Abstract
Objective
Type II mixed cryoglobulinemia (MC) is a systemic vasculitis usually associated with hepatitis C virus (HCV). The present trial was performed to investigate the efficacy of therapy with pegylated interferon alfa-2a (PEG-IFN alfa-2a) plus ribavirin in patients with HCV-related MC vasculitis and evaluate the factors associated with clinical remission of MC.
Methods
A total of 46 consecutive patients with HCV-related Type II MC received PEG-IFN alfa-2a (standard dose 180 mg/week) subcutaneously plus oral ribavirin (800–1,200 mg/day) for 48 weeks. The response to treatment was analyzed by comparing clinical, immunologic, and virologic parameters at the initial evaluation with those observed at the end of follow-up. Logistic regression was used to assess the factors associated with clinical remission.
Results
A total of 22 patients (48%) had a sustained virologic response and were complete clinical responders. Serum cryoglobulin disappeared in 26 of 46 patients (56%), and complement levels normalized in 70% of the patients. In univariate analysis, factors associated with complete clinical response were early virologic response at 4 weeks [OR 1.4 (95% CI 0.1–17.1)], proteinuria [OR 1.4 (95% CI 0.2–8.2)] and the fibrosis score [OR 1.09 (95% CI 0.6–1.9)], peripheral neuropathy [OR 0.9 (95% CI 0.1–6.5)], arthralgia [OR 0.7 (95% CI 0.1–3.9)], sicca syndrome [OR 0.6 (95% CI 0.1–3.2)], cryoglobulin [OR 0.2 (95% CI 0.07–1.09)], and purpura [OR 0.1 (95% CI 0.01–1.3)]. In multivariate analysis, only cryoglobulinemia was independently associated with complete clinical response. No patient had side effects for which discontinuation of therapy was required.
Conclusion
The results indicated that treatment with PEG-IFN alfa-2a plus ribavirin can achieve a complete clinical response in patients with HCV-related MC. Complete clinical response correlates with the eradication of HCV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mixed cryoglobulinemia (MC) is a systemic vasculitis which leads to clinical manifestations ranging from the MC syndrome (purpura, arthralgia, and asthenia) to more serious lesions with neurologic and renal involvement. 60–80% of patients with MC are hepatitis C virus (HCV) infected [1]. The primary role of HCV in the mechanism of cryoprecipitation is mainly suggested by its selective concentration in cryoglobulins [2].
Therapeutic strategies can target either the viral trigger (HCV if present) or pathogenic events downstream the triggering infection, for example, the proliferation B-cells directly. Antiviral therapy should be considered as a first-line treatment in many HCV-positive patients. However, it may prove ineffective, contraindicated, or poorly tolerated in some cases. The other available treatment, such as cytotoxic agents and steroids, may lead to life-threatening complications and may be difficult to manage in the long term [3–5].
Clinical improvement of HCV-related vasculitis correlates with virologic response, that is, negative or significant decrease in the serum HCV RNA level. The combination of pegylated interferon (PEG-IFN) plus ribavirin is the current standard for the treatment of patients with chronic HCV infection [6]. This combination therapy effectively treated HCV-related vasculitis in patients with HCV genotype 2, 3 [7, 8].
The present trial was performed to investigate the efficacy of PEG-IFN alfa-2a plus ribavirin in patients with HCV genotype 4-related MC vasculitis.
Patients and methods
Patients
Our study group comprised of 46 consecutive patients with HCV-related MC (Table 1). The patients showed positivity for MC in serum (cryoglobulin level 0.05 g/l) on at least two determinations. All patients were MC type II associated with the triad of purpura–arthralgia–asthenia and sometimes with renal or neurologic involvement. All patients were positive for HCV RNA and negative for hepatitis B surface antigen. All of the patients had histologically proven chronic active liver disease. Inclusion criteria for our study were as follows: (1) chronic active HCV infection, (2) signs of MC vasculitis in the absence of any alternative condition known to cause vasculitis, (3) treatment with PEG-IFN plus ribavirin for 48 weeks, and (4) a minimum of 6 months of follow-up after stopping anti-HCV treatment.
