weeks in Chinese patients compared with conventional IFN-α2b [68].
HBeAg-positive chronic hepatitis B: A study of Peg-IFN-α2a with or without LAM in 814 patients (>85 % were Asian) showed that a 48-week course of Peg-IFN-α2a monotherapy at 180 μg weekly achieved ALT normalization in 41 %, HBeAg seroconversion in 32 %, HBV DNA level <105 copies/mL in 32 %, HBV DNA <400 copies/mL in 14 %, and HBsAg seroconversion in 3 % at 6-month post-treatment [69]. Sub-analyses in Asian patients found a similar HBeAg seroconversion rate of 31 % [70]. Similar results were observed in a trial in Hong Kong using Peg-IFN-α2b [71]. The most recent 4-arm NEPTUNE study, comparing 90 versus 180 μg weekly and 24 versus 48 weeks Peg-IFN-α2a treatment, showed that the highest HBeAg seroconversion rate at 6-month post-treatment was achieved in patients who received Peg-IFN-α2a at a dose of 180 μg weekly for 48 weeks (36.2 vs. 25.8 %, 22.9 and 14.1 % in those who received 90 μg for 48 weeks, 180 μg for 24 weeks, and 90 μg for 24 weeks, respectively) [72]. The results from the NEPTUNE study confirm that the recommended dosage of Peg-IFN-α2a treatment should be 180 μg once weekly for 48 weeks. At 1 year after the end of Peg-IFN-α2a treatment, delayed HBeAg seroconversion was achieved in 14 % of the initial non-responders, and durability of HBeAg seroconversion was seen in 86 % of initial responders [70]. A long-term follow-up (mean duration 3 years) study in 172 HBeAg-positive patients treated with Peg-IFN-α2b with or without LAM showed a sustained response in 81 % of initial responders and delayed response in 27 % of initial non-responders. In addition, HBsAg clearance was achieved in 11 and 30 % of overall patients and initial responders, respectively, at the last visit [73]. Another long-term follow-up (mean duration 6 years) study in 85 Chinese patients treated with Peg-IFN-α2b at a dose of 1.5 μg/kg weekly for 32 weeks and LAM 100 mg daily for 52 or 104 weeks showed that 37 % achieved initial response, and 77 and 57 % of the initial responders had sustained HBeAg seroconversion and virologic response (HBV DNA <10,000 copies/mL), respectively, at 5 years. Together with delayed HBeAg seroconversion in 69 % of the initial nonresponders, the HBeAg seroconversion rate was 60 % at 5 years [74].
HBeAg-negative chronic hepatitis B: A study (61 % Asians) on treatment with Peg-IFN-α2a 180 μg weekly for 48 weeks showed ALT normalization in 59 %, HBV DNA level <20,000 copies/mL in 43 %, HBV DNA <400 copies/mL in 19 %, and HBsAg clearance in 3 % of the patients at 6 months after the end of treatment [75]. The combined response (ALT normalization and HBV DNA <20,000 copies/mL) at 6 months after treatment was 45 % in Asians, as compared with 36 % in overall patients [70]. A study of Peg-IFN-α2b ± LAM treatment for 48 weeks showed virologic response (HBV DNA <60 IU/mL) in 43 % and ALT normalization in 40 % of treated patients at 6-month post-treatment [76]. Long-term follow-up of 230 patients treated with Peg-IFN-α2a ± LAM showed a sustained virological response (HBV DNA <10,000 copies/mL) in 21 % at 5 years after the end of treatment. The rate of HBsAg seroclearance increased overtime from 5 % at 1-year post-treatment to 12 % (or 35 % among responders) at 5-year post-treatment [77]. An Italian multicenter study of extended therapy with Peg-IFN-α2a in 128 HBeAg-negative genotype D patients showed that 48 weeks treatment with Peg IFN-α2a 180 μg weekly, followed by another 48 weeks of 135 μg weekly doses resulted in a significantly higher virological response (HBV DNA <2,000 IU/mL) rates at 1-year post-treatment than in those treated for 48 weeks only (29 vs. 12 %, p = 0.03), as well as a higher HBsAg seroclearance rate (6 vs. 0 %). Extended treatment was well tolerated and did not result in more adverse events than the 48-week treatment course [78].
Chronic hepatitis B with cirrhosis: Treatment with a 52-week course of Peg-IFN-α2b with or without LAM in 24 HBeAg-positive patients with well-compensated cirrhosis showed a higher rate of sustained virological response (HBeAg seroconversion and HBV DNA <10,000 copies/mL) at 26-week post-treatment, than that achieved in patients without cirrhosis (30 vs. 14 %, p = 0.02). In addition, improvement in liver fibrosis occurred more frequently in patients with advanced fibrosis than in those without advanced fibrosis (66 vs. 22 %, p < 0.001). The side effects, including serious side effects, in patients with and without advance fibrosis were comparable [79].
Combination therapy with IFN or Peg-IFN and LAM in HBeAg-positive and HBeAg-negative chronic hepatitis B patients had greater on-treatment HBV DNA suppression, but there was no difference in sustained off-treatment response when compared to IFN or Peg-IFN alone [60, 62, 66]. Combination therapy with Peg-IFN and adefovir dipivoxil also showed a similar rate of sustained virologic response at 6-month post-treatment to that achieved with Peg-IFN alone [80]. A clinical trial of sequential therapy with LAM 100 mg daily for 4 weeks followed by Peg-IFN-α2b 1.0 μg/kg weekly for a further 24 weeks in 36 patients with HBeAg-positive chronic hepatitis B showed a significantly higher rate of HBV DNA undetectability (<4,700 copies/mL, 50 vs. 14.8 %), and higher rates of HBeAg loss (38.9 vs. 14.8 %) at 6-month post-therapy, as compared with Peg-IFN-α2b monotherapy for 24 weeks in 27 patients [81]. Sequential therapy with adefovir or entecavir followed by Peg-IFN-α2a has also showed promising results in small studies [82, 83]. Currently, there are on-going clinical trials of different kinds of combination therapy with Peg-IFN plus nucleos(t)ide analogues, including sequential or intermittent combination therapy. However, combination therapy cannot be recommended in clinical practice until its benefit has been confirmed in future studies.
Predictors of response to IFN-based therapy include baseline and on-treatment factors. High baseline ALT and low baseline HBV DNA are associated with a higher response to IFN and Peg-IFN treatment. A recent study in 205 HBeAg-positive patients treated with Peg-IFN showed that IL28B genotype AA (vs. AG/GG) at rs12980275 and CC (vs. CT/TT) at rs 12979860 was significantly associated with HBeAg seroconversion and HBsAg seroclearance [84]. Earlier studies showed that HBV genotypes A and B had a better response to IFN and Peg-IFN treatment than genotypes D and C, respectively [62]. Patients with genotype C had a lower response than those with genotype B when treated with a lower dosage of Peg-IFN-α2a (90 μg) or for a shorter duration (24 weeks) [67]. However, the most recent NEPTUNE study has confirmed a comparable response to Peg-IFN-α2a 180 μg weekly for 48 weeks between genotype B and C patients [72]. The results from these studies suggest that Peg-IFN-α2a 180 μg for 48 weeks could improve the response in patients with genotype C. Pooled data from the two largest studies of HBeAg-positive patients with Peg-IFN treatment showed that genotype A patients with higher levels of baseline ALT or lower levels of HBV DNA, and genotype B and C patients with both higher ALT levels and lower HBV DNA levels had a high predicted probability of treatment response [85]. It is obvious that more extensive and prospective investigations are required to confirm the findings related to the host genetic factors and the interplay between host and viral factors.
An on-treatment ALT flare followed by a decrease in HBV DNA and HBeAg levels during Peg-IFN treatment was found to be predictors of response at the end of treatment and during post-treatment follow-up [86, 87]. On-treatment HBsAg levels or decline has been shown to be a strong predictor of sustained response to Peg-IFN treatment. Studies of quantitative HBsAg levels during a 48-week course of 180 μg Peg-IFN-α2a weekly treatment in HBeAg-positive patients showed that higher rates of HBeAg seroconversion at 6-month post-treatment in patients with HBsAg levels <1,500 IU/mL at weeks 12 and 24 than in those with HBsAg levels >20,000 IU/mL at the same time points (57 vs. 16 % at week 12, and 54 vs. 15 % at week 24) [72, 88]. A study in HBeAg-positive Caucasian patients with predominantly HBV genotype D and A treated with Peg-IFN-α2b ± LAM for 52 weeks found that patients who achieved no decline of HBsAg at week 12 had a 97 % probability of non-response during post-treatment follow-up [89]. However, a study in predominantly (88 %) HBV genotype C and B Asians treated with Peg-IFN-α2a ± LAM for 48 weeks found that patients who achieved no HBsAg decline at week 12 had only 82 % probability of non-response [90]. A study of a 48-week course Peg-IFN-α2a ± LAM in HBeAg-negative patients found that patients who achieved an HBsAg decline of >10 % from the baseline at weeks 12 and 24 had a sustained virologic response (HBV DNA <10,000 copies/mL) at 1-year post-treatment in 47 and 43 % of cases, respectively, and an HBsAg seroclearance rate of 23 % at 5 years after treatment [36].
Side effects of IFN-based therapy: The most frequently reported side effects are flu-like symptoms, headache, fatigue, myalgia, alopecia, and local reaction at the injection site. IFN and Peg-IFN have myelosuppressive effects, but neutropenia <1,000/mm3 and thrombocytopenia <50,000/mm3 are uncommon unless patients have cirrhosis or low cell counts prior to treatment. Neutropenia and thrombocytopenia induced by IFN or Peg-IFN do not significantly increase the risk of infection and bleeding, except in patients with cirrhosis or immunosuppression. Although IFN and Peg-IFN have many side effects, they are mostly mild and usually well tolerated. Premature discontinuation due to patient intolerability has been reported in 2–8 % of patients treated with Peg-IFN.
Thymosin α1
A few studies have evaluated the efficacy of thymosin α1 treatment in patients with chronic hepatitis B. Treatment with thymosin α1 1.6 mg twice weekly for 6 months achieved a complete response (ALT normalization with HBeAg loss and undetectable HBV DNA by solution hybridization assay at 12 months after the end of therapy) in 40–45 % patients, being higher in genotype B-infected than in genotype C-infected HBeAg-positive patients (52 vs. 24 %, p = 0.036) [91, 92]. In a Japanese study in which 95 % of the patients were infected with genotype C virus, 1.6 mg thymosin α1 therapy achieved HBeAg seroconversion rate of 21.5 % [93], similar to 24 % in Taiwanese patients infected with genotype C [92]. A meta-analysis including 353 patients from five trials showed that the odds ratios for virological response to thymosin α1 at the end of treatment, and 6 and 12-month post-treatment were 0.56 (0.2–1.52), 1.67 (0.83–3.37), and 2.67 (1.25–5.68), respectively, with a significantly increasing virological response over time after thymosin therapy [94]. A randomized controlled trial of lymphoblastoid IFN 5 MU in combination with thymosin α1 1.6 mg three times weekly compared with lymphoblastoid IFN 5 MU three times weekly for 24 weeks was conducted in 96 patients with HBeAg-positive chronic hepatitis B. The study found a marginally higher HBeAg loss rate (45.8 and 28 %; p = 0.067) in the patients undergoing combination therapy at 1 year after the end of treatment as well as a higher but non-significant difference in HBeAg seroconversion rate (43.8 vs. 28 %) [95]. A randomized controlled trial comparing thymosin α1 and LAM for 24 weeks followed by continuous LAM therapy versus LAM monotherapy showed higher HBeAg seroconversion rate (26.5 vs. 6.1 %, p = 0.024) at week 24, but the difference became non-significant (26.5 vs. 21.2 %) at week 52 [96]. In HBeAg-negative patients, a randomized controlled study of treatment with thymosin α1 1.6 mg twice weekly for 6 months in Chinese patients showed a complete response (ALT normalization and undetectable HBV DNA by PCR assay) in 11 (42.3 %) of 26 patients at 6 months after the end of treatment [97]. The main advantages of thymosin α1 are the fixed duration of treatment and minimal side effects. However, the number of patients included in thymosin α1 trials was far smaller in comparison with recent trials using Peg-IFN or nucleoside analogues. More well-designed large-scale studies are needed to confirm its efficacy.
Immunomodulating agents: overall conclusions
A finite duration of IFN-based treatment results in increased sustained virological, biochemical, and serological response, improvement of liver histology, prevention or reduction of liver disease progression, and higher overall and hepatic complication-free survival rates. Peg-IFN will eventually replace conventional IFN because of a better pharmacokinetic profile, more convenient once weekly dosing and superior efficacy. HBeAg seroconversion and HBsAg seroclearance can increase over time during post-IFN treatment follow-up. IFN-based therapy is as effective as or even better in patients with well-compensated cirrhosis than in patients without cirrhosis and has comparable side effects. IFN is contraindicated in patients with decompensated cirrhosis. Baseline and on-treatment response predictors may be potential tools to optimize and improve IFN-based treatment in the future. Future studies to identify how to optimize treatment strategy according to on-treatment predictors are needed. Thymosin α1 is effective in the treatment of HBeAg-positive and HBeAg-negative chronic hepatitis B patients, with a significantly increasing virological response over time after the end of therapy.
Nucleos(t)ide analogs (nucs)
LAM, adefovir dipivoxil, entecavir, and telbivudine have been approved in all Asia–Pacific countries. Tenofovir disoproxil fumarate has been approved in some countries and awaiting approval in others. Clevudine has been approved in Korea and the Philippines, while its development has been stopped in other countries due to myopathy.
