Abstract
Veno-venous extracorporeal membrane oxygenation (VV-ECMO) is a form of extracorporeal life support that provides total gas exchange (CO2 and O2) within the central venous circulation. The bicaval dual lumen cannula (DLC) is an option for patients requiring respiratory support with VV-ECMO. The catheter is inserted via the internal jugular vein into the superior and inferior vena cava, drains blood into the ECMO circuit for gas exchange, and then returns arterialized blood to the right heart for circulation. The DLC facilitates physical therapy, ambulation, and early extubation. This chapter will review the uses, advantages, and unique complications of the DLC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extracorporeal membrane oxygenation (ECMO) provides cardiopulmonary support for critically ill patients who fail to respond to conventional treatment and are expected to recover within days to weeks; or after receiving curative therapy (surgical procedure, ventricular assist device, or organ transplant) [1]. Patients in severe respiratory failure are initiated on temporary ECMO support as rescue for acute deterioration, or as a bridge to a decision on a treatment course, recovery, lung transplant, primary graft dysfunction, or post-transplant infection [2]. The Extracorporeal Life Support Organization (ELSO), an international voluntary database capturing over 100,000 ECMO cases to date, reports overall survival to discharge of 66% for neonates, 53% for pediatrics, and 48% for adults. ECMO outcomes vary with patient population, mode of ECMO support, and indication. Survival for veno-venous (VV)-ECMO is approximately 80% for neonates, 65% for pediatrics, and 60% for adults when used for elective or urgent respiratory applications [3, 4]. Extracorporeal cardiopulmonary resuscitation (ECPR) utilizes primarily veno-arterial ECMO and is used emergently for acute cardiac and pulmonary arrest or resuscitation.
Neonatal patients placed on VV-ECMO typically have improved outcomes compared to adults, which can be attributed to a decreased incidence of parenchymal disease, chronic lung damage, other comorbidities, as well as an ability to better recover from major physiologic insult [5]. In the mid-1980s to early 1990s, prospective randomized control trials in the United States and Great Britain comparing ECMO to conventional ventilator management in neonates with respiratory failure demonstrated ECMO was a safe alternative with better survival than conventional management [6,7,8]. By the late 1980s, veno-arterial ECMO (VA-ECMO) via the internal jugular (IJ) vein and carotid artery was standard therapy for neonates with refractory respiratory failure [9]. When only respiratory support is required, however, VV-ECMO is safer than VA-ECMO because it spares the carotid artery, directs micro emboli toward pulmonary circulation, and perfuses pulmonary vasculature with oxygenated blood. Early experience with neonatal VV-ECMO placed a venous drainage cannula in the IJ vein and a reinfusion cannula in the femoral vein [5]. Unfortunately, the femoral vein is often too small and fragile to accommodate an ECMO cannula in patients less than 10 kg limiting application for neonatal VV-ECMO [10]. The first dual lumen cannula (DLC) was developed to provide simultaneous drainage and reinfusion through a single cannula placed via the IJ vein in neonates [11,12,13]. Neonatal DLCs provide adequate flow with a single 13–14 French (Fr.) cannula, where 3 Fr. is equivalent to 1 mm diameter. The original design provided a 2:1 ratio between the drainage portion cross-sectional area and the reinfusion portion to allow adequate venous drainage by gravity fluid relative to positive pressure reinfusion [11]. In 1989, the first clinical experience with a 14 Fr. neonatal DLC was reported [9]. Of the 21 neonates with respiratory failure, 17 were successfully cannulated with a DLC placed in the right atrium (RA) via the IJ vein. One patient was immediately converted to VA-ECMO for hypotension with asystole and another was converted after 24 h for insufficient gas exchange with maximum flow. The remaining 15 were supported with 100–150 ml/kg/min of veno-venous dual lumen extracorporeal membrane oxygenation (VVDL-ECMO) flow and successfully decannulated [9]. Initial success was amplified in a 27-center retrospective study comparing neonatal respiratory failure managed with VA-ECMO or VVDL-ECMO. Anderson et al. reported 87% survival for 135 VA-ECMO cases and 95% survival for 108 VVDL-ECMO cases, fueling expansion of VVDL-ECMO [14]. Widespread availability of the DLC and establishment of the ELSO database in 1989 led to larger propensity matched studies and database reviews confirming the utility of VVDL-ECMO for neonatal respiratory failure [3, 15,16,17].