Thirty of the 46 patients had histologically confirmed systemic vasculitis. The remaining patients without histological confirmation of systemic vasculitis presented with typical signs of “essential” MC vasculitis, that is, arthralgia, asthenia, purpura of the lower extremities, and/or polyneuropathy [by electromyography (EMG) study]. For each patient, clinical and biologic data were recorded at the time of the initial evaluation, at the end of antiviral treatment, 6 months after stopping antiviral treatment, and at the end of follow-up. The clinical data included age, sex, neurologic involvement, recent-onset hypertension, cutaneous involvement (Raynaud’s phenomenon, purpura, livedo, and distal ulcers), arthralgia, myalgia, and clinical signs of hepatic involvement. The laboratory evaluation included a complete hemogram, a serum chemistry profile and determination of the C3 and C4 fractions of complement, rheumatoid factor (RF), and cryoglobulin. A urinalysis was completed to screen for hematuria, and a 24-h urine collection was performed to quantify daily excretion of protein.
Virologic and immunologic serum markers
HCV antibodies and RNA were detected by specific third-generation immunoassay. Serum HCV RNA was measured by reverse transcription-polymerase chain reaction (RT-PCR) (Roche diagnostics, Neuilly-sur-Serine, France). HCV genotyping was performed using a second-generation line probe assay (LiPA; Innogenetics, Brussels, Belgium).
Liver biopsy specimens were evaluated according to the previously validated Metavir scoring system [9]. Cryoglobulin levels were measured and classified as previously described [10]. The immunologic evaluation included determination of RF and complement components, using standard methods.
Treatment and criteria of response
All patients received PEG-IFN alfa-2a at a standard dosage of 180 mg/week subcutaneously plus oral ribavirin (800–1,200 mg/day) for 12 months. In cases of renal insufficiency and/or severe polyneuropathy (seven patients), corticosteroids were given in a short-term, low-dose regimen (prednisone 0.5 mg/kg/day for 2 weeks, with a rapid decrease to 10 mg/day within 6 weeks). The responses to treatment were analyzed by comparing clinical, virological, and immunological parameters at initial evaluation and at 6 months after the end of therapy. Clinical response was defined by the evolution of the main clinical signs: purpura, peripheral neuropathy (clinical and electrophysiologic improvement in two successive examinations), arthralgia, and renal involvement. Relapse was defined as the reappearance of clinical signs of vasculitis. A sustained virologic response was defined by the absence of detectable serum HCV RNA 6 months after the discontinuation of antiviral treatment; the remaining patients were classified as virologic non-responders. Patients with partial responses were not considered as clinical responders in our study. A partial immunologic response was defined as a decrease in the serum cryoglobulin level of >50% compared with baseline. Patients that cleared cryoglobulins without clearance of the virus were considered immunological responders with virologic relapse.
Exclusion criteria
-
Recent (within 4 weeks) initiation of or increase in immunosuppressive therapy.
-
Active systemic infection (other than hepatitis C).
-
Pregnancy or breast feeding.
-
Prior treatment with rituximab.
-
Significant renal insufficiency (creatinine clearance less than 30 ml/min).
-
Presence of life-threatening vasculitis, such as rapidly progressive glomerulonephritis, CNS vasculitis, cardiac disease due to active vasculitis, or GI vasculitis (defined by ischemic bowel, perforation, or infarction).
-
Significant hepatic insufficiency: Child-Pugh classification of B or C, history of variceal bleeding, encephalopathy, or history of liver transplantation.
-
Co-infection with either HBV or HIV.
Statistical analysis
Continuous variables were dichotomized using median values (age, duration of HCV infection, HCV RNA level, alanine aminotransferase (ALT) level, cryoglobulin level, duration of anti-HCV therapy, and ribavirin dosage). Odds ratios (ORs) and their 95% confidence intervals (95% CIs) were also computed. Multivariate models were performed using multiple logistic regressions. All factors with a p value lower than 0.1 in the univariate analyses were initially included. Factor selection was determined using a backward procedure based on the Akaike criteria. Comparisons of variables before and after antiviral therapy were performed using Wilcoxon’s paired test and McNemar’s test. All tests were two-sided at the 0.05 significance level.
Results
A total of 46 patients with HCV-related MC type ll (36 men and 10 women) with a mean age of 42 ± 12.5 years (range 29–59 years) were included. The mode of transmission of HCV was suggested to be due to blood transfusion in 4 patients, after surgical procedures in 10 patients, occupational in 6 patients, after sexual exposure in 1 patient, after parentral treatment in 7 patients, intravenous drug use in 1 patient, and unknown in 15 patients.