Nucs can be structurally grouped as L-nucleoside, acyclic nucleotide phosphonate, or D-cyclopentanes, which reflect their patterns of antiviral drug resistance (Table 3). For example, resistance to L-nucleosides is mainly associated with HBV codon substitutions at rtM204V/I and occasionally at rtA181T; resistance to acyclic phosphonate is associated with HBV codon substitutions at rtA181T/V and/or at rtN236T; and resistance to the D-cyclopentane group in association with a change at rTI169 or rtT184 or rtS202 or rM250V in combination with rtL180M plus rtM204I/V. The rtA181T is a multi-drug resistance change affecting both the L-nucleoside and acyclic phosphonate nucleotide groups (Table 3).
L-Nucleoside analogues
Lamivudine
LAM, an L-nucleoside analogue, at a daily dose of 100 mg, is effective in HBV suppression, ALT normalization, and histologic improvement.
HBeAg-positive patients: In the Asian LAM trial and a multi-center trial in China, HBeAg seroconversion was achieved in approximately 44–47 % after 4–5 years of LAM therapy [98, 99]. The rate of HBeAg seroconversion is proportional to the pre-treatment ALT level and is highest among patients with ALT over 5 times the ULN [100]. Children treated with LAM for 1 year with dosages adjusted for body weight (3 mg/kg) achieved similar efficacy and safety to that in adults [101]. Sustained HBeAg seroconversion was documented in ~80 % of patients after cessation of LAM therapy in earlier studies, but the relapse rate was much higher if HBeAg-negative HBV reactivations were counted. In the largest study thus far, the relapse (defined as HBV DNA >140,000 copies/mL) rate after 12 months consolidation was 8.7 % in 5 years, in contrast to 61.9 % in those with consolidation therapy <12 months [102]. The relapse (HBV DNA level >104 copies/mL) rate was 54 % in 1 year and 68 % in 2 years in a Taiwanese study [103]. In a study involving 71 Taiwanese patients who had achieved HBeAg seroconversion with undetectable HBV DNA (<300 copies/mL) and stopped LAM therapy after 6–15 months consolidation therapy, 19 patients (27 %) encountered clinical relapse (20 % HBeAg-negative relapse, 7 % HBeAg reversion) within 6–15 months after therapy [104]. The relapse rate was significantly higher in patients with genotype C HBV infection [odds ratio 5.92 (1.6–21.7) vs. genotype B] in one earlier study involving a total of 82 patients [105], but no difference was found in a more recent study using a more stringent stopping rule [104]. In pediatric patients, the durability of HBeAg seroconversion increased from 82 % to more than 90 % in those who had received LAM for 52 weeks and more than 2 years, respectively [101]. It seems appropriate to conclude that LAM can be stopped after HBeAg seroconversion, provided that HBV DNA is undetectable by PCR assay and consolidation therapy has been administered for at least 12 months [102, 104]. After stopping therapy, close monitoring of the patients is mandatory.
HBeAg-negative patients: A randomized controlled trial in Hong Kong and China among 89 HBeAg-negative chronic hepatitis B patients showed that 2-year LAM treatment resulted in a maintained complete response (HBV DNA <2,000 IU/mL and normal ALT) rate of 56 % at 2 years, and 26 % achieved a sustained response [a sustained response rate of 36/56 (46.4 %)] 6 months after stopping LAM [106]. Studies among Chinese patients (genotype C dominant) who stopped LAM treatment after a minimum of 24 months with at least 3 HBV DNA undetectable results 6 months apart showed a post-treatment relapse (HBV DNA ≥104 copies/mL) rate of 37–50 % at 1 year [107, 108]. In a study involving 85 Taiwanese patients (genotype B 73 %) with pre-therapy ALT >5 × ULN, 81 % achieved maintained virologic response (HBV DNA <105 copies/mL and normal ALT) during 6–12 months LAM therapy, and 39 % achieved sustained virologic response [a sustained response rate of 33/69 (48 %)] at 1-year post-therapy [109]. Overall, the relapse rate in those who had a consolidation therapy >12 months was up to 50 % at 1-year post-therapy.
A recent study from Hong Kong including 53 HBeAg-negative patients treated with LAM for a mean of 34 (12–76) months and who stopped LAM therapy for 47 ± 35 months showed that both end-of-treatment HBsAg ≤100 IU/mL and reduction by >1 log from the baseline were associated with sustained response (HBV DNA ≤200 IU/mL) of 78 % at 1 year, with an NPV of 96 % [110].
LAM is well tolerated, even in patients with decompensated cirrhosis and in pediatric patients. Long-term therapy in viremic patients with advanced fibrosis or early cirrhosis delays clinical progression by reducing the rate of hepatic decompensation and HCC development, even in patients with low or normal ALT levels [111]. However, after 6–9 months of LAM therapy, viral breakthrough may occur due to the emergence of drug resistance. The key LAM-resistant mutant is at the YMDD locus in the catalytic domain of the HBV polymerase gene (rtM204I/V ± rtL180M). Another LAM-resistant mutation, rtA181T/V, has also been reported. Compensatory codon substitutions that may increase viral replication may also be found, such as rtL80V/I, rtI169T, rtV173L, rtT184S/G, rtS202I, and rtQ215S [112]. The incidence of rtM204 V/I substitution increased from 24 % in 1 year to 70 % in 5 years [99, 113]. The substitutions of rtM204I/V do not confer cross-resistance to adefovir or tenofovir, but do so to entecavir. LAM resistance is associated with virological breakthrough, biochemical breakthrough, and sometimes hepatic decompensation [114]. Development of drug resistance may also reverse the histologic benefit and diminish the benefit in reducing disease progression among patients with advanced fibrosis and early cirrhosis [111]. Higher body mass index, male gender, and higher baseline HBV DNA are independent baseline predictors of LAM resistance [113]. Patients who have undetectable HBV DNA at month 6 of LAM treatment have a lower risk of LAM resistance. In the GLOBE study, undetectable HBV DNA at week 24 was associated with LAM resistance in 9 and 5 % patients at 2 years among HBeAg-positive and HBeAg-negative patients, respectively [115].
Telbivudine
Telbivudine (LdT) is an L-nucleoside analogue with potent and specific anti-HBV activity. LdT 600 mg daily has been shown to have more potent HBV suppression than LAM and ADV [116, 117]. In the phase III international trial of LdT versus LAM (GLOBE study), 55.6 % of HBeAg-positive patients and 77.5 % of HBeAg-negative patients achieved undetectable HBV DNA (<300 copies/mL), and 29.6 % of patients had HBeAg seroconversion after 2 years of LdT treatment [115]. Two other randomized studies have confirmed an HBeAg seroconversion rate of 25–28 % after 1 year treatment with LdT [116, 117]. Among the 39 patients who achieved HBeAg seroconversion and off LdT per protocol (treatment >52 weeks, HBeAg-negative >24 weeks, and HBV DNA <5 log10 copies/mL) in the GLOBE study, 80 % had sustained HBeAg seroconversion, 66 % had HBV DNA <2,000 IU/mL, and 29 % had undetectable HBV DNA after a median follow-up of 29 weeks [115]. After excluding patients who had drug resistance at year 2 in the GLOBE study, continuation of LdT till year 3 was associated with undetectable HBV DNA in 76 % of HBeAg-positive and 86 % of HBeAg-negative patients, HBeAg seroconversion in 37 % of HBeAg-positive patients, and HBsAg loss in 1.6 % of HBeAg-positive patients, but no HBsAg loss in HBeAg-negative patients [118]. Rapid reduction of serum HBsAg (>1 log decline in year 1) during LdT therapy was associated with a higher chance of HBsAg clearance at year 3 [119]. In another small study in China (N = 17), serum HBsAg <100 IU/mL at the end of 2-year LdT treatment was associated with sustained HBeAg seroconversion with undetectable HBV DNA up to 2-year post-treatment (sensitivity 75 %, specificity 100 %) [120].
LdT is generally well tolerated, even in patients with decompensated liver cirrhosis. In the 2-year report of the GLOBE study, increase in creatine kinase levels was observed more frequently in recipients of LdT, of whom 12.9 % (vs. 4.1 % in LAM-treated controls) had grade 3 or 4 elevation (>7 × ULN). The majority of grade 3 or 4 creatine kinase elevations decreased spontaneously to grade 2 or lower during continued treatment; they did not correlate with musculoskeletal side effects, and no cases of rhabdomyolysis were reported. Symptomatic myopathy was reported in two patients, and in both, resolved after stopping LdT [115].
The commonest LdT-resistant substitution is rtM204I, and rtA181T/V also confers resistance to LdT [112]. The 2-year risk of LdT resistance was 25.1 % in HBeAg-positive patients and 10.8 % in HBeAg-negative patients [115]. The risk of drug resistance was lower than that with LAM in both the international (GLOBE) and China phase III studies [115, 116]. In the subgroup that had no genotypic resistance at year 2 and received LdT up to year 3, an incremental 1.0 % HBeAg-positive and 2.1 % HBeAg-negative patients developed genotypic resistance to LdT [118]. Early viral suppression with undetectable HBV DNA at week 24 was associated with improved clinical outcome and lower risk of drug resistance [115]. Among HBeAg-positive patients with favorable baseline factors (ALT > 2 × ULN and HBV DNA < 9 log copies/mL), 71 % have undetectable HBV DNA at week 24, and 89, 52, 81, and 1.8 % of these patients will have undetectable HBV DNA, HBeAg seroconversion, normal ALT, and drug resistance at the end of 2 years’ LdT treatment, respectively. Among HBeAg-negative patients with favorable baseline factors (HBV DNA <7 log copies/mL), 95 % have undetectable HBV DNA at week 24, and 91, 83, and 2 % of them will have undetectable HBV DNA, normal ALT, and drug resistance at the end of 2 years’ LdT treatment, respectively [121].
Acyclic nucleotide phosphonates
Adefovir dipivoxil
Adefovir dipivoxil (ADV) is an acyclic adenine nucleotide analogue and a potent inhibitor of HBV reverse transcriptase. ADV 10 mg daily for 48 weeks has been shown to effectively suppress HBV DNA replication, normalize ALT, and improve liver histology in two large, international multicenter double-blinded, placebo-controlled studies.
In HBeAg-positive patients, HBeAg seroconversion can be achieved in 30–37 % after 3–5 years of ADV treatment [122, 123]. In HBeAg-negative patients, 67 % of patients had HBV DNA <200 IU/mL after 240-week treatment with ADV [124]. There was no difference in the response to ADV across different HBV genotypes or ethnic groups. Long-term ADV therapy with HBV suppression was associated with fibrosis regression in 73 % of patients [124].
The safety profile of 10 mg ADV daily was similar to placebo in patients with compensated chronic hepatitis B. Renal laboratory abnormalities reported with 30 mg daily ADV were not observed with the 10 mg dosage during the 1-year study period. Reversible increase in serum creatinine of more than 0.5 mg/dL (maximum 1.5 mg/dL) was reported in up to 3 % of patients when the therapy was extended to 5 years [122, 124]. In patients with decompensated chronic hepatitis B, the rate of serum creatinine increase by more than 0.5 mg/dL among patients treated with ADV was up to 24 %, similar to a rate of 17 % in patients treated with entecavir in a randomized control trial [125].
The primary drug resistance mutations against ADV are rtA181 V/T at domain B and rtN236T at domain D of the HBV polymerase gene. The substitution rtA181T is associated with a stop codon substitution at the S gene (sW172*), which leads to intracellular HBV retention [126]. Hence, patients with rtA181T substitution may not have the classical virological breakthrough as patients with LAM resistance. The cumulative incidence of genotypic resistance to ADV was 0, 3, 11, 18, and 29 % at the end of each successive year of therapy in HBeAg-negative patients [124]. HBV with substitutions rtA181T/V has partial cross-resistance to tenofovir, LAM, and LdT but remains sensitive to entecavir. The substitution rtN236T has partial cross-resistance to tenofovir, but it is sensitive to LAM, LdT, and entecavir [112]. HBV DNA <200 IU/mL at week 48 can predict a lower risk of ADV resistance (6 %, vs. 49 % of those with HBV DNA >200 IU/mL) during 192 weeks of ADV treatment in HBeAg-negative patients [124].
ADV is effective in suppressing HBV DNA in patients with rtM204I/V HBV substitution. Switching to ADV monotherapy is associated with a high risk of ADV resistance among patients with LAM resistance. In a Korean report among 320 LAM-resistant patients switching to ADV monotherapy, the 5-year cumulative probability of ADV resistance was 65.6 % [127]. Add-on ADV therapy for LAM resistance resulted in better HBV DNA suppression (undetectable HBV DNA: 82–87 %) and lower risk of ADV resistance (4–8 %) in 3–4 years [128]. Add-on ADV in patients with HBV DNA >107 copies/mL (>200,000 IU/mL) is associated with insufficient virologic response [129]. In a small report from China, stopping ADV among LAM-resistant patients was associated with a high risk of virological relapse (80 % in 1 year), even after achieving HBeAg seroconversion with good HBV DNA suppression [130].
Tenofovir disoproxil fumarate
Tenofovir disoproxil fumarate (TDF) is an acyclic adenine nucleotide analogue effective for both HBV and HIV. In a phase III randomized trial, TDF 300 mg daily has been shown to have superior HBV DNA suppression than ADV 10 mg daily in both HBeAg-positive and HBeAg-negative patients [131]. TDF treatment for 3 years was associated with 72 % undetectable HBV DNA (<400 copies/mL) and 26 % HBeAg seroconversion in HBeAg-positive patients, and 87 % undetectable HBV DNA in HBeAg-negative patients. HBsAg clearance developed in 8 % of HBeAg-positive patients, but they were all non-Asians infected by genotype A, D, and F HBV. No HBsAg clearance was observed in HBeAg-negative patients [132]. TDF treatment for 5 years was associated with sustained viral suppression (2.8 % remained viremic) and significant regression of fibrosis (44 %)/cirrhosis (76 %), whereas no resistance to TDF was detected (Marcellin P, et al. Hepatology 2011;54: 480A and 1011A).