The initial commercial DLC (Kendall Healthcare Products Co.) was inserted through the internal jugular vein into the right atrium (unicaval) and included a series of drainage ports facing the superior vena cava/RA junction opposite reinfusion ports directed distally roughly toward the tricuspid valve. The drainage and reinfusion lumens both traveled the length of the cannula [9, 13]. This design drained 10–65% (average 20%) of reinfused oxygenated blood back into the oxygenator before reaching systemic circulation, a phenomenon known as recirculation [9]. The unicaval DLCs available today include the OriGen DLC (Origen Biomedical, Austin, TX) and NovaPort twin (Xenios, Heilbronn, Germany). Several published reviews show recirculation is flow dependent with higher flows directly proportional to recirculation ranging from 20 to 40%.
A new generation of single site access DLC with bicaval venous drainage from the superior vena cava (SVC) and inferior vena cava (IVC) repositioned the drainage ports into the SVC and IVC, significantly reducing recirculation to as low as 2%. This design was marketed as the Avalon Elite DLC in Europe in 2008 and was quickly incorporated into the practice of adult ECMO [18]. The cannula release coincided with the H1N1 worldwide outbreak and the conventional ventilation or ECMO for severe adult respiratory failure (CESAR) trial which stimulated use of single venous bicaval access for total gas exchange in otherwise relatively healthy young adults for acute respiratory distress syndrome (ARDS) [19,20,21]. The bicaval DLC became available in the USA in 2009 [22, 23]. Shortly after introduction, these more efficient DLCs exploded in use comprising 71% of all pediatric ECMO cannulas used in 2011 [24]. In 2018 the crescent bicaval DLC (MC3, Dexter, MI) named for the crescent moon-shaped division made by the reinfusion lumen inside the drainage lumen was introduced with Food and Drug Administration (FDA) clearance for prolonged use with VV-ECMO.
Advantages of single site cannulation via the IJ vein in pediatric and adult patients include increased ambulation, easier participation in physical therapy, and improved patient comfort after extubation. Likewise, the risk of infection is potentially lower with a single cannulation site, especially the absence of groin cannulation. The downside is the need for image guided insertion, and the risk of displacement or perforation if improperly inserted, and the higher cost of a DLC compared to single lumen catheters. This chapter will review how the DLC is used during ECMO, insertion and decannulation, and common complications.
Modes of ECMO support using the dual lumen cannula
The DLC can be used for both traditional (4–6 L/min) and low-flow (0.4–1 L/min) VV-ECMO [25]. Low-flow VV-ECMO facilitates carbon dioxide removal by diffusion for hypercapnic patients and is known as extracorporeal carbon dioxide removal (ECCO2R; pronounced “ee-kor”) [26]. Kolobow and colleagues pioneered the understanding of carbon dioxide removal as a separate physiologic process from oxygenation. Carbon dioxide removal is dependent on gas diffusion via the concentration gradient either through the native lung alveoli or with ECMO created by sweep gas of the membrane oxygenator. Uncoupling the ideas of oxygenation and carbon dioxide, Kolobow et al. championed what is now known as low tidal volume protective mechanical ventilation to allow lung rest (with resultant CO2 retention) combined with ECCO2R for carbon dioxide removal in ARDS patients [25, 27, 28]. Recently, ECCO2R has been studied in situations of status asthmaticus and acute chronic obstructive pulmonary disease (COPD) exacerbations, facilitating extubation and in some cases avoiding intubation [29,30,31]. An unblinded trial of adults (n = 5) with acute COPD exacerbations were treated with single site ECCO2R using the Avalon Elite catheter (20 Fr and 23 Fr for bicaval drainage and minimal recirculation) to achieve early extubation. The pilot trial showed feasibility of extubation within 6.8±8.3 h after initiation of ECCO2R [29]. This is also supported by case reports of ECCO2R in near fatal asthma case series by Brenner and Schneider [30, 31]. Use of ECCO2R is a subject of ongoing discussion in the literature because it carries the same risk as VV-ECMO without the benefit of oxygenation or the option to add hemodynamic support in cases of acute deterioration.