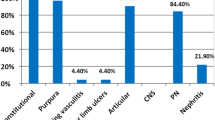
Clinical manifestations of MC included purpura in 33 patients (71.7%), peripheral neuropathy in 30 (65.2%), arthralgia in 33 (71.7%), arthritis in 21 (45.6%), renal involvement in 18 (39%), sicca syndrome in 28 (60.9%), and Raynaud’s phenomenon in 2 (4.3%). 10 patients (21.7%) had proteinuria. The mean cryoglobulin level was 1.03 g/l (range 0.25–2.9). The serum C4 level was low in 36 patients (78.3%), and 34 patients (74%) had rheumatoid factor activity. The mean ± SD HCV RNA level was 4.8 ± 0.7 log copies/ml (range 4–6.2). All patients were genotype 4. Liver biopsy revealed that all patients had signs of chronic active hepatitis, with a mean ± SD Metavir activity score of 1.6 ± 0.7 and a mean ± SD fibrosis score of 1.6 ± 1.4.
Following antiviral therapy, there was a significant improvement in the percentage of patients with purpura (p < 0.007), peripheral neuropathy (p < 0.001), arthralgia (p < 0.003), and arthritis (p < 0.003) (Table 1) was noticed.
The main virologic parameters (i.e., HCV RNA and ALT) improved significantly. Sustained viral response was achieved in 48% of patients (Table 1). The cryoglobulin level decreased from a mean of 1.03 ± 0.69 to 0.24 ± 0.33 gm/liter (<0.001). The C4 complement level normalized in 30 of 36 patients (<0.001). The percentage of patients with cryoglobulin positive significantly decreased from 100 to 44% (<0.001) (Table 1; Fig. 1).
Complete clinical response correlates with the eradication of HCV (p < 0.005) (Fig. 2).
In univariate analysis, factors associated with complete clinical response were early virologic response at 4 weeks [OR 1.4 (95% CI 0.1–17.1)], proteinuria [OR 1.4 (95% CI 0.2–8.2)], and the fibrosis score [OR 1.09 (95% CI 0.6–1.9)]; factors negatively associated with complete clinical response were peripheral neuropathy [OR 0.9 (95% CI 0.1–6.5)], arthralgia [OR 0.7 (95% CI 0.1–3.9)], sicca syndrome [OR 0.6 (95% CI 0.1–3.2)], cryoglobulin [OR 0.2 (95% CI 0.07–1.09)], and purpura [OR 0.1 (95% CI 0.01–1.3)] (Table 2). In multivariate analysis, only cryoglobulinemia was independently associated with complete clinical response.
At the end of the follow-up, regardless of the virologic response no significant changes in the mean serum level of creatinine or the mean GFR were seen.
Treatment was well tolerated in 78.3% of patients; in the remaining, patient side effects included fatigue in 13%, fever in 8.7%, myalgia in 21.7%, anemia in 15.2%, thrombocytopenia in one patient, neutropenia in one patient, and depression in two patients. No therapy interruptions were needed. A dosage reduction of antiviral therapy was required in two patients because of hematologic complications.
Discussion
The current standard initial therapy for patients with chronic HCV infection is combination therapy (PEG-IFN plus ribavirin) with a response rate of 48–88% according to HCV genotype [6]. In our study, the sustained viral response rate was only 48% because all our patients were genotype 4 which is predominant in the Middle East and Africa, although recent evidence suggests that genotype 4 is spreading in Western countries [11].
PEG-IFN, alone or combined with ribavirin, has proven to be more effective than IFN-α alone or in combination with ribavirin, in patients with HCV infection, particularly those infected with genotype 4. Patients with HCV-related MC also seemed to benefit from this new combination therapy, even though 44% of patients relapsed a few weeks after the end of therapy in Mazzaro’s study [12]. Cacoub et al. and Saadoun et al. [7, 8] obtained remarkable results in clinical, virological, and serologic parameters of patients with cryoglobulinemic vasculitis.
Nowadays, the therapeutic strategy for HCV eradication in chronic hepatitis patients undergoing treatment with the combination of PEG-IFN and ribavirin takes into account genotype. Patients with genotype 2 or 3 could be treated for 24 weeks or even shorter duration [13–15]. High viral load, advanced fibrosis, obesity, black ethnicity, and male gender are negative prognostic indexes. In these cases, a more prolonged treatment could be considered (up to 48 weeks). The presence of genotype 1 calls for a 48-week course of therapy with weekly injections of either PEG-IFN-α2a or -α2b, and oral ribavirin 1,000 mg/day for patients weighing 75 kg or less, or 1,200 mg/day for patients weighing greater than 75 kg. While we are waiting for adequate clinical trials on genotype 4, patients infected with this genotype should be treated the same as those infected genotype 1. So in our study, patients were treated for 48 weeks.