TDF is generally well tolerated, even in patients with decompensated liver disease [133]. No case of lactic acidosis has been reported. Over 3 years of TDF treatment, only 1 (of 641) patient had mild renal impairment, which resolved after dosage reduction to every other day [132]. A comparative study has shown that TDF therapy of HBV mono-infection results in a yearly median change of estimated glomerular filtration rate (GFR) of −0.92 mL/min, similar to the −0.92 mL/min with LAM, −1.02 mL/min with ADV, −1.00 mL/min with entecavir therapy, and lower than the −2.05 mL/min in untreated HBV patients and −2.64 mL/min with TDF therapy in HIV-HBV patients [134]. Decrease in bone mineral density and osteomalacia have been reported among patients with HIV infection treated with TDF-containing antiretroviral regimens, but similar bone problems have not been reported in HBV mono-infected patients [135]. Although the coexistence of several factors related to disease and adverse drug effects make it difficult to directly apply the experience of TDF in HIV to that of HBV mono-infected patients, close observation on proximal tubular injury and bone toxicity must be maintained.
No TDF resistance has been reported during treatments of up to 3 years [132]. TDF is highly effective in the treatment of patients with rtM204I/V HBV substitutions, but rtA181 V/T and rtN236T confer intermediate resistance to TDF [112]. Among patients who have A181T/V and/or rtN236T substitution, viral suppression by TDF is reduced [136, 137]. In a German study of 113 patients who had experienced failure of previous LAM and/or ADV therapy and then switched to TDF treatment for a median of 23 months, the probability of undetectable HBV DNA (<400 copies/mL) was 52 % for those with ADV resistance versus 100 % for those without ADV resistance, and patients with a baseline HBV DNA >7 log10 copies/mL had the lowest response [136]. Other studies have confirmed that switching to TDF is more effective than switching to entecavir in patients with prior failure of or resistance to LAM and ADV, but the virological responses were often suboptimal [137, 138]. A recent study further showed that rescue therapy with ETV plus TDF achieved undetectable HBV DNA (<80 IU/mL) after a median of 6 months in 51 (89.5 %) of 57 patients, in whom Nuc therapy (LAM + ADV, ETV + ADV, TDF + LAM) had failed and who had multi-drug resistance rtA181V/T or other multiple drug resistance mutations [139].
D-cyclopentanes
Entecavir
Entecavir (ETV) is a cyclopentyl guanosine analogue with potent selective inhibition of the priming, DNA-dependent synthesis and reverse transcription functions of HBV polymerase. ETV 0.5 mg daily has been shown to have greater HBV DNA suppression than LAM and ADV, with HBV DNA becoming undetectable (<300 copies/mL) in 60–71 % of HBeAg-positive patients and 88–90 % of HBeAg-negative patients at weeks 48–52. In the 5-year report of an international trial among HBeAg-positive patients, who switched from ETV 0.5 mg daily to 1 mg daily since year 3, the cumulative probability of HBV DNA <300 copies/mL was 89, 91, and 94 % in years 3, 4, and 5, respectively. In addition to a HBeAg seroconversion rate of 31 % by year 2, the HBeAg seroconversion rate in 141 HBeAg-positive patients was 23 % from weeks 96–240 [140]. This result was confirmed by other studies among patients treated with ETV 0.5 mg daily, with 83–90 % patients having undetectable HBV DNA, and 24–44 % patients having HBeAg seroconversion at year 3 of treatment [141–143]. HBsAg seroclearance occurs in 0–1.4 % of HBeAg-positive patients after 3–5 years of ETV treatment [140–142]. Continuous HBV DNA suppression by ETV was associated with improvement of hepatic necroinflammation and fibrosis [144]. A recent large study showed that there was a significant overall decline in HBsAg level from baseline to year 1 to year 2, but only 30 % of the patients had a decline >0.5 log10 IU/mL. It was also found that baseline HBsAg level and decline >0.5 log10 IU/mL at week 12 or 24 were not predictive of HBeAg seroconversion at 2 years [145].
Among HBeAg-negative patients who discontinued ETV therapy after achieving undetectable HBV DNA levels had been documented on 3 occasions each 6 months apart [1], 47 % of 61 patients did not experience relapse during 12 months off-ETV therapy (Jeng WJ, et al. Hepatology 2011;54:S1014A abst 1379). Approximately 21 % of patients will have partial virologic response to ETV 0.5 mg daily, defined as >1 log decline from baseline but a detectable HBV DNA at week 48 of treatment. On continuation of ETV for 2–3 years without adaptation, 81 % of the partial virologic responders achieved undetectable HBV DNA, and none developed ETV resistance. Hence, treatment adaptation is generally not required for partial virologic response to ETV [142].
ETV is well tolerated. In decompensated patients, a German study showed that 5 of 16 patients with a model of end-stage liver disease (MELD) score >22 developed lactic acidosis and 1 died [146]. In a multicenter study with 93 patients with cirrhosis with Child’s class B or C, one patient with a MELD score of 21 developed lactic acidosis, which resolved spontaneously [125]. No lactic acidosis was reported in a Korean cohort of 70 patients with decompensated cirrhosis and another Hong Kong cohort of 36 patients with severe acute exacerbation of chronic hepatitis B [147, 148].
ETV has a high genetic barrier of resistance. Drug resistance requires at least 3 codon substitutions, including rtL180 M, rtM204I/V, plus a substitution at one of the following amino acids: rtT184S/G, rtS202I/G, and/or rtM250 V [112]. Among treatment-naïve patients, ETV resistance is very rare. In the long-term follow-up of an international trial on HBeAg-positive and HBeAg-negative patients, the cumulative probability of ETV resistance was 1.2 % after 5 years of ETV treatment [149]. This is confirmed by studies in Japan and Hong Kong, where ETV resistance was detected in 0.8–3.3 % of patients who received ETV for 2–3 years [143, 145].
ETV is effective in the treatment of ADV and TDF resistance. Switching to ETV monotherapy (1 mg daily) is initially effective in LAM-resistant patients (rtM204I/V), but the subsequent risk of ETV resistance is high. The presence of rtM204I/V and rtl180 M reduces the genetic barrier to ETV and resulted in a cumulative genotypic resistance and virological breakthrough of 51 and 43 % at year 5, respectively [149].
Other direct antiviral agents
Clevudine is an L-nucleoside pyrimidine analogue with potent antiviral activity against HBV. Clevudine 30 mg daily for 24 weeks has been shown to be associated with 59 % undetectable HBV DNA (<300 copies/mL) and 7.6 % HBeAg seroconversion in HBeAg-positive patients; with 92 % undetectable HBV DNA in HBeAg-negative patients. The virologic relapse was slow and gradual within 24 weeks of stopping treatment. Mutations at rtA181T could be detected in 2.7 % of HBeAg-positive patients at week 24. Both rtM204I/V and rtA181 V/T mutations confer resistance to clevudine. The global development of clevudine was terminated in 2009 because of case reports of serious myopathy related to myonecrosis [150].
LB80830 is a new acyclic nucleotide phosphonate with chemistry similar to ADV and TDF. In a phase II, open-label, multicenter study among 65 LAM-resistant patients, a dose-dependent reduction in HBV DNA was observed up to −3.92 log copies/mL at the optimal dose of 150 mg daily at week 12 [151]. No significant adverse event was observed. Further clinical trials are warranted to confirm the efficacy and safety of this drug.
De novo combination treatment of direct antiviral agents
In a phase II study, combination of LdT and LAM was found to be inferior to LdT monotherapy in terms of HBV DNA suppression and risk of drug resistance. In another study comparing combination of LAM and ADV versus LAM monotherapy for 2 years, combination therapy was associated with a lower risk of LAM resistance, but similar HBV DNA suppression. However, LAM resistance still developed in 15 % of patients who received combination LAM and ADV therapy [152]. A most recent European study involving 78 Nuc-experienced patients has shown that combination of TDF and emtricitabine (FTC) achieved undetectable HBV DNA at week 96 in >94 % of the patients [153]. So far, there is no evidence that combination of two direct antiviral agents results in better viral suppression as compared to a single agent.
Therapy with nucs: overall conclusions
The cross-trial comparisons of antiviral efficacy in randomized trials are presented in Table 4. ETV and TDF have superior viral suppression and drug-resistance profiles compared to LAM or ADV. Although LdT has similar viral suppression as compared to ETV and TDF, it has significantly higher risk of drug resistance. Continuous viral suppression is associated with histologic improvement and regression of liver fibrosis and cirrhosis. Increased antiviral potency is not correlated with a higher chance of HBeAg seroconversion or HBsAg clearance. On-treatment monitoring and adaptation of the drug regimen is recommended if LAM, ADV, or LdT is used to reduce the risk of developing drug resistance. Cohort studies have shown that up to 40 % virological breakthroughs are not associated with drug resistance and are likely due to the lack of medication adherence [154]. Rescue therapy using nuc(s) without cross-resistance (Table 3) should be administered as soon as genotypic drug resistance is confirmed. The indications of drug therapy in patients with renal insufficiency or failure are similar to those in ordinary patients. Since all of the currently available nucs are excreted unchanged in the urine, the nuc dose should be adapted in patients with GFR <50 mL/min: half dose daily or full dose on alternate day if GFR is 30–49 mL/min; one dose every 3 days if GFR is 10–29 mL/min; one dose a week after dialysis [155]. Stopping treatment among HBeAg-positive patients can be considered if HBeAg seroconversion with undetectable HBV DNA by PCR persists for more than 12 months. Stopping treatment in HBeAg-negative patients after demonstration of undetectable HBV DNA >12 months results in a relapse rate of 50 % at 1-year post-therapy. HBsAg clearance is a remote ideal endpoint to stop antiviral agents, and quantitative HBsAg will be a potential marker to guide treatment cessation. Since most patients on nuc treatment require long-term therapy, drug resistance is a great concern. In choosing a direct antiviral agent to initiate therapy, antiviral potency, resistance profile, and drug cost should be considered [5]. In general, the first-line therapy should be either ETV or TDF, and the second-line therapy should be LdT, ADV, and LAM. Nonetheless, pharmacoeconomic studies will be helpful in individual countries in the Asia–Pacific region, because cost is one of the most important factors in the choice of drug for initial therapy [5].
Special groups of patients
Pregnancy
When women in the childbearing age require antiviral therapy, the issue of pregnancy must be discussed before starting treatment. For anti-HBV therapy, IFN-based therapy is preferable, and pregnancy is discouraged during IFN therapy. In pregnant women with chronic HBV infection who need antiviral therapy, the liver disease stage of the mother and potential benefit of treatment must be weighed against the risk to the fetus. IFN-based therapy is contraindicated because of its antiproliferative effect; the only choice is a nuc with small risk to the fetus. Among the nucs, LdT and TDF are classified as category B drugs (no risk in animal studies, but unknown in humans), whereas LAM, ADV, and ETV are classified as category C drugs (teratogenic in animals, but unknown in humans) by the US FDA [156].
For prevention of vertical transmission, a meta-analysis of randomized controlled trials (238 LAM-treated and 232 untreated patients) showed a 13.0–23.7 % lower incidence of intrauterine infection, indicated by newborn HBsAg (OR: 0.38, 95 % CI: 0.15–0.94; p = 0.04) and HBV DNA (OR: 0.22, 95 % CI: 0.12–0.40; four RCTs, p < 0.001) seropositivity. The LAM-treated group showed a 1.4–2.0 % mother-to-child transmission rate as assessed at 9–12 months, indicated by infant HBsAg (OR: 0.31, 95 % CI: 0.15–0.63; four RCTs, p < 0.01) and HBV DNA (OR: 0.20, 95 % CI: 0.10–0.39; two RCTs, p < 0.001) seropositivity. No significant higher adverse effects or complications in pregnancy were observed [157]. In a more recent prospective and open-label study conducted in China, 229 mothers with HBeAg positivity and HBV DNA >107 copies/mL received LdT 600 mg daily from week 20 to week 32 of gestation (n = 135) or served as untreated controls (n = 94). Forty-three (33 %) of the treated mothers and none of the untreated controls had undetectable HBV DNA (<500 copies/mL) at delivery. With standard HBV vaccination and hepatitis B immunoglobulin (HBIg), the incidence of perinatal transmission was lower in the infants born to the treated mothers than to the controls (0 vs. 8 %, p = 0.002). No serious adverse events were noted in the treated mothers or their infants [158]. According to an antiretroviral pregnancy registry, TDF therapy in 942 pregnant women (including 606 in the first trimester) resulted in a rate of birth defects similar to the background rate [159]. It was also shown that TDF was superior to LAM in the prevention of mother to child transmission (Lawler J, et al. Hepatology 2011;54:S892A, abst 1117). These studies have shown that antiviral therapy administered in late pregnancy may further reduce the risk of perinatal HBV infection from highly viremic mothers, as compared with passive-active immunization alone. However, the extent of benefit, the threshold of serum HBV DNA level for initiating therapy, the optimal time to start therapy, the appropriate choice of antiviral agent, and the optimal duration of therapy have not been determined.