Traditional VV-ECMO is the preferred mode of support for respiratory failure refractory to conventional management with stable hemodynamics because it provides oxygenation and carbon dioxide removal. Emergent cannulation at the bedside is percutaneous via the IJ and femoral vein. Preferred non-emergent cannulation involves a single DLC via the right IJ under fluoroscopic guidance [32]. Typical pre-ECMO ventilator settings include 80–100% FiO2, 10–20 cmH2O positive end expiratory pressure (PEEP), 30–40 cm of water (cmH2O) peak airway pressure, and respiratory rate of 40 with persistent hypoxia and/or hypercapnia [32]. VV-ECMO minimizes injury from high pressure and volume settings or prolonged excessive fraction of inspired oxygen (FiO2), allowing for lung protective mechanical ventilation settings or extubation [33]. Spontaneously breathing, nonsedated VV-ECMO patients are ambulatory and can participate in preoperative rehabilitation to maximize their clinical status. The recent Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA) trial randomized ARDS patients to VV-ECMO (n = 124) or conventional management (n = 125) with 35 patients crossed over to the VV-ECMO group for refractory hypoxemia. Even after crossover, intent-to-treat analysis found VV-ECMO was non-inferior to conventional management with 60-day survival of 35% in the VV-ECMO group and 46% in the conventional management group [34]. A retrospective review of VV-ECMO patients bridged to lung transplantation showed 6-month survival of those who underwent successful lung transplant was 80% (n = 13/16) in the non-intubated ECMO group versus 50% (n = 12/24) in the mechanical ventilation group (p = 0.02) [35].
Beyond VV-ECMO, DLCs are used for “hybrid approaches” to ECMO that add venous or arterial access to complement the existing system. Hybrid approaches accommodate for the changing physiology and clinical conditions in ECMO patients allowing for a more fluid approach to patient management. For example, if VV-ECMO is initiated preoperatively, the Avalon catheter supplies adequate venous drainage for cardiopulmonary bypass [36]. In cases of worsening cardiac dysfunction, hemodynamic support is achieved with the addition of another cannula to the subclavian artery, converting the VV-ECMO circuit to a veno-arterial-venous (VAV) circuit [37]. Likewise, converting a VA-ECMO circuit to VAV by exchanging the femoral venous drainage cannula for a DLC placed in the internal jugular can improve oxygenation. Overall, hybrid ECMO only comprises 2% of all ECMO runs [38].
Hybrid configurations are indicated if adequate perfusion is not achieved or if complications arise secondary to cannulation strategy such as differential hypoxia in VA-ECMO. Emergent VA-ECMO cannulation via the femoral vein-femoral artery relies on native cardiopulmonary function to adequately perfuse the coronary arteries, head, and upper extremity perfusion, which is dependent on the location of the mixing cloud in the aorta. As cardiopulmonary function becomes compromised, the differential hypoxia in peripheral VA-ECMO worsens, with arterialized blood reinfused at the groin mixing unpredictably with forward flow. This differential hypoxia known as Harlequin syndrome or north-south syndrome can be managed by switching the patient to a VAV hybrid configuration. A sheep model of compromised lung function with a left ventricular oxygen saturation (LV O2 Sat) of 70 ± 8% on VA-ECMO was increased to 96 ± 6% when the ECMO configuration was changed to a DLC in the right internal jugular and an arterial reinfusion cannula in the femoral artery [39]. This highlights the ability of the addition of a DLC to help solve differential hypoxia presenting in an existing VA-ECMO circuit or the addition of an arterial cannula to an existing VV DLC ECMO configuration to aid cardiac compromise. The “sport model,” an ambulatory modified VA-ECMO circuit cannulated via the right jugular vein and the subclavian or axillary artery can use a single or dual lumen cannula for venous drainage [40].