The presumed efficacy of the antiviral therapy with PEG-IFN in MC, which is drawn from limited observations of small cohorts of patients, should be the target of future trials. At present, some data suggest that patients with HCV and circulating cryoglobulins do respond at least as well as HCV-positive patients without cryoglobulinemia [16].
Regarding the clinical response, in our study there was a significant improvement in percentage of patients with purpura (p < 0.007), peripheral neuropathy (p < 0.001), arthralgia(p < 0.003), and arthritis (p < 0.003). Similar results were reported in a large cohort study by Saadoun et al. [8]. Many other studies found also a significant clinical response to antiviral therapy [17–19].
Type II mixed cryoglobulin syndrome is a systemic vasculitis mainly linked to immune complex deposition in several organs and to HCV infection [20]. In our study, the overall rates of complete clinical and virological responses of HCV-MC vasculitis were closely correlated. But few patients with complete clinical response had no sustained viral response (viral relapse). According to the previous reports [8, 21, 22], HCV-MC vasculitis may remain in clinical remission despite the persistence of viremia.
In our study, as indicated by univariate analysis, many factors were associated with clinical response including early virologic response, proteinuria, the fibrosis score, sicca syndrome, cryoglobulin, and purpura. Only cryoglobulin level was associated with complete clinical response in multivariate analysis. Clearance of cryoglobulins is a major goal of therapy in MC, since recurrence of vasculitis, sometimes in association with B cell lymphoma, has been reported in patients with persistent cryoglobulins despite successful treatment of HCV [3]. In our study, clearance of cryoglobulins was noted in 26 (56%) out of 46 patients and this was in accordance with other studies [7, 8].
Corticosteroids and immunosuppressive drugs were not associated with an improved clinical outcome of MC as compared with antiviral therapy alone [4, 7, 23]. However, our study was not designed to assess their efficacy in HCV-MC vasculitis, since only patients who had more severe disease (renal insufficiency, severe polyneuropathy, and/or life-threatening complications) received additional therapy with corticosteroids and, less commonly, immunosuppressive agents and they were not enrolled in our study. More recently, data on the efficacy of rituximab, an anti-CD20 monoclonal antibody, have been reported in patients with HCV-MC vasculitis. It appears that rituximab is very efficacious against cryoglobulin production and its clinical consequences (i.e., inflammatory vascular lesions). However, those studies did not allow conclusions to be drawn concerning the efficacy of anti-CD20 monoclonal antibody on peripheral neuropathy and nephropathy. The lack of efficacy in the clearance of HCV virus as well as the potential increase in HCV viral load emphasizes the need for combined antiviral therapy to block the HCV infection [4, 24, 25].
The proportion of HCV-MC vasculitis patients reporting any adverse events to anti-HCV therapy was 32% in our study. This was similar to the proportions previously reported in HCV-infected patients without MC [6, 26]. The most frequent adverse events observed were fatigue, fever, cytopenia, and myalgia. However, no therapy interruptions were needed.
In summary, the findings of our study underscore the idea that PEG-IFN alfa-2a plus ribavirin should be considered for induction therapy in patients with HCV-MC vasculitis. Inducing a sustained virologic and clinical response and minimizing the use of immunosuppressive drugs are the main goals in the treatment of patients with HCV-MC vasculitis. Although this approach affords a satisfactory response rate, additional therapy (i.e., anti-CD20 monoclonal antibody or new immunosuppressive agents) may be needed in MC patients with partial or no response. Further studies may be needed to assess the combination therapy.