Patients with concurrent HCV, HDV, or HIV infection
Patients with concurrent HCV, HDV, or HIV infections tend to have a higher incidence of cirrhosis, HCC, and mortality. Insufficient data exist to reach firm conclusions on the management of patients with HCV and/or HDV infections. However, it is generally agreed that the dominant virus should be identified before designing therapeutic strategy. If HBV is dominant, treatment should be aimed toward this virus. If HCV is dominant, Peg-IFN therapy in combination with ribavirin can achieve a sustained HCV clearance rate comparable to that in HCV mono-infection. This has been demonstrated in an open-label, comparative, multicenter study involving 321 Taiwanese patients with active HCV infection, in which patients with HCV genotype 1 infection received Peg-IFN-α2a 180 μg weekly and ribavirin (1,000–1,200 mg) daily for 48 weeks. Patients with HCV genotypes 2 or 3 received Peg-IFN-α2a 180 μg weekly and ribavirin 800 mg daily for 24 weeks. The sustained virologic response in HCV genotype 1-infected patients was comparable between 161 HBV + HCV patients and 160 HCV mono-infection patients (72.2 vs. 77.3 %). For patients with HCV genotype 2/3 infections, the sustained virologic response values were 82.8 and 84.0 %, respectively [160].
LAM is ineffective in patients with chronic HDV infection. Small randomized controlled trials using 3–9 MU IFN for 3–24 months showed a biochemical and virologic response in up to 70 % of patients with chronic HDV infection. Higher doses of IFN (9 MU thrice weekly) for 12 months have been found to inhibit HDV-RNA, normalize ALT, and improve histology in patients with chronic HDV infection. ALT response was sustained in 50 % of patients, and the long-term outcomes and survival improved significantly even in patients with liver cirrhosis [161]. A recent randomized trial using Peg-IFN-α2a with or without ADV combination or ADV monotherapy was conducted in 90 patients with chronic HDV infection. End-of-treatment serum HDV RNA negativity rate was 23, 24, and 0 % in Peg-IFN + ADV, Peg-IFN, and ADV groups, respectively, but the virologic response was sustained in only 28, 28, and 0 % at the end of 24 weeks follow-up, respectively [162].
In patients with concurrent HIV and HBV infection, the accepted threshold for initiation of HBV therapy in HIV-HBV coinfected individuals is HBV DNA >2,000 IU/mL [163]. In patients with concurrent HIV infection and CD4+ counts of more than 500 cells/μL, treatment options include agents without anti-HIV activity: IFN, ADV, and LdT. IFN-based therapy or ADV is preferred because of the absence of resistance in the former and a low resistance profile in the latter. In practice, TDF is always the treatment of choice, and thus a regimen of TDF, FTC, and a third active antiretroviral drug should be proposed to prevent the selection of HIV-resistant mutants. Both LAM and TDF are active against both HBV and HIV and can be used in combination as part of highly active antiretroviral therapy (HAART) in patients who need both anti-HBV and anti-HIV therapies. HBV-active HAART should contain TDF in all individuals (provided there is no TDF contraindication), and this is usually co-prescribed with FTC as Truvada or with LAM. TDF in HIV-HBV coinfected individuals has been shown to result in high rates of HBV DNA suppression (>90 %), HBeAg loss (46 %), and HBsAg loss (12 %) in HBeAg-positive patients after 5 years of treatment, without evidence of resistance [164]. If TDF-associated renal toxicity occurs, the dose of TDF should be adjusted according to renal clearance. If TDF is absolutely contraindicated, then there is little data on the best alternative treatment. In patients with low CD4 count and active liver disease, HBV should be treated first to avoid the risk of immune reconstitution syndrome that usually occurs with HIV treatment.
Patients with hepatic decompensation
Patients with acute hepatitis B, chronic hepatitis B, or HBV-cirrhosis who develop hepatic decompensation should be treated immediately, because it may both improve their clinical status and even remove them from liver transplant lists. Interferons are generally contraindicated in patients with Child B or C cirrhosis, because significant adverse effects due to serious bacterial infections and possible exacerbation of liver disease occur even with low doses.
LAM is well tolerated and results in clinical improvement or stabilization, especially in patients who have completed a minimum of 6 months’ treatment [165, 166]. Early treatment is recommended to improve outcomes. Selection of resistant mutants with resultant biochemical dysfunction, reduction in efficacy, and rapid clinical deterioration in this group of patients is a real concern with early treatment.
Other nucs have also been evaluated in several studies. A Korean study in 70 patients with decompensated liver cirrhosis showed that 55 (78.5 %) ETV-treated patients survived >1 year and had improved Child-Turcotte-Pugh (CTP) and MELD scores; 36 (66 %) of them achieved CTP class A and 49 % showed improvement in the CTP score by >2 points [147]. A multinational, multicentered, randomized, open-label comparative study of ETV 1 mg/day versus ADV 10 mg/day for up to 96 weeks was conducted in 199 patients with hepatic decompensation (CTP scores ≧7). ETV demonstrated significant superiority to ADV for mean reduction in serum HBV DNA and greater reduction of MELD score (−2.6 vs. −1.7) at week 48 [125]. In a phase 2, double-blind study of 112 patients with chronic hepatitis B and decompensated liver disease randomized to receive either TDF (n = 45), emtricitabine (FTC)/TDF (fixed-dose combination; n = 45), or ETV (n = 22), CTP and MELD scores improved in all groups [133]. A randomized control trial comparing LdT and LAM therapy for 104 weeks in patients with decompensated cirrhosis showed that LdT had better response in all aspects than LAM (Gane EJ, et al. Abstract J Hepatol 2010;52 Suppl 1:S4). All studies consistently show that the earlier the therapy starts, the better the prognosis. Since patients with severe decompensated liver diseases are at risk of renal dysfunction or lactic acidosis, and patients with a MELD score >21 may develop lactic acidosis during ETV therapy, close monitoring of renal function and lactic acidosis is required in this group of patients.
Pediatric patients
Children with elevated ALT levels respond to IFN and LAM in a similar manner to adults. A recent pediatric liver specialists meeting report concluded that LAM may be used starting at 3 years of age, ADV is approved for those aged 12 years and older, and ETV for age 16 years and older. IFN-α is approved for use in children as young as 12 months of age [167].
Newer agents such as Peg-IFN and TDF have not yet been studied, but are likely to be as effective in children as in adults with chronic HBV infection. Long-term safety and drug resistance are more important concerns in children than in adults. A long-term follow-up study showed that IFN therapy provided little benefit in comparison with untreated children [168]. Therefore, drug therapy is usually not recommended in pediatric patients because of the apparent lack of long-term benefits and the attending risks of starting drug therapy, unless there is an absolute indication such as in the setting of ensuing or overt hepatic decompensation [169], or in those who have evidence of severe liver disease or advanced fibrosis/cirrhosis.
Patients with drug resistance
Patients treated with low genetic barrier nuc(s) may experience drug resistance even in the first year of therapy. Once drug resistance is confirmed in patients with virologic breakthrough, rescue therapy using nuc(s) without cross-resistance (Table 3) should be administered as soon as possible and before HBV DNA increases over 2 × 106 IU/mL [129]. A randomized control trial showed that Peg-IFN-α2a therapy was effective in patients with LAM resistance [170].
Patients undergoing immunosuppression or chemotherapy
Reactivation of HBV replication with decompensation has been reported in 20–50 % of patients with chronic HBV infection undergoing cancer chemotherapy or immunosuppressive therapy, especially in those receiving high-dose steroid regimen. Reactivation commonly occurs after the first 2–3 cycles of chemotherapy. High viral load at baseline is the most important risk factor for HBV reactivation [171]. Following transarterial chemoembolization in patients with HCC, HBV reactivation was also observed in 30 % of patients, and HBV DNA >2,000 IU/mL was a risk factor; increasing intensity of therapy was associated with increasing risk and severity of HBV reactivation [172].
LAM is effective in the treatment of HBV reactivation in HBsAg-positive organ transplantation recipients and cancer patients undergoing chemotherapy, particularly, if it is used preemptively. A number of meta-analyses have confirmed that preemptive LAM therapy reduces reactivation of HBV with a risk reduction estimated to be between 79 and 89 %. One meta-analysis of 21 studies (324 LAM-treated and 599 untreated patients), two of which were randomized controlled trials, showed significant reduction of clinical and virological reactivation in the LAM group (OR: 0.09; 95 % CI: 0.05–0.15 and OR: 0.04, 95 % CI: 0.01–0.14, respectively). All-cause mortality was significantly reduced in the LAM group (OR: 0.36, 95 % CI: 0.23–0.56) [173]. These studies indicate that prophylactic use of LAM within 1 week before the start of chemotherapy and continued for at least 24 weeks after the end of chemotherapy when the white blood cell count has normalized, can reduce HBV reactivation frequency and severity of flares and improve survival [171]. Of note is that both ETV and TDF are more attractive candidates given their high potency and extremely low resistance rates. A recent study did show that ETV was more effective than LAM in preventing hepatitis B reactivation (0 % of 34 vs. 12.4 % of 89; p = 0.024) in lymphoma patients receiving chemotherapy [174]. Further randomized studies using these drugs for prophylaxis in the setting of chemotherapy are awaited.
The impact of immunosuppressive therapy on patients with occult HBV infection is poorly characterized. In a study involving 244 consecutive HBsAg-negative lymphoma patients who received chemotherapy, 8 (3.3 %) developed de novo HBV-related hepatitis and 3 developed fulminant hepatic failure, following a 100-fold increase in serum HBV DNA levels. These patients responded to LAM, but one died of hepatic failure. These findings suggest that even in an HBV endemic area, the occurrence of de novo HBV-related hepatitis after chemotherapy is low [175]. It was suggested that HBsAg-negative, anti-HBc-positive patients, especially those receiving biologic agents such as rituximab or etanercept plus steroid-containing regimens, should be closely monitored to facilitate early commencement of nuc therapy [171]. Abatacept, a soluble fusion protein that links to CTL antigen-4, was recently reported to be associated with HBV reactivation in a HBsAg-negative, anti-HBc and anti-HBs-positive patient [176].
Immunosuppression in solid organ transplantation other than the liver in HBsAg-positive recipients is associated with more frequent and more rapid liver disease progression, and may occasionally be associated with fibrosing cholestatic hepatitis. It was therefore recommended that all HBsAg-positive transplant recipients receive nuc to maintain HBV suppression, preferably using ETV or TDF [177].
Liver transplantation for chronic HBV infection
Liver transplantation has become a cost-effective treatment of liver failure and HCC with excellent 5-year survival. Improving economics and live related liver donation have allowed a rapid expansion of liver transplantation within the Asia–Pacific region where hepatitis B is the most common indication for both acute and chronic liver failure. Acute or chronic HBV infection accounts for most cases of acute liver failure in this region, and more than 80 % of cases of chronic liver failure and HCC are caused by chronic HBV infection. Although HBV recurrence can be prevented in 60 % of cases by high-dose (10,000 U/month) intravenous hepatitis B immunoglobulin (HBIg), this therapy is prohibitively expensive (US$ 50,000 per annum, lifelong) and is less effective in transplant candidates with detectable serum HBV DNA at the time of transplant, of whom almost 40 % develop either early recurrence because of insufficient neutralizing antibody in the immediate perioperative phase, or late recurrence because of more rapid selection of surface escape mutants in the “a” determinant of the HBV pre-S/S genome. The addition of an oral nuc will suppress the circulating viral load prior to transplantation, thereby preventing early recurrence and reducing the required perioperative neutralizing dose of anti-HBs. Co-administration of nucs with HBIg following transplantation should help prevent or delay late HBV recurrence. Late recurrence does still occur in 5–10 % of patients during combination HBIg plus LAM prophylaxis due to selection of variants conferring resistance to both HBIg and LAM, which is possible because of overlapping reading frames of major catalytic regions of the HBV polymerase gene and neutralization domains of the surface gene.
Combination LAM/HBIg prophylaxis reduces recurrence rates of HBV infection and is associated with 5-year patient and graft survival rates of 85 and 80 %, respectively. A meta-analysis involving two prospective and four retrospective studies has shown that the risk reduction (odds ratio) in HBV recurrence with HBIg and LAM treatment (n = 193) versus HBIg treatment (n = 124) was 0.08 (95 % CI: 0.03–0.21). The odds ratios showing HBV-related death and all-cause mortality reduction, assessed in three studies, with HBIg and LAM versus HBIg alone were 0.08 (95 % CI: 0.02–0.33) and 0.02 (95 % CI: 0.06–0.82), respectively [178]. A long-term (median, 62 months) follow-up study involving 147 patients has shown that LAM plus low-dose intramuscular HBIg (400–800 U daily for 1 week, then monthly) appears as effective as LAM plus high-dose intravenous HBIg, but is associated with <10 % of the cost (US$ 4,000) [179]. Another study suggested that late HBIg substitution by ADV (at least 12-month post-transplant) can prevent late HBV recurrence at less cost [180]. There is emerging data that HBIg ± LAM prophylaxis can be replaced by LAM monotherapy at 12-month post-transplant in certain ‘low-risk’ patient groups. These include patients who were HBV-DNA negative (hybridization assay) before transplant or those in whom LAM therapy was started and patients with sustained protective levels of anti-HBs production following post-transplant vaccination.