Venous drainage is often the limiting factor in achieving satisfactory blood flow in peripheral ECMO. Additional venous drainage cannulas (single or dual lumen) allow for increased drainage to improve overall ECMO flow. When a DLC VV-ECMO circuit fails to achieve adequate oxygenation, a secondary venous drainage cannula is added to the circuit in a configuration known as veno-veno-venous (VVV) ECMO. Venous drainage can be further increased by modifying the DLC to drain venous blood from the reinfusion port directed toward the right atrium as well as the SVC and IVC. In this scenario, a Y-connector is used to connect both lumens of the DLC. Reports of veno-veno-veno-arterial (VVVA) and veno-veno-arterial-venous (VVAV) ECMO exist in the literature; however, they are rare, and the DLC is used as a source of venous drainage [41].
Choosing a bicaval dual lumen cannula
Cannula size is chosen according to patient needs determined by body habitus, cardiac index (cardiac output divided by body surface area), and required flow. Table 1 compares the commercially available DLCs. DLC sizes range from 13 to 31 Fr. with the smallest 13 Fr. cannulas used in neonates > 2.5 kg. The Avalon Elite (Getinge) does not make a size smaller than 13 Fr. Adult sizes range from 20 to 31 Fr., with the smaller 13 –19 Fr. sizes also available. The Crescent (Medtronic) is designed for the adult market and the smallest size is 24 Fr. The most common bicaval dual lumen cannula sizes used in adult patients are 27–31 Fr. with larger cannulas capable of maintaining higher flow rates, but associated with increased complications and bleeding at the cannulation site [42]. The Crescent 30 Fr. supports up to 7 L/min of flow, and the Avalon 27 Fr. supports up to 5 L/min of flow. The smaller 19 Fr. Avalon supports up to 2.5 L/min of flow. The unicaval NovaPort twin DLC used for extracorporeal CO2 removal (ECCO2R) is optimized for lower flows with the 22 Fr. supporting up to 1.5 L/min and the 18 Fr. supporting up to 1.0 L/min of flow.
When choosing an appropriate cannula, compatibility with the ECMO circuit pump must be considered. Currently, ECMO circuits control blood flow with roller pumps and centrifugal pumps. There has been a shift away from positive pressure roller pumps because they are not afterload sensitive, so there is potential for circuit rupture when a kink or other occlusion occurs upstream of a roller pump [43]. Afterload sensitive centrifugal pumps make circuit rupture unlikely but put more stress on drainage cannula. While positive pressure roller pumps rely on passive venous drainage, centrifugal pumps utilize low pressures generated at the pump inlet. DLCs originally designed for use with roller pumps collapsed from the pressure drop created by centrifugal pumps and were redesigned with wire reinforcement. Non-reinforced DLCs are still manufactured for use with roller pumps but are not recommended with centrifugal pumps.
VV-ECMO using a bicaval DLC with an atrial septostomy or preexisting atrial septal defect (ASD) is used to relieve right ventricular or pulmonary failure [44]. This was shown in two adult cases with preexisting ASD and Eisenmenger’s syndrome using a 23 Fr Avalon Elite catheter to relieve acute pulmonary hypertensive crisis. After ECMO cannulation the patients’ arterial O2 saturation went from 64 to 92% and from 75 to 98%, respectively [45]. Kon et al. showed the feasibility of using a bicaval DLC and ASD (created or congenital) to create a physiologic right to left shunt allowing oxygenated blood to be delivered directly to the left heart. In 2016 a man presenting with right ventricular dysfunction secondary to severe pulmonary fibrosis was treated with an atrial septostomy and a 31F Avalon Elite catheter. This patient required a repeat atrial septostomy on postcannulation day 12 which relieved the patient’s hypoxia and vasopressor requirement. The use of atrial septostomy and VV-ECMO with the Avalon catheter in this case improved the man’s RV dysfunction, hypoxia, and hypercarbia [46].