References
Cacoub P, Poynard T, Ghillani P, for the MULTIVIRC Group, et al. Extrahepatic manifestations of chronic hepatitis C. Arthritis Rheum 1999;42:2204–2212
Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med 1992;327:1490–1495
Misiani R, Bellavita P, Fenili D, et al. Interferon alfa-2a therapy in cryoglobulinemia associated with hepatitis C virus. N Engl J Med 1994;330:751–756
Dammacco F, Sansonno D, Han JH, et al. Natural interferon versus its combination with 6-methyl-prednisolone in the therapy of type II mixed cryoglobulinemia: a long-term, randomized, controlled study. Blood 1994;84:3336–3343
Tarantino A, Campise M, Banfi G, et al. Long-term predictors of survival in essential mixed cryoglobulinemic glomerulonephritis. Kidney Int 1995;47:618–623
Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975–982
Cacoub P, Saadoun D, Sene D, Limal N, Piette JC. Treatment of hepatitis C virus-related systemic vasculitis. J Rheumatol 2005;32:2078–2082
Saadoun D, Resche-Rigon M, Thibault V, Piette V, Cacoub P. Antiviral therapy for hepatitis C virus-associated mixed cryoglobulinemia vasculitis: a long-term followup study. Arthritis Rheum 2006;54(11):3696–3706
The French METAVIR, Group CooperativeStudy, Bedossa P. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994;20:15–20
Musset L, Diemert MC, Taibi F, et al. Characterization of cryoglobulins by immunoblotting. Clin Chem 1992;38:798–802
Rustgi VK. The epidemiology of hepatitis C infection in the United States. J Gastroenterol 2007;42:513–521
Mazzaro C, Zorat F, Caizzi M, et al. Treatment with peg-interferon α-2b and ribavirin of hepatitis C virus-associated mixed cryoglobulinemia: a pilot study. J Hepatol 2005;42:632–638
Hauser SC, Pardi DS, Poterucha JJ. Mayo Clinic Gastroenterology and Hepatology Board Review. Mayo Clinic Scientific Press, MN (2006)
Liang TJ. Shortened therapy for hepatitis C virus genotype 2 or 3. Is less more? N Engl J Med 2007;357:176–178
Ali S, Meidinger RR, Kayali Z et al. Outcomes of pegylated interferon and ribavirin therapy for HCV patients with cryoglobulinemia as compared to patients without cryoglobulinemia. Gastroenterology 2007;132:A790
Cacoub P, Lidove O, Maisonobe T, et al. Interferon and ribavirin treatment in patients with hepatitis C virus-related systemic vasculitis. Arthritis Rheum 2002;46:3317–3326
Naarendorp M, Kallemuchikkal U, Nuovo GJ, Gorevic PD. Long-term efficacy of interferon for extra hepatic disease associated with hepatitis C virus infection. J Rheumatol 2001;28:2466–2473
Zuckerman E, Keren D, Slobodin G, et al. Treatment of refractory, symptomatic, hepatitis C virus related mixed cryoglobulinemia with ribavirin and interferon. J Rheumatol 2000;27:2172–2178
Tallarita T, Gagliano M, Corona D, et al. (2009) Successful combination of Rituximab and plasma exchange in the treatment of cryoglobulinemic vasculitis with skin ulcers: a case report. Cases J 2:7859
Lamprecht P, Moosig F, Gause A, et al. Immunological and clinical follow up of hepatitis C virus associated cryoglobulinaemic vasculitis. Ann Rheum Dis 2001;60:385–390
Alric L, Plaisier E, Thebault S, et al. Influence of antiviral therapy in hepatitis C virus-associated cryoglobulinemic MPGN. Am J Kidney Dis 2004;43:617–623
Gota C, Fessler BJ, Calabrese LH, Cooper SM. Persistent cryoglobulinemic vasculitis following successful treatment of hepatitis C virus. J Rheumatol 2005;32:1164–1167
Roccatello D, Baldovino S, Rossi D, et al. Long-term effects of anti-CD20 monoclonal antibody treatment of cryoglobulinaemic glomerulonephritis. Nephrol Dial Transplant 2004;19:3054–3061
Tallarita T, Gagliano M, Corona D, et al. Successful combination of Rituximab and plasma exchange in the treatment of cryoglobulinemic vasculitis with skin ulcers: a case report. Cases J 2009;2:7859
Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of Chronic hepatitis C: a randomized trial. Lancet 2001;358:958–965
Dammacco F, Tucci FA, Lauletta G, et al. Pegylated interferon-alpha, ribavirin, and rituximab combined therapy of hepatitis C virus-related mixed cryoglobulinemia: A long-term study. Blood 2010;116(3):343–353
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El Khayat, H.R., Fouad, Y.M., Ahmad, E.A. et al. Hepatitis C virus (genotype 4)-associated mixed cryoglobulinemia vasculitis: effects of antiviral treatment. Hepatol Int 6, 606–612 (2012). https://doi.org/10.1007/s12072-011-9303-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-011-9303-x