Although LAM is safe and well tolerated before and after transplantation, LAM monotherapy without HBIg is associated with late post-transplant HBV recurrence from the emergence of LAM resistance. The observed HBV recurrence rate with LAM monotherapy appears higher in European series (45 %) than Asian series (20 % at 5 years; Fung J, et al. Hepatology 2011; 54:450A). This difference in recurrence rates may reflect differences in HBV genotype, or possibly a beneficial effect of adoptive immunity from live related HBV-immune donors in Asia. ADV, TDF, and ETV are available for rescue therapy for LAM resistance, and de novo use of these agents may minimize the problems of drug resistance. In a recent Hong Kong study, ETV monotherapy was used as antiviral prophylaxis in 80 patients undergoing transplantation for HBV-cirrhosis. The cumulative rate of HBsAg loss was 88 % at 12 months following liver transplantation, with 98.8 % achieving undetectable HBV DNA levels [181]. Well-designed studies are needed to determine whether prophylactic monotherapy with a potent anti-HBV agent, such as ETV or TDF, or combined with HBIg might be effective in HBV transplant patients.
Of note, less than half the patients had started nuc therapy prior to transplant and most remained viremic at the time of transplantation. In comparison, in a recent Australasian prospective open-labeled study, 62 HBsAg-positive patients received combination LAM plus ADV from the time they were put on the transplant list. This combination prevented emergence of LAM resistance and suppressed HBV DNA to undetectable levels in most patients prior to transplant. Following transplant, HBV DNA remained undetectable, and all patients had cleared serum HBsAg within 6 months [182]. These data suggest that antiviral prophylaxis with either a single potent nuc with a high genetic barrier (ETV or TDF) or a combination of nucs without cross-resistance (LAM/ADV, TDF/FTC) will provide similar safety and efficacy to current LAM plus HBIg prophylaxis, but without the cost and inconvenience of long-term monthly HBIg administration. The future use of HBIg immunoprophylaxis may become limited to perioperative use only in patients with high viral load at the time of transplant.
Adoptive immune transfer may result in de novo anti-HBs production in recipients of live related liver grafts from HBV-immune donors. A liver from an anti-HBc(+) donor carries a significant risk of de novo HBV infection if transplanted into an HBV-naïve recipient [183]. This risk becomes negligible if the recipient receives long-term prophylaxis with either LAM or HBIg, or if the recipient is positive for anti-HBs through either natural immunity from previous infection or through vaccination.
Antiviral therapy before and/or after curative or local–regional therapy of HCC
Since HCC surveillance has been widely implemented, an increasing number of patients with HCC may be receiving curative therapy, such as HCC resection or percutaneous radiofrequency ablation (RFA). As most HCCs develop in patients with cirrhosis or advanced fibrosis, their underlying liver diseases should be managed or treated as in their counterparts without HCC [21].
Recently, a study involving 193 HBV-related HCC patients who underwent tumor resection and were followed up for a mean duration of 58 months concluded that tumor factors were associated with early (<2 years) HCC recurrence, while high viral load and hepatic inflammatory activity were associated with late (>2 years) HCC recurrence [184]. A recent study from Japan has also shown that the 3-year HCC recurrence rate after RFA was much higher (67 vs. 28 %) in those with HBV DNA >4 log10 copies/mL [185]. Based on these findings, pre- and post-operative antiviral therapies may theoretically reduce late HCC recurrence. However, only a few studies have addressed this issue. A randomized controlled trial showed that pre-emptive LAM therapy reduced the incidence of HBV-reactivation hepatitis from 30 to 3 % in HCC patients undergoing transarterial chemolipiodolization [186]. A meta-analysis involving 9 cohort studies with 551 HCC patients who had received curative therapy showed that LAM therapy in 204 patients achieved a 41 % reduction in HCC recurrence and an 87 % reduction in liver-related mortality, as compared with 347 untreated patients [187]. Of a recent study involving 136 patients who underwent curative HCC resection, 42 received LAM or ETV therapy and had higher 3 and 5 year HCC-free survival (both 51.4 vs. 33.8 %; p = 0.05) as compared with the untreated group [188].
To date, the number of studies reporting use of nucs for the prevention of HCC recurrence is limited [187], and each involved a small case number and short treatment duration, so that the results of these studies are inconclusive. It is anticipated that well-designed studies using better antiviral regimens will prove that antiviral therapy may achieve tertiary prevention of HCC recurrence.
Issues and recommendations
Based on this background information, the following issues and recommendations for management of chronic HBV infection are listed. The recommendations were based on evidence graded as I (at least 1 well-designed, randomized, control trial), II (well-designed cohort or case–control studies), III (case series, case reports, or flawed clinical trials), and IV (opinions of respected authorities based on clinical experience, descriptive studies, or reports of expert committees). The recommendations were graded into A: strong recommendation and B: weak recommendation.
General management
Before active therapy, a thorough evaluation of the patient is essential. A complete blood count, biochemical tests, and HBV replication status should be part of the initial evaluation. The severity of liver fibrosis needs to be evaluated before therapy. HBV genotype and HBsAg level may provide additional information, especially in the setting of IFN-based therapy. Besides drug therapy directed at liver disease, counseling of the patient is also very important and even crucial for successful antiviral therapy. This should include information on the infectivity/transmission of HBV and preventive measures for family members and sexual contacts (e.g., vaccination); advice on lifestyle such as activity, diet, alcohol use, risk behaviors, and factors that predispose to superinfection with other hepatitis virus(es) and their prevention; the importance and need for careful follow-up and long-term monitoring, and possible therapy. Health-related quality of life assessment has shown that patients with chronic HBV infection attribute a wide range of negative psychological, social, and physical symptoms to their condition even in the absence of cirrhosis or cancer [189]. These symptoms should be considered in the counseling process. The indications, the risks/benefits, advantages/disadvantages, cost, and possible problems of each therapeutic option should be explained in detail. The importance of compliance, persistence, adherence, and monitoring on and off therapy should also be stressed [154, 190]. The therapy should be tailored for individual needs. Careful assessment on an individual basis, including likelihood of response and economic factors of individual patients, is absolutely essential before starting therapy.
Recommendation 1
Thorough evaluation and counseling are mandatory before considering drug therapy (IIA).
Indications for treatment
Available information suggests that patients with PNALT or minimally raised ALT usually have minimal histological changes and respond poorly, in terms of HBeAg seroconversion, when treated with currently available drugs. Therefore, no drug treatment is recommended for this group of patients unless they have evidence of advanced fibrosis or cirrhosis [191]. However, they should be followed up every 3 months for the first year and then monitored every 3 months if HBeAg positive and every 6 months if HBeAg negative. HBeAg-negative patients with serum HBV DNA >20,000 IU/mL and PNALT should also be followed up every 3 months. Surveillance for HCC using ultrasonography and serum α-fetoprotein every 3–6 months is also important for high-risk HBV-infected persons (males, age >40 years, cirrhosis, positive family history of serious liver disease) [192]. A liver biopsy should be considered in viremic patients older than 40 years [193], especially those with high normal or minimally raised ALT levels [8]. A recent systemic review has shown that histologically significant liver disease is rare in HBeAg-negative carriers with PNALT and HBV DNA between 2,000 and 20,000 IU/mL. Thus, these carriers require neither liver biopsy nor immediate antiviral therapy but need more frequent monitoring during the following 3 years [194].
Patients with active HBV replication (positive HBeAg and/or HBV DNA >2,000–20,000 IU/mL) and raised ALT levels are candidates for treatment. Liver biopsy is recommended before therapy to assess the necroinflammatory grade, determine the fibrotic stage, and exclude other possible causes of raised ALT levels as a guide to the indication for antiviral treatment. If liver biopsy is not feasible, non-invasive assessment of liver fibrosis is an alternative [56, 57].
Recommendation 2
Patients with viral replication but persistently normal or minimally elevated ALT levels should not be treated, except for those with advanced fibrosis or cirrhosis. They need adequate follow-up and HCC surveillance every 3–6 months (IA).
Recommendation 3
Assessment of liver fibrosis is recommended in viremic patients with high normal or minimally raised ALT levels and patients older than 40 years, except for patients with clinical evidence of cirrhosis (IIA).
Time to start treatment (Figs. 1, 2, 3)
Treatment may be started if patients have persistently elevated ALT levels ≥2 times ULN (at least 1 month between observations).
Patients with a rising trend in ALT (from normal or minimally elevated levels) or with ALT >5 times ULN may be developing an exacerbation, and severe hepatitis or hepatic decompensation may follow. They should be monitored closely with weekly or biweekly serum ALT, bilirubin, and prothrombin time measurement. Treatment must be initiated in time, particularly in those with increasing serum HBV DNA > 3 × 108 IU/mL [169] or in patients with advanced fibrosis [21] to prevent the development or deterioration of hepatic decompensation. Such exacerbations, particularly in patients with declining serum HBV DNA level or a level <200,000 IU/mL, may also precede spontaneous HBeAg seroconversion and may be followed by disease remission [9]. Thus, it is reasonable to delay treatment for an observation period of 3 months, if there is no concern about hepatic decompensation.
Recommendation 4
Chronic HBV-infected patients with ALT ≥2 times ULN, and HBV DNA ≥2.0 × 104 IU/mL if HBeAg positive and ≥2.0 × 103 IU/mL if HBeAg-negative as well as patients with advanced fibrosis or cirrhosis with any ALT level should be considered for treatment (IA). Treatment should be started as early as possible in case of impending or overt hepatic decompensation (IA). Otherwise, 3–6 months’ observation is recommended to ensure the need of therapy (IIA). Indications are similar for retreatment.
Which drugs or strategy?
Drugs currently approved for the treatment of chronic HBV infection have relatively limited sustained long-term efficacy. Therefore, age of patient, severity of liver disease, probability of sustained response, likelihood of drug resistance, adverse events, and complications need to be carefully considered. Conventional-IFN or Peg-IFN, thymosin-α, LAM, ADV, ETV, LdT, and TDF can all be considered for initial therapy in patients without liver decompensation. IFN-based therapy is preferred in younger patients. ETV and TDF are the preferred nucs. The rates of sustained response seem to be higher with IFN-based therapy than with direct antiviral agents, and response can be achieved with a defined duration of treatment. Cirrhotic patients respond to IFN-based therapy better than, or at least as well as, their non-cirrhotic counterparts. IFN-based therapy has more side effects and requires closer monitoring.
For viremic patients (both HBeAg positive and HBeAg negative, adults and children) with ALT level >5 times ULN, ETV, TDF, LdT, or LAM are recommended if there is a concern about hepatic decompensation. IFN-based therapy is also effective in patients with higher ALT level if there is no concern about hepatic decompensation.
For HBeAg-positive patients with an ALT level between 2 and 5 times ULN, the choice between IFN-based therapy and nucs is less clear, and either agent may be used. Theoretically, this group of patients has not mounted a high enough immune response against HBV and thus needs immunomodulation.
Twelve-month Peg-IFN induced higher sustained response rates than nucs in HBeAg-negative patients with intermittent or persistent ALT elevation, moderate to severe inflammation, fibrosis on biopsy, and serum HBV DNA >2,000 IU/mL (104 copies/mL). Nucs provide other options, but long-term therapy is required, and therefore the drug-resistance profile of the drug to be used should be considered. The long-term (>5 years) effect of IFN therapy is better known than that of nucs.
The decision as to which agent to be used should be an individual one, based on disease severity, history of flares, hepatic function, the rapidity of drug action, resistance profile, side effects, drug costs, and patient choice. Cost-effectiveness of drug therapy is specific for each country and should be studied independently to guide the choice of drug.
Recommendation 5
Treatment-naïve patients can be treated with conventional IFN 5–10 MU 3 times per week (IB) or Peg-IFN-α2a 180 μg weekly or Peg-IFN-α2b 1–1.5 μg/kg weekly (IA), ETV 0.5 mg daily (IA), TDF 300 mg daily (IA), ADV 10 mg daily (IB), LdT 600 mg daily (IB), or LAM 100 mg daily (IB). Thymosin α 1.6 mg 2 times per week can also be used (IB). ETV or TDF is the preferred nuc.
How to monitor?
To achieve the most cost-effective treatment, adequate monitoring during and after treatment is crucial. HBV DNA should be measured using assays standardized/validated to report against the WHO IU/mL reference standard. If affordable, drug-resistance testing should also be considered.
Recommendation 6
During therapy, ALT, HBeAg, and/or HBV-DNA should be monitored at least every 3 months (IA). Renal function should be monitored if TDF or ADV is used (IA). Muscle weakness should be monitored, especially if LdT is used (IIIA). During IFN-based therapy, monitoring of blood cell counts and other adverse effects are mandatory (IA).
Recommendation 7
After the end of therapy, levels of ALT and HBV DNA should be monitored monthly for the first 3 months to detect early relapse, and then every 3 months in the first year after therapy. If uneventful, monitor every 3 months (for cirrhotic patients) to 6 months (for responders) thereafter (IIA). For non-responders, further monitoring of HBV markers is required to both recognize a delayed response or to plan retreatment when indicated (IIA).
When to stop therapy?
The recommended duration of IFN-based therapy is finite, irrespective of whether or not response has been achieved. A 6–12 month observation period after the end of IFN-based therapy is also recommended to both detect a delayed response and establish whether the response is sustained, and thus whether retreatment or other therapy is required. The recommended duration of thymosin α1 therapy is 6 months, with 12 months’ observation after the end of therapy.
Since the incidence of drug resistance increases with increasing duration of nuc therapy, therapy can be stopped if the patient has undergone HBeAg seroconversion with HBV DNA loss (PCR) for at least 12 months. For those who remain HBeAg positive, the decision to continue or stop therapy should be evaluated individually on the basis of clinical/virological response and disease severity. If resistant mutations emerge, early rescue therapy with another agent is indicated. For HBeAg-negative patients, the optimal duration of nuc treatment is unknown, unless HBsAg seroclearance has occurred, and the decision to stop therapy should be determined by clinical response and severity of the underlying liver disease.