Insertion
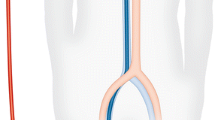
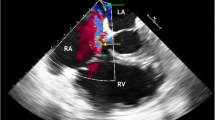
Right internal jugular Avalon cannulas are inserted 31 cm into the central vascular system using the Seldinger technique, percutaneously placed in the vessel using a guidewire and serial dilations under fluoroscopic and/or echocardiographic insertion guidance [18] (Fig. 1). The catheter traverses the superior vena cava, right atrium, with the tip placed in the inferior vena cava. Venous drainage ports positioned in the superior and inferior vena cava draw blood to supply the oxygenator. After gas exchange, the blood is returned to the infusion lumen of the catheter, terminating at a port directed toward the tricuspid valve that delivers a jet of arterialized blood to the right heart (Fig. 2). Insertion of the OriGen DLC is also percutaneous into the right IJ vein, but the tip of the cannula is inserted into the RA. Likewise, the 18 and 22 Fr. NovaPort twin DLCs are inserted 17 cm via the IJ vein into the RA. The largest NovaPort twin 24 Fr. DLC is inserted into the femoral vessel.
Weaning trial and decannulation
Weaning protocols are a matter of ongoing discussion and vary by center [47]. Our center has developed an algorithm for pre-weaning evaluation of all ECMO support modes and disease etiologies. Before ECMO is weaned, the patient is typically afebrile, euvolemic, and has an improved chest radiograph and acceptable resolution or treatment of the initial indication. The VV-ECMO trial off consists of weaning and clamping the sweep gas while maintaining VV pump flow and monitoring the patient’s respiratory function on post-ECMO mechanical ventilation settings. Lung function is monitored for 4 to 24 h. If oxygen saturation (SaO2) and partial pressure of carbon dioxide (PCO2) remain stable, the patient is ready to be weaned.
Care must be taken to avoid air embolism. During decannulation, the sedated patient is placed in Trendelenburg position with a short ventilator pause. Pressure is applied to the exit site and continuously maintained while purse-string suture is used to close the skin.
Complications
The DLC is considered technically difficult to insert with a 10% rate of cannula migration and malpositioning [48]. The distal tip of bicaval DLC is inserted into the IVC in close proximity to the right atrium and hepatic vessels distally, introducing risk of injury by perforation and cannula migration [49]. Perforation and tamponade occur (3–15%) when the guidewire is misplaced across the tricuspid valve into the right ventricle [49, 50]. A stiff guidewire can directly perforate the heart wall, and a flexible guidewire can loop inside the right heart chamber and cause perforation secondary to cannula insertion. User inexperience and unfamiliarity with bedside fluoroscopy and transesophageal echocardiography (TEE) have been highlighted as a cause of complication during DLC insertion. Fluoroscopy and TEE should be used to visualize the guidewire traverse the SVC, RA, and IVC and confirm placement beyond the level of the renal veins prior to cannulation [51]. Visualization of the guidewire in the SVC and IVC does not exclude looping within the right atrium/right ventricle [52]. A single-center chart review of 72 patients cannulated with the Avalon Elite DLC reported two cases of cardiac perforation during insertion despite use of fluoroscopic guidance [49]. A single-center chart review (2008–2010) of 25 neonates cannulated with either the non-wire reinforced OriGen DLC or wire reinforced Avalon Elite DLC under TEE guidance showed an increased risk of cardiac perforation with the wire reinforced Avalon (n = 2/14) versus the non-wire reinforced OriGen (n = 0/11) [50]. However, a review of the ELSO registry (1998–2011) that included 1323 pediatric VV-ECMO cases showed similar rates of perforation between wire and non-wire reinforced DLCs (6.6 vs. 4.8%, p = 0.441) [24]. To date, no studies have compared DLC complications between adults and children. In general, it is suspected that neonates and children are more susceptible to perforation and tamponade due to smaller and thinner vessels and heart chambers when compared to adults [53].
Cannula migration has been reported in 6–10% of pediatric and adult cases of DLC VV-ECMO [54, 55]. Malpositioning of the drainage and reinfusion ports result in inadequate ECMO flow and hypoxia. The 13 Fr. cannula (neonate) only has a distance of 2.5 cm from the inferior IVC port to the right atrium infusion port and can dislodge into the right heart when the patient moves [50]. It is suggested to use the largest cannula possible or to place the inferior tip of a 13 Fr. cannula 1 cm lower in the IVC. A single-center chart review of 25 pediatric Avalon Elite VVDL-ECMO patients demonstrated use of fluoroscopy during insertion decreased the need for cannula repositioning (n = 3/14, 21% vs. n = 7/11, 64%; p = 0.05) when compared to using echocardiography alone [56].
In adults the risk of cannula migration is related to increased ambulation. To allow for patient comfort and stabilization of the ECMO lines, a 30-in. hold n place foley leg band should be placed across the patient’s forehead and lines secured by the green Velcro strap [48]. There is hesitance to transport ECMO patients cannulated with a DLC between hospitals. The largest series (n = 170) of patients transported on mobile ECMO (2010–2014) compared adult ECMO patients cannulated at a referral center and transported (n = 46) to those cannulated on site (n = 126). All patients were placed on VV-ECMO under fluoroscopic guidance. Authors reported no deaths on transport, no cannula misplacement, and no significant difference in complication rate (bleeding, arrythmia, tamponade, pneumothorax), or survival (to discharge or 6 months) compared to on-site cannulation. This high-volume center experience demonstrates the safety of transport while on DLC cannulated VV-ECMO when the mobile ECMO team is comfortable with fluoroscopy at the bedside. The study further demonstrates that mobile ECMO is resource and expertise intense, requiring a team of two congenital and one adult cardiothoracic surgeon, one intensivist, and one full-time dedicated ECMO consultant [57].
Other complications of DLCs include cannula site bleeding (20%), cannula site infection (10%), and neurologic complications (7%) [49]. Intracranial hemorrhage is the most common neurologic complication in VV-ECMO patients and is thought to result from thrombocytopenia, anticoagulation, and intracranial venous hypertension from cannula obstruction of the internal jugular vein and superior vena cava [42, 58]. Common DLC sizes (27–31 Fr.) are larger than two site return cannulas (19–23 Fr.) for adult patients. No animal studies have confirmed that DLC cannulas increase cerebral venous pressure; however, a review of the ELSO registry (2011–2016) comparing propensity matched VV-ECMO patients cannulated with the Avalon Elite 27 Fr. (n = 372) or 31 Fr. (n = 372) found rate of intracranial hemorrhage was 3-times higher in those cannulated with the 31 Fr. compared to the 27 Fr. (n = 16, 4.3% vs. n = 6, 1.6%, p = 0.03). There was no difference in hemolysis, cannula related complications, or mortality. These results suggest the use of the smallest cannula able to achieve adequate ECMO flow to minimize risk of intracranial hemorrhage [42]. Likewise, a 4 year (2009–2012) single-center case series of 72 patients cannulated with the Avalon Elite reported cannulation site bleeding (n = 15), cannulation site infection (n = 7), intracranial bleeding (n = 5), cannula movement (n = 4), pneumothorax (n = 2), and tamponade (n = 2) [49].