Recommendation 8
For conventional IFN, the current recommended duration of therapy is 4–6 months for HBeAg-positive patients (IA) and at least a year for HBeAg-negative patients (IA). For Peg-IFN, the recommended duration is 12 months (IA). For thymosin α1, the recommended duration of therapy is 6 months for both HBeAg-positive (IA) and HBeAg-negative patients (IIB).
Recommendation 9
For nucs: In HBeAg-positive patients, treatment can be stopped when HBeAg seroconversion with undetectable HBV DNA has been maintained for at least 12 months (IIA). In HBeAg-negative patients, it is not clear how long treatment should be continued if HBsAg remains positive, but treatment discontinuation can be considered if patients have been treated for at least 2 years with undetectable HBV DNA documented on three separate occasions 6 months apart (IIA). In compliant patients with primary treatment failure at month 3 or suboptimal viral response at month 6, switch to a more potent drug or add a drug without cross resistance if LAM, LdT, or ADV was used (IIIA).
What to do for patients in special circumstances?
Female patients of child-bearing age
When treatment is indicated in women of childbearing age, both the drug property and the duration of dosing should be considered. Supplemental use of LAM or LdT in the third trimester of pregnancy in women with HBV DNA >2 × 106 IU/mL is safe and effective in preventing mother-to-child HBV transmission and seems to be cost-effective [195].
Recommendation 10-1
For female patients of child-bearing age, IFN-based therapy is preferred for non-pregnant women. Pregnancy is discouraged during IFN therapy (IA). Pregnant women who need treatment can be treated with category B nucs (IIA).
Recommendation 10-2
For the prevention of mother-to-child transmission, pregnant women with high HBV DNA (>2 × 106 IU/mL) can be treated with LdT in the third trimester (IIA). TDF is an alternative (IIIA).
Patients with concurrent HIV infection
All HIV-infected patients with HBV DNA > 2,000 IU/mL and/or significant necroinflammation or fibrosis should be considered for HBV treatment. Treatment needs to be individualized according to the patient’s HIV status. All HIV-HBV coinfected patients requiring HBV treatment with CD4 count <500 cells/mm3 should receive ART containing TDF + FTC/LAM. For patients with CD4 >500 cells/mm3, treatment options include IFN-based and ADV therapy. In practice, ART containing TDF + FTC/LAM should still be considered as an alternative option.
Recommendation 11
ART containing TDF + FTC/LAM is the treatment of choice for the majority of HIV-HBV coinfected individuals. If the CD4 count is greater than 500 and ART is not warranted, ADV or PEG-IFN can be considered (IIA).
Patients with concurrent HCV or HDV infection
It is important to determine which virus is dominant before designing the treatment strategy.
Recommendation 12
In patients with concurrent HCV or HDV infection, determine which virus is dominant and treat the patients accordingly (IA).
Patients with decompensated liver disease
IFN is usually contraindicated in patients with decompensated liver disease. Nucs with potent and prompt HBV suppressive action should be used immediately.
Recommendation 13
ETV or TDF is the agent of choice for patients with obvious or impending hepatic decompensation (IA). LdT, LAM, or ADV can also be used in nuc-naïve patients (IB). Renal function and lactic acidosis should be monitored in this group of patients, especially those with MELD score greater than 20 (IIIA).
Patients with drug resistance
Patients with viral breakthrough evident by more than 1 log IU/mL increase of HBV DNA from the nadir should be tested to confirm viral resistance, even in a self-declared compliant patient. Rescue therapy should be instituted as early as possible in case of drug resistance.
Recommendation 14
For patients who develop drug resistance while on LAM, add-on ADV therapy (IA) or switching to TDF is indicated (IIA); switching to ETV (1 mg/day) is an option (IB) but not preferred. For patients who develop drug resistance while on ADV, add-on LAM, LdT, ETV, or switching to TDF is indicated (IIIA). For patients who develop drug resistance while on LdT, add-on ADV therapy or switching to TDF is indicated (IIIA). For patients who develop drug resistance while on ETV, add-on TDF or ADV is indicated (IIIA). For patients with prior failure of or resistance to LAM or LdT and ADV, switching to ETV plus TDF is indicated (IIA).
Switching to IFN-based therapy is an option for patients with resistance to LAM (IA) or other nucs (IIIA).
Patients undergoing immunosuppression or chemotherapy
HBV reactivation is a serious complication in patients undergoing immunosuppression or chemotherapy. LAM therapy is effective when instituted early, before the occurrence of clinical jaundice and decompensation. Results are significantly better if LAM is used before starting chemotherapy. Prophylactic treatment using other antiviral agents has not been reported.
Recommendation 15-1
Before receiving immunosuppression or chemotherapy, patients should be screened for HBsAg (IVA). If they are HBsAg positive, start nuc treatment if clinically indicated (IA). Otherwise, prophylactic therapy with LAM before the start and up to at least 6 months after the end of immunosuppression or chemotherapy is recommended (IA). ETV and TDF can also be used for prophylaxis (IIIA).
Recommendation 15-2
Patients who are going to receive biologic agent such as anti-CD 20 rituximab or anti-tumor necrosis factor-α etanercept should be screened for anti-HBc. If they are anti-HBc positive, HBV DNA should be closely monitored and treated with nuc when needed (IVA).
Patients in the setting of organ transplantation
Nucs are effective in HBV suppression in patients undergoing organ transplantation, prevention (in combination with HBIg) of HBV recurrence after liver transplant, and treatment of HBV-related allograft infection. Adequate use of these agents has improved patient outcomes.
Recommendation 16-1
Nuc(s) treatment should be commenced in all HBV patients who are listed for organ transplantation and have detectable HBV DNA (IIA).
For liver transplantation, LAM plus low dose HBIg (400–800 U, i.m. daily for 1 week, followed by 400–800 U monthly over the long-term) provide safe and effective prophylaxis against HBV reinfection of the allograft (IIA). Alternatively, LAM + ADV or ETV prophylaxis can be considered (IIA).
Recommendation 16-2
Late (at least 12-month post-transplant) HBIg substitution by ADV provides safe and cost-effective prophylaxis (IIA). Late conversion to LAM monotherapy may be considered in ‘low-risk’ patients (IA).
Recommendation 16-3
HBV-naïve patients receiving a liver from an anti-HBc (+) donor should receive long-term prophylaxis with either LAM or HBIg (IIIA).
Patients before and/or after curative or local–regional therapy of HCC
Since most HCCs develop in patients with cirrhosis or advanced fibrosis, their underlying liver diseases should be managed or treated as in their counterparts without HCC.
Recommendation 17
Nuc treatment should be commenced in all HCC patients with HBV DNA >2,000 IU/mL before and/or after curative therapy of HCC as in their counterparts without HCC (IIIB). Preemptive nuc therapy should be initiated in all HCC patients who are to undergo transarterial chemoembolization (IIA).
Unresolved issues and areas for further study
Despite recent advances in the treatment of chronic HBV infection, the results of treatment are still unsatisfactory. In particular, the following issues remain unsettled:
-
1.
Should HBV genotyping be routinely used in designing interferon-based treatment plans?
-
2.
What should be the treatment strategy for children with chronic HBV infection? ‘Necessity’ or ‘likelihood to respond’?
-
3.
Is there more effective therapy for patients with chronic HDV infection?
-
4.
What is the role for corticosteroid withdrawal, nuc pulse therapy, or other immunomodulating agents and modes of immunomodulation?
-
5.
What is the optimal combination therapy to enhance efficacy?
-
6.
What is the role of qHBsAg in formulating the treatment strategy, such as early stopping rules?
-
7.
Which is the best non-invasive test for liver fibrosis? More comparative studies are needed.
-
8.
What is the optimal treatment for multi-drug resistance?
The development of new drugs and new strategies, especially combination or sequential antiviral therapy, is the highest priority in further improving the outcomes of treatment.
References
Liaw YF, Leung N, Kao JH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int 2008;2:263–283
Keeffe EB, Dieterich DT, Han SH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol 2008;6:1315–1341
Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009;50:661–662
European Association For The Study Of The Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol 2009;50:227–242
Liaw YF. Antiviral therapy of chronic hepatitis B: opportunities and challenges in Asia. J Hepatol 2009;51:403–410
Liaw YF, Chu CM. Hepatitis B virus infection. Lancet 2009;373:582–592
Hui CK, Leung N, Yuen ST, et al. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase. Hepatology 2007;46:395–401
Lai M, Hyatt BJ, Nasser I, et al. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol 2007;47:760–767
Liaw YF. Hepatitis flares and hepatitis B e antigen seroconversion: Implication in anti-hepatitis B virus therapy. J Gastroenterol Hepatol 2003;18:246–252
Lin CL, Kao JH. The clinical implications of hepatitis B virus genotype: recent advances. J Gastroenterol Hepatol 2011;26(Suppl 1):123–130
Chu CM, Hung SJ, Lin J, et al. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med 2004;116:829–834
Hsu YS, Chien RN, Yeh CT, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology 2002;35:1522–1527
Chu CM, Liaw YF. Predictive factors for reactivation of hepatitis B following hepatitis B e antigen seroconversion in chronic hepatitis B. Gastroenterology 2007;133:1458–1465
Chen YC, Chu CM, Liaw YF. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B. Hepatology 2010;51:435–444
Chu CM, Liaw YF. Incidence and risk factors of progression to cirrhosis in inactive carriers of hepatitis B virus. Am J Gastroenterol 2009;104:1693–1699
Feld JJ, Ayers M, El-Ashry D, et al. Hepatitis B virus DNA prediction rules for hepatitis B e antigen-negative chronic hepatitis B. Hepatology 2007;46:1057–1070
Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology 1988;8:493–496
Lin SM, Yu ML, Lee CM, et al. Interferon therapy in HBeAg positive chronic hepatitis reduces cirrhosis and hepatocellular carcinoma. J Hepatol 2007;46:45–52
Park BK, Park YN, Ahn SH, et al. Long-term outcome of chronic hepatitis B based on histological grade and stage. J Gastroenterol Hepatol 2007;22:383–388
Wu CF, Yu MW, Lin CL, et al. Long-term tracking of hepatitis B viral load and the relationship with risk for hepatocellular carcinoma in men. Carcinogenesis 2008;29:106–112
Chen CF, Lee WC, Yang HI, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology 2011;141:1240–1248
Chen YC, Chu CM, Yeh CT, Liaw YF. Natural course following the onset of cirrhosis in patients with chronic hepatitis B: a long term follow-up study. Hepatol Int 2007;1:267–273
Chu CM, Liaw YF. Hepatitis B virus-related cirrhosis: natural history and treatment. Semin Liver Dis 2006;26:142–152
Yang HI, Lu SN, Liaw YF, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med 2002;347:168–174
Iloeje UH, Yang HI, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006;130:678–686
Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65–73
Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology 2008;135:111–121