A retrospective review of the ELSO database comparing VV-ECMO implanted at a single site using a DLC versus two cannulas showed the DLC group had significantly higher incidences of cannula-related complications (22.3 vs. 14.2%, p < 0.001) acidosis (30.3 vs. 19.6%, p = 0.019), seizures (35.1 vs. 14.7%, p = 0.001), and cardiovascular complications (24.4 vs 21.7%, p = 0.032). The multi-site cannulation group had more renal complications (15.8 vs. 12.9%, p = 0.005) and hyperglycemia (47.4 vs. 30.8%, p = 0.001). No difference was seen in the complications of cannula site bleeding, tamponade, pneumothorax, or survival to discharge [24].
Decannulation poses less risk than insertion of a DLC. Echocardiography should be used after cannula removal to ensure no complications have occurred. A retrospective study (2009–2014, n = 28) of an academic medical center showed that decannulation done at bedside by an intensivist using a purse string suture technique did not increase complications when compared to the procedure being performed by a surgeon in the operating room [59]. This highlights the ability of an intensivist to reduce resources required to remove a DLC.
Conclusions
The DLC is an option for patients who are electively initiated on VV-ECMO. Insertion requires technical skill and image guidance to ensure proper placement. VVDL-ECMO allows for maximum clinical conditioning through early extubation and physical therapy (with or without ambulation) while bridging to recovery, decision, or definitive therapy.
References
Extracorporeal Life Support Organization (ELSO). General guidelines for all ECLS cases. August 2017. https://www.elso.org/Resources/Guidelines.aspx. Accessed June 12 2019.
Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015;7:E166–76.
Lorusso R, Alexander P, Rycus P, Barbaro R. The extracorporeal life support organization registry:update and perspectives. Ann Cardiothorac Surg. 2019;8:93–8.
ECMO. Registry of the Extracorporeal Life Support Organization (ELSO). Ann Arbor: Michigan; April, 2019.
Bartlett RH, Gazzaniga AB, Toomasian J, Coran AG, Roloff D, Rucker R. Extracorporeal membrane oxygenation (ECMO) in neonatal respiratory failure. 100 cases. Ann Surg. 1986;204:236–45.
Bartlett RH, Roloff DW, Cornell RG, Andrews AF, Dillon PW, Zwischenberger JB. Extracorporeal circulation in neonatal respiratory failure: a prospective randomized study. Pediatrics. 1985;76:479–87.
O'Rourke PP, Crone RK, Vacanti JP, et al. Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: a prospective randomized study. Pediatrics. 1989;84:957–63.
No authors listed. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. UK Collaborative ECMO Trail Group. Lancet. 1996;348:75-82.
Anderson HL, Otsu T, Chapman RA, Barlett RH. Venovenous extracorporeal life support in neonates using a double lumen catheter. ASAIO Trans. 1989;35:650–3.
Harvey C. Cannulation for Neonatal and Pediatric Extracorporeal Membrane Oxygenation for Cardiac Support. Front Pediatr. 2018;6:17.
Zwischenberger JB, Toomasian JM, Drake K, Andrews AF, Kolobow T, Bartlett RH. Total respiratory support with single cannula venovenous ECMO: double lumen continuous flow vs. single lumen tidal flow. Trans Am Soc Artif Intern Organs. 1985;31:610–5.
Andrews AF, Zwischenberger JB, Cilley RE, Drake KL. Venovenous extracorporeal membrane oxygenation (ECMO) using a double-lumen cannula. Artif Organs. 1987;11:265–8.
Otsu T, Merz SI, Hultquist KA, et al. Laboratory evaluation of a double lumen catheter for venovenous neonatal ECMO. ASAIO Trans. 1989;35:647–50.
Anderson HL, Snedecor SM, Otsu T, Barlett RH. Multicenter comparison of conventional venoarterial access versus venovenous double-lumen catheter access in newborn infants undergoing extracorporeal membrane oxygenation. J Pediatr Surg. 1993;28:530–4.
Delius R, Anderson H, Schumacher R, et al. Venovenous compares favorably with venoarterial access for extracorporeal membrane oxygenation in neonatal respiratory failure. J Thorac Cardiovasc Surg. 1993;106:329–38.