Yu MW, Shih WL, Lin CL, et al. Body-mass index and progression of hepatitis B: a population-based cohort study in men. J Clin Oncol 2008;26:5576–5582
Tai DI, Lin SM, Sheen IS, et al. Long-term outcome of hepatitis B e antigen-negative hepatitis B surface antigen carriers in relation to changes of alanine aminotransferase levels over time. Hepatology 2009;49:1859–1867
Chu CM, Chen YC, Tai DI, Liaw YF. Level of hepatitis B virus DNA in inactive carriers with persistently normal levels of alanine aminotransferase. Clin Gastroenterol Hepatol 2010;8:535–540
Chen YC, Huang SF, Chu CM, Liaw YF. Serial HBV DNA levels in patients with persistently normal transaminase over 10 years following spontaneous HBeAg seroconversion. J Viral Hepat 2012;19:138–146
Chen JD, Yang HI, Iloeje UH, et al. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology 2010;138:1747–1754
Chu CM, Liaw YF. Hepatitis B surface antigen seroclearance during chronic HBV infection. Antivir Ther 2010;15:133–143
Liu J, Yang HI, Lee MH, et al. Incidence and determinants of spontaneous hepatitis B surface antigen seroclearance: a community-based follow-up study. Gastroenterology 2010;139:474–482
Chu CM, Liaw YF. Prevalence of and risk factors for hepatitis B viremia after spontaneous hepatitis B surface antigen seroclearance in hepatitis B carrier. Clin Infect Dis 2012;54:88–90
Liaw YF. Clinical utility of hepatitis B surface antigen quantitation in patients with chronic hepatitis B: a review. Hepatology 2011;53:2121–2129
Chan HL, Thompson A, Martinot-Peignoux M, et al. Hepatitis B surface antigen quantification: Why and how to use it in 2011—A Core Group Report. J Hepatol 2011;55:1121–1131
Nguyen T, Thompson AJ, Bowden S, et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol 2010;52:508–513
Jaroszewicz J, Calle Serrano B, Wursthorn K, et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol 2010;52:514–522
Chan HL, Wong VW, Wong GL, et al. A longitudinal study on the natural history of serum hepatitis B surface antigen changes in chronic hepatitis B. Hepatology 2010;52:1232–1241
Tseng TC, Liu CJ, Su TH, et al. Serum hepatitis B surface antigen levels predict surface antigen loss in hepatitis B e antigen seroconverters. Gastroenterology 2011;141:517–525
Chan HL, Wong GL, Tse CH, et al. Viral determinants of hepatitis B surface antigen seroclearance in hepatitis B e antigen-negative chronic hepatitis B patients. J Infect Dis 2011;204:408–414
Tseng TC, Liu CJ, Yang HC, et al. Determinants of spontaneous surface antigen loss in HBeAg-negative patients with a low viral load. Hepatology 2012;55:68–76
Chen YC, Jeng WJ, Chu CM, Liaw YF. Decreasing levels of HBsAg predict HBsAg seroclearance in patients with inactive chronic hepatitis B virus infection. Clin Gastroenterol Hepatol 2012;10:297–302
Livingston SE, Simonetti JP, Bulkow LR, et al. Clearance of hepatitis B e antigen in patients with chronic hepatitis B and genotypes A, B, C, D, and F. Gastroenterology 2007;133:1452–1457
Yu MW, Yeh SH, Chen PJ, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 2005;97:265–272
Thakur V, Guptan RC, Kazim SN, et al. Profile, spectrum and significance of HBV genotypes in chronic liver disease patients in the Indian subcontinent. J Gastroenterol Hepatol 2002;17:165–170
Tanaka Y, Mukaide M, Orito E, et al. Specific mutations in enhancer II/core promoter of hepatitis B virus subgenotypes C1/C2 increase the risk of hepatocellular carcinoma. J Hepatol 2006;45:646–653
Chan HL, Tse CH, Mo F, et al. High viral load and hepatitis B virus subgenotype Ce are associated with increased risk of hepatocellular carcinoma. J Clin Oncol 2008;26:177–182
Zhang HW, Yin JH, Li YT, et al. Risk factors for acute hepatitis B and its progression to chronic hepatitis in Shanghai, China. Gut 2008;57:1713–1720
Chen CH, Hung CH, Lee CM, et al. Pre-S deletion and complex mutations of hepatitis B virus related to advanced liver disease in HBeAg-negative patients. Gastroenterology 2007;133:1466–1474
Yang HI, Yeh SH, Chen PJ, et al. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst 2008;100:1134–1143
Lim SG, Cheng Y, Guindon S, et al. Viral quasi-species evolution during hepatitis Be antigen seroconversion. Gastroenterology 2007;133:951–958
Wang HY, Chien MH, Huang HP, et al. Distinct hepatitis B virus dynamics in the immunotolerant and early immunoclearance phases. J Virol 2010;84:3454–3463
Jung KS, Kim SU, Ahn SH, et al. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology 2011;53:885–894
Wong GL, Wong VW, Choi PC, et al. Development of a non-invasive algorithm with transient elastography (Fibroscan) and serum test formula for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther 2010;31:1095–1103
Fung J, Lai CL, Seto WK, Yuen MF. The use of transient elastography in the management of chronic hepatitis B. Hepatol Int 2011;5:868–875
Yuen MF, Tanaka Y, Fong DY, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol 2009;50:80–88
Wong VW, Chan SL, Mo F, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol 2010;28:1660–1665
Yang HI, Sherman M, Su J, et al. Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J Clin Oncol 2010;28:2437–2444
Yang HI, Yuen MF, Chan HL, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol 2011;12:568–574
Chien RN. Current therapy for hepatitis C or D or immunodeficiency virus concurrent infection with chronic hepatitis B. Hepatol Int 2008;2:296–303
Piratvisuth T. Immunomodulator therapy of chronic hepatitis B. Hepatol Int 2008;2:140–146
Luo K, Mao Q, Karayiannis P, et al. Tailored regimen of interferon alpha for HBeAg-positive chronic hepatitis B: a prospective controlled study. J Viral Hepat 2008;15:684–689
Lampertico P, Del Ninno E, Vigano M, et al. Long-term suppression of hepatitis B e antigen-negative chronic hepatitis B by 24-month interferon therapy. Hepatology 2003;37:756–763
Papatheodoridis GV, Dimou E, Dimakopoulos K, et al. Outcome of hepatitis B e antigen-negative chronic hepatitis B on long-term nucleos(t)ide analog therapy starting with lamivudine. Hepatology 2005;42:121–129
Cooksley WGE, Piratvisuth T, Lee SD, et al. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis Be antigen-positive chronic hepatitis B. J Viral Hepatitis 2003;10:298–305
Zhao H, Kurbanov F, Wan MB. Genotype B and younger patient age associated with better response to low-dose therapy: A trial with pegylated/nonpegylated interferon-alpha-2b for hepatitis B e antigen-positive patients with chronic hepatitis B in China. Clin Infect Dis 2007;44:541–548
Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–2695
Piratvisuth T, Lau GKK, Chao YC, et al. Sustained response to peginterferon alfa-2a (40 kD) with or without lamivudine in Asian patients with HBeAg-positive and HBeAg-negative chronic hepatitis B. Hepatol Int 2008;2:102–110
Chan HL, Leung NW, Hui AY, et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-alpha2b and lamivudine with lamivudine alone. Ann Intern Med 2005;15(142):240–250
Liaw YF, Jia JD, Chan HLY, et al. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior HBeAg seroconversion rates in HBV genotypes B or C. Hepatology 2011;54:1591–1599
Buster EH, Flink HJ, Cakaloglu Y, et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology 2008;135:459–467
Wong VW, Wong GL, Yan KK, et al. Durability of peginterferon alfa-2b treatment at 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology 2010;51:1945–1953
Marcellin P, Lau GKK, Bonino F, et al. Peginterferon alfa-2 alone, lamivudine alone and the two in combination combination in patients with HBeAg negative chronic hepatitis B. N Engl J Med 2004;351:1206–1217
Papadopoulos VP, Chrysagis DN, Protopapas AN, et al. Peginterferon alfa-2b as monotherapy or in combination with lamivudine in patients with HBeAg-negative chronic hepatitis B: a randomised study. Med Sci Monit 2009;15:CR56–CR61
Marcellin P, Bonino F, Lau GK, et al. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology 2009;136:2169–2179
Lampertico P, Viganò M, Colombo M. Treatment of HBeAg-negative chronic hepatitis B with pegylated interferon. Liver Int 2011;31(Suppl 1):90–94
Buster EH, Hansen BE, Buti M, et al. Peginterferon alpha-2b is safe and effective in HBeAg-positive chronic hepatitis B patients with advanced fibrosis. Hepatology 2007;46:388–394
Piccolo P, Lenci I, Demelia L, et al. A randomized controlled trial of pegylated interferon-alpha2a plus adefovir dipivoxil for hepatitis B e antigen negative chronic hepatitis B. Antivir Ther 2009;14:1165–1174
Sarin SK, Sood A, Kumar M, et al. Effect of lowering HBV DNA levels by initial antiviral therapy before adding immunomodulator on treatment of chronic hepatitis B. Am J Gastroenterol 2007;102(1):96–104
Moucari R, Boyer N, Ripault MP, et al. Sequential therapy with adefovir dipivoxil and pegylated interferon alfa-2a for HBeAg-negative patients. J Viral Hepat 2011;18:580–586
Chen CC, Wang PC, Chang HW, Chen CF. Safety and efficacy of two-step peginterferon a-2a treatment in patients of chronic hepatitis B with acute exacerbation. J Viral Hepat 2012;19:161–192
Sonneveld MJ, Wong VW, Woltman AM, et al. Polymorphisms near IL28B and serological response to peginterferon in HBeAg-positive patients with chronic hepatitis B. Gastroenterology 2012;142:513–520
Buster EH, Hansen BE, Lau GK, et al. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology 2009;137:2002–2009
ter Borg MJ, Hansen BE, Bigot G, et al. ALT and viral load decline during PEG-IFN alpha-2b treatment for HBeAg-positive chronic hepatitis B. J Clin Virol 2008;42:160–164
Liaw YF. On-treatment outcome prediction and adjustment during chronic hepatitis B therapy: now and future. Antivir Ther 2009;14:13–22
Piratvisuth T, Marcellin P, Popescu M, et al. Hepatitis B surface antigen: Association with sustained response to peginterferon alfa-2a in hepatitis B e antigen-positive patients. Hepatol Int 2011; doi:10.1007/s12072-011-9280-0.
Sonneveld MJ, Rijckborst V, Boucher CA, et al. Prediction of sustained response to peginterferon alfa-2b for hepatitis B e antigen-positive chronic hepatitis B using on-treatment hepatitis B surface antigen decline. Hepatology 2010;52:1251–1257
Piratvisuth T, Marcelin P. Further analysis is required to identify an early stopping rule for peginterferon therapy that is valid for all hepatitis B e antigen–positive patients. Hepatology 2011;53:1054–1055
Chien RN, Liaw YF, Chen TC, et al. Efficacy of thymosin alpha-1 in patients with chronic type B hepatitis: a randomized controlled trial. Hepatology 1998;27:1383–1387
Chien RN, Lin CY, Yeh CT, et al. Hepatitis B virus genotype B is associated with better response to thymosin alpha-1 therapy than genotype C. J Viral Hepat 2006;13:845–850
Iino S, Toyota J, Kumada H, et al. The efficacy and safety of thymosin alpha-1 in Japanese patients with chronic hepatitis B; results from a randomized clinical trial. J Viral Hepat 2005;12:300–306
Chan HL, Tang JL, Tam W, et al. The efficacy of thymosin in the treatment of chronic hepatitis B virus infection: a meta-analysis. Aliment Pharmacol Ther 2001;15:1899–1905
Lim SG, Wai CT, Lee YM, et al. A randomized, placebo-controlled trial of thymosin-alpha1 and lymphoblastoid interferon for HBeAg-positive chronic hepatitis B. Antivir Ther 2006;11:245–253
Lee HW, Lee JI, Um SH, et al. Combination therapy of thymosin alpha-1 and lamivudine for HBeAg positive chronic hepatitis B: a prospective randomized, comparative pilot study. J Gastroenterol Hepatol 2008;23:729–735
You J, Zhuang L, Cheng HY, et al. A randomized, controlled, clinical study of thymosin alpha-1 versus interferon-alpha in Chinese patients with chronic hepatitis B lacking hepatitis B envelope antigen. J Chin Med Assoc 2005;68:65–72.
Chang TT, Lai CL, Chien RN, et al. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol 2004;19:1276–1282
Yao GB, Zhu M, Cui AY. A 7-year study of lamivudine therapy for chronic hepatitis B virus e antigen-positive chronic hepatitis B patients in China. J Dig Dis 2009;10:131–137
Chien RN, Liaw YF, Atkins M, for Asian Hepatitis Lamivudine Trial Group. Pretherapy alanine transaminase level as a determinant for hepatitis B e antigen seroconversion during lamivudine therapy in patients with chronic hepatitis B. Hepatology 1999;30:770–774
Jonas MM, Little NR, Gardner SD, Members of the International Pediatric Lamivudine Investigator Group. Long-term lamivudine treatment of children with chronic hepatitis B: Durability of therapeutic responses and safety. J Viral Hepat 2008;15:20–27
Lee HW, Lee HJ, Hwang JS, et al. Lamivudine maintenance beyond one year after HBeAg seroconversion is a major factor for sustained virologic response in HBeAg-positive chronic hepatitis B. Hepatology 2010;51:415–421
Kuo YH, Chen CH, Wang JH, et al. Extended lamivudine consolidation therapy in hepatitis B e antigen-positive chronic hepatitis B patients improves sustained hepatitis B e antigen seroconversion. Scand J Gastroenterol 2010;45:75–81
Yeh CT, Hsu CW, Chen YC, Liaw YF. Withdrawal of lamivudine in HBeAg-positive chronic hepatitis B patients after achieving effective maintained virological suppression. J ClinVirol 2009;45:114–118
Chien RN, Yeh CT, Tsai SL, et al. The determinants for sustained HBeAg response to lamivudine therapy. Hepatology 2003;38:1267–1273
Chan HL, Wang H, Niu J, et al. Two-year lamivudine treatment for hepatitis B e antigen-negative chronic hepatitis B: a double-blind, placebo-controlled trial. AntivirTher 2007;12:345–353
Fung SK, Wong F, Hussain M, Lok AS. Sustained response after a 2-year course of lamivudine treatment of hepatitis e antigen-negative chronic hepatitis B. J Viral Hepat 2004;11:432–438
Liu F, Wang L, Li XY, et al. Poor durability of lamivudine effectiveness despite stringent cessation criteria: a prospective clinical study in hepatitis B e antigen-negative chronic hepatitis B patients. J Gastroenterol Hepatol 2011;26:456–460
Chien RN, Liaw YF. Short-term lamivudine therapy in HBeAg-negative chronic active hepatitis B in Taiwan. Antivir Ther 2006;11:947–952
Chan HL, Wong GL, Chim AM, et al. Prediction of off-treatment response to lamivudine by serum hepatitis B surface antigen quantification in hepatitis B e antigen-negative patients. Antivir Ther 2011;16:1249–1257
LiawYF Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521–1531
Zoulim F, Locarnini S. Management of treatment failure in chronic hepatitis B. J Hepatol 2012;56 Suppl 1:S112–122
Lai CL, Dienstag J, Schiff E, et al. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis 2003;36:687–696
Liaw YF, Chien RN, Yeh CT, et al. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology 1999;30:567–572
Liaw YF, Gane E, Leung N, et al. 2-year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology 2009;136:486–495
Hou J, Yin YK, Xu D, et al. Telbivudine versus lamivudine in Chinese patients with chronic hepatitis B: results at 1 year of a randomized, double-blind trial. Hepatology 2008;47:447–454
Chan HL, Heathcote EJ, Marcellin P, et al. Treatment of hepatitis B e antigen-positive chronic hepatitis B with telbivudine or adefovir: a randomized trial. Ann Intern Med 2007;147:745–754
Gane E, Wang Y, Liaw YF, et al. Efficacy and safety of prolonged 3-year telbivudine treatment in patients with chronic hepatitis B. Liver Int 2011;31:676–684
Wurthorn K, Jung M, Riva A, et al. Kinetics of hepatitis B surface antigen decline during 3 year of telbivudine treatment in hepatitis B e antigen-positive patients. Hepatology 2010;52:1611–1620
Cai W, Xie Q, An B, et al. On-treatment serum HBsAg level is predictive of sustained off-treatment virologic response to telbivudine in HBeAg-positive chronic hepatitis B patients. J ClinVirol 2010;48:22–26
Zeuzem S, Gane E, Liaw YF, et al. Baseline characteristics and early on-treatment response predict the outcomes of 2 years of telbivudine treatment of chronic hepatitis B. J Hepatol 2009;51:11–20
Marcellin P, Chang TT, Lim SG, et al. Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. Hepatology 2008;48:750–758
Zeng M, Mao Y, Yao GB, et al. Five years of treatment with adefovir dipivoxil in Chinese patients with HBeAg-positive chronic hepatitis B. Liver Int 2012;32:137–146
Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology 2006;131:1743–1751
Liaw YF, Gigi-Raptopoulou M, Cheinquer H, et al. Efficacy and safety of entecavir versus adefovir in chronic hepatitis B patients with hepatic decompensation: a randomized, open-label study. Hepatology 2011;54:91–100
Warner N, Locarnini S. The antiviral drug selected hepatitis B virus rtA181T/sW172* mutant has a dominant negative secretion defect and alters the typical profile of viral rebound. Hepatology 2008;48:88–98
Lee JM, Park JY, Kim DK, et al. Long-term adefovir dipivoxil monotherapy for up to 5 years in lamivudine-resistant chronic hepatitis B. Antivir Ther 2010;15:235–241
Yatsuji H, Suzuki F, Sezaki H, et al. Low risk of adefovir resistance in lamivudine-resistant chronic hepatitis B treated with adefovir plus lamivudine combination therapy: two-year follow-up. J Hepatol 2008;48:923–931
Lampertico P, Vigano M, Manenti E, et al. Adefovir rapidly suppresses hepatitis B in HBeAg-negative patients developing genotypic resistance to lamivudine. Hepatology 2005;42:1414–1419
Liang Y, Jiang J, Su M, et al. Predictors of relapse in chronic hepatitis B after discontinuation of antiviral therapy. Aliment Pharmacol Ther 2011;34:344–352
Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxilfumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008;359:2442–2455
Heathcote EJ, Marcellin P, Buti M, et al. Three-year efficacy and safety of tenofovir disoproxilfumarate treatment for chronic hepatitis B. Gastroenterology 2011;140:132–143
Liaw YF, Sheen IS, Lee CM, et al. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology 2011;53:62–72
Mauss S, Berger F, Filmann N, et al. Effect of HBV polymerase inhibitors on renal function in patients with chronic hepatitis B. J Hepatol 2011;55:1235–1240
Gutiérrez F, Masiá M. The role of HIV and antiretroviral therapy in bone disease. AIDS Rev 2011;13:109–118
Van Bommel F, de Man R, Wedemeyer H, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology 2010;51:73–80
Patterson SJ, George J, Strasser SI, et al. Tenofovir disoproxilfumarate rescue therapy following failure of both lamivudine and adefovir dipivoxil in chronic hepatitis B. Gut 2011;60:247–254
Ong A, Wong VW, Wong GL, et al. Management options for lamivudine-resistant chronic hepatitis B patients with suboptimal virological suppression by adefovir. Aliment Pharmacol Ther 2011;34:972–981
Petersen J, Ratziu V, Buti M, et al. Entecavir plus tenofovir combination as rescue therapy in pretreated chronic hepatitis B patients. An international multicenter cohort study. J Hepatol 2012;56:520–526
Chang TT, Lai CL, Yoon SK, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology 2010;51:422–433
Yuen MF, Seto WK, Fung J, et al. Three years of continuous entecavir therapy in treatment-naïve chronic hepatitis B patients: viral suppression, viral resistance and clinical safety. Am J Gastroenterol 2011;106:1264–1271
Zoutendijk R, Peijnders JG, Brown A, et al. Entecavir treatment for chronic hepatitis B: adaptation is not needed for the majority of naïve patients with a partial virological response. Hepatology 2011;54:443–451
Yokosuka O, Takaguchi K, Fujioka S, et al. Long-term use of entecavir in nucleoside-naïve Japanese patients with chronic hepatitis B infection. J Hepatol 2010;52:791–799
Chang TT, Liaw YF, Wu SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010;52:886–893
Fung J, Lai CL, Young J, et al. Quantitative hepatitis B surface antigen levels in patients with chronic hepatitis B after 2 years of entecavir treatment. Am J Gastroenterol 2011;106:1766–1773
Lange CM, Bojunga J, Hofmann WP, et al. Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function. Hepatology 2009;50:2001–2006
Shim JH, Lee JC, Kim KM, et al. Efficacy of entecavir in treatment-naïve patients with hepatitis B virus-related decompensated cirrhosis. J Hepatol 2010;52:176–182
Wong VW, Wong GL, Yiu KK, et al. Entecavir treatment in patients with severe acute exacerbation of chronic hepatitis B. J Hepatol 2011;54:236–242
Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology 2009;49:1503–1514
Flischer RD, Lok AS. Myopathy and neuropathy associated with nucleos(t)ide analog therapy for hepatitis B. J Hepatol 2009;51:787–791
Yuen MF, Han KH, Um SH, et al. Antiviral activity and safety of LB80380 in hepatitis B e antigen-positive chronic hepatitis B patients with lamivudine-resistant disease. Hepatology 2010;51:767–776
Sung JJ, Lai JY, Zeuzem S, et al. Lamivudine compared with lamivudine and adefovir dipivoxil for the treatment of HBeAg-positive chronic hepatitis B. J Hepatol 2008;48:728–735
Si-Ahmed SN, Pradat P, Zoutendijk R, et al. Efficacy and tolerance of a combination of tenofovir disoproxil fumarate plus emtricitabine in patients with chronic hepatitis B: a European multicenter study. Antiviral Res 2011;92:90–95
Hongthanakorn C, Chotiyaputta W, Oberhelman K, et al. Virological breakthrough and resistance in patients with chronic hepatitis B receiving nucleos(t)ide analogues in clinical practice. Hepatology 2011;53:1854–1863
Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology 2009;49(5 Suppl):S185–S195
Giles M, Visvanathan K, Sasadeusz J. Antiviral therapy for hepatitis B infection during pregnancy and breastfeeding. Antivir Ther 2011;16:621–628
Shi Z, Yang Y, Ma L, et al. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus, A systematic review and meta-analysis. Obs Gyn 2010;116:147–159
Han GR, Cao MK, Zhao W, et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol 2011;55:1215–1221
Petersen J. HBV treatment and pregnancy. J Hepatol 2011;55:1171–1173
Liu CJ, Chuang WL, Lee CM, et al. Peginterferon alfa-2a plus ribavirin for the treatment of dual chronic infection with hepatitis B and C viruses. Gastroenterology 2009;136:496–504
Farci P, Chessa C, Balestrieri C, et al. Treatment of chronic hepatitis D. J Viral Hepat 2007;45:1056–1075
Wedemeyer H, Yurdaydin C, Dalekos GN, et al. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med 2011;364:322–331
Rockstroh JK, Bhagani S, Benhamou Y, et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Med 2008;9:82–88
de Vries-Sluijs TE, Reijnders JG, Hansen BE, et al. Long-term therapy with tenofovir is effective for patients co-infected with human immunodeficiency virus and hepatitis B virus. Gastroenterology 2010;139:1934–1941
Chien RN, Lin CH, Liaw YF. The effect of lamivudine therapy in hepatic decompensation during acute exacerbation of chronic hepatitis B. J Hepatol 2003;38:322–327
Fontana RJ, Hann HW, Perrillo RP, et al. Determinants of early mortality in patients with decompensated chronic hepatitis B treated with antiviral therapy. Gastroenterology 2002;123:719–727
Jonas MM, Block JM, Haber BA, et al. Treatment of children with chronic hepatitis B virus infection in the United States: patient selection and therapeutic options. Hepatology 2010;52:2192–2205
Iorio R, Giannattasio A, Cirillo F, et al. Long-term outcome in children with chronic hepatitis B: a 24-year observation period. Clin Infect Dis 2007;45:943–949
Jeng WJ, Sheen IS, Liaw YF. Hepatitis B virus DNA level predicts hepatic decompensation in patients with acute exacerbation of chronic hepatitis B. Clin Gastroenterol Hepatol 2010;8:541–545
Sun J, Hou JL, Xie Q, et al. Randomised clinical trial: Efficacy of peginterferon alfa-2a in HBeAg positive chronic hepatitis B patients with lamivudine resistance. Aliment Pharmacol Ther 2011;34:424–431
Liu CJ, Chen PJ, Chen DS, Kao JH. Hepatitis B virus reactivation in patients receiving cancer chemotherapy: natural history, pathogenesis, and management. Hepatol Int 2011; doi: 10.1007/s12072-011-9279-6.
Jang JW, Kwon JH, You CR, et al. Risk of HBV reactivation according to viral status and treatment intensity in patients with hepatocellular carcinoma. Antivir Ther 2011;16:969–977
Katz LH, Fraser A, Gafter-Gvili A, et al. Lamivudine prevents reactivation of hepatitis B and reduces mortality in immune suppressed patients: Systematic review and meta-analysis. J Viral Hepat 2008;15:89–102
Li HR, Huang JJ, Guo HQ, et al. Comparison of entecavir and lamivudine in preventing hepatitis B reactivation in lymphoma patients during chemotherapy. J Viral Hepat 2011;18:877–883
Hui CK, Cheung WW, Zhang HY, et al. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology 2006;131:59–68
Germanidis G, Hytiroglou P, Zakalka M, Settas L. Reactivation of occult hepatitis B virus infection following treatment of refractory rheumatoid arthritis with abatacept. J Hepatol 2011; doi:10.1016/j.jhep.2011.10.011
Vallet-Pichard A, Fontaine H, Mallet V, Pol S. Viral hepatitis in solid organ transplantation other than liver. J Hepatol 2011;55:474–482
Loomba R, Rowley AK, Wesley R, et al. Hepatitis B immunoglobulin and lamivudine improve hepatitis B-relaated outcomes after liver transplantation: meta-analysis. Clin Gastroenterol Hepatol 2008;6:696–700
Gane EJ, Angus PW, Strasser S, et al. Lamivudine plus low-dose hepatitis B immunoglobulin to prevent recurrent hepatitis B following liver transplantation. Gastroenterology 2007;132:931–937
Angus PW, Patterson SJ, Strasser SI, et al. A randomized study of adefovir dipivoxil in place of HBIG in combination with lamivudine as post-liver transplantation hepatitis B prophylaxis. Hepatology 2008;48:1460–1466
Fung J, Cheung C, Chan SC, et al. Entecavir monotherapy is effective in suppressing hepatitis B virus after liver transplantation. Gastroenterology 2011;141:1212–1219
Patterson SJ, Angus PW. Post-liver transplant hepatitis B prophylaxis: the role of oral nucleos(t)ide analogues. Curr Opin Organ Transplant 2009;14:225–230
Cholongitas E, Papatheodoridis GV, Burroughs AK. Liver grafts from anti-hepatitis B core positive donors: a systematic review. J Hepatol 2010;52:272–279
Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol 2009;51:890–897
Goto T, Yoshida H, Tateishi R, et al. Influence of serum HBV DNA load on recurrence of hepatocellular carcinoma after treatment with percutaneous radiofrequency ablation. Hepatol Int 2011;5:767–773
Jang JW, Choi JY, Bae SH, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology 2006;43:233–240
Wong JS, Wong GL, Tsoi KK, et al. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther 2011;33:1104–1112
Chan AC, Chok KS, Yuen WK, et al. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg 2011;146:675–681
Kim JH, Kwon SY, Lee YS, et al. Virologic response to therapy increases health-related quality of life for patients with chronic hepatitis B. Clin Gastroenterol Hepatol 2012;10:291–296
Chotiyaputta W, Peterson C, Ditah FA, et al. Persistence and adherence to nucleos(t)ide analogue treatment for chronic hepatitis B. J Hepatol 2011;54:12–18
Han KH, Kim DY. Chronic HBV infection with persistently normal ALT: not to treat. Hepatol Int 2008;2:185–189
Liaw YF. Prevention and surveillance of hepatitis B virus-related hepatocellular carcinoma. Semin Liver Dis 2005;25(Suppl 1):40–47
Chu CM, Liaw YF. Chronic hepatitis B virus infection acquired in childhood: special emphasis on prognostic and therapeutic implication of delayed HBeAg seroconversion. J Viral Hepat 2007;14:147–152
Papatheodoridis GV, Manolakopoulos S, Liaw YF, Lok A. Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: a systematic review. J Hepatol 2012. doi:10.1016/j.hep.2011.11.030
Hung HF, Chen HH. Cost-effectiveness analysis of prophylactic lamivudine use in preventing vertical transmission of hepatitis B virus infection. Pharmacoeconomics 2011;29:1063–1073
Acknowledgements
The expert pre-meeting was organized and sponsored by the Taiwan Association for the Study of Liver and the Prosperous Foundation, Taipei, Taiwan. The authors thank the secretarial assistance of Ms. Su-Chiung Chu in preparing this statement. The authors also wish to thank Associate Professor Gail Matthews from the Kirby Institute, Sydney, Australia, for her assistance with the sections on HIV-HBV coinfection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liaw, YF., Kao, JH., Piratvisuth, T. et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int 6, 531–561 (2012). https://doi.org/10.1007/s12072-012-9365-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-012-9365-4