Gauger PG, Hirschl RB, Delosh TN, Dechert RE, Tracy T, Bartlett RH. A matched pairs analysis of venoarterial and venovenous extracorporeal life support in neonatal respiratory failure. ASAIO J. 1995;41:M573–9.
Zwischenberger JB, Nguyen TT, Upp JR, et al. Complications of neonatal extracorporeal membrane oxygenation. Collective experience from the extracorporeal life support organization. J Thorac Cardiovasc Surg. 1994;107:838–48.
Wang D, Zhou X, Liu X, Sidor B, Lynch J, Zwischenberger JB. Wang-Zwische double lumen cannula-toward a percutaneous and ambulatory paracorporeal artificial lung. ASAIO J. 2008;54:606–11.
Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–95.
Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306:1659–68.
Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374:1351–63.
Bermudez CA, Rocha RV, Sappington PL, Toyoda Y, Murray HN, Boujoukos AJ. Initial experience with single cannulation for venovenous extracorporeal oxygenation in adults. Ann Thorac Surg. 2010;90:991–5.
Javidfar J, Brodie D, Wang D, et al. Use of bicaval dual-lumen catheter for adult venovenous extracorporeal membrane oxygenation. Ann Thorac Surg. 2011;91:1763–8.
Zamora IJ, Shekerdemian L, Fallon SC, et al. Outcomes comparing dual-lumen to multisite venovenous ECMO in the pediatric population: The extracorporeal life support registry experience. J Pediatr Surg. 2014;49:1452–7.
Abrams D, Brodie D. Emerging indications for extracorporeal membrane oxygenation in adults with respiratory failure. Ann Am Thorac Soc. 2013;10:371–7.
Taccone FS, Malfertheiner MV, Ferrari F, et al. Extracorporeal CO2 removal in critically ill patients: A systematic review. Minerva Anestesiol. 2017;83:762–72.
Gattinoni L, Kolobow T, Agostoni A, et al. Clinical application of low frequency positive pressure ventilation with extracorporeal CO2 removal (LFPPV-ECCO2R) in treatment of adult respiratory distress syndrome (ARDS). Int J Artif Organs. 1979;2:282–3.
Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:295–305.
Abrams DC, Brenner K, Burkart KM, et al. Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10:307–14.
Brenner K, Abrams DC, Agerstrand CL, Brodie D. Extracorporeal carbon dioxide removal for refractory status asthmaticus: experience in distinct exacerbation phenotypes. Perfusion. 2014;29:26–8.
Schneider TM, Bence T, Brettner F. “Awake” ECCO2R superseded intubation in a near-fatal asthma attack. J Intensive Care. 2017;5:53.
Lynch W. ECLS Cannulation for adults with respiratory failure. In: Brogan T, editor. Extracorporeal life support: The ELSO red book. Ann Arbor: ELSO; 2017. p. 429–36.
Schmid C, Philipp A, Hilker M, et al. Venovenous extracorporeal membrane oxygenation for acute lung failure in adults. J Heart Lung Transplant. 2012;31:9–15.
Goligher EC, Tomlinson G, Hajage D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc bayesian analysis of a randomized clinical trial. JAMA. 2018;320:2251–9.
Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med. 2012;185:763–8.
Bacchetta M, Javidfar J, Sonett J, Kim H, Zwischenberger J, Wang D. Ease of conversion from venovenous extracorporeal membrane oxygenation to cardiopulmonary bypass and venoarterial extracorporeal membrane oxygenation with a bicaval dual lumen catheter. ASAIO J. 2011;57:283–5.
Kohler K, Valchanov K, Nias G, Vuylsteke A. ECMO cannula review. Perfusion. 2013;28:114–24.
Sorokin V, MacLaren G, Vidanapathirana PC, Delnoij T, Lorusso R. Choosing the appropriate configuration and cannulation strategies for extracorporeal membrane oxygenation: The potential dynamic process of organ support and importance of hybrid modes. Eur J Heart Fail. 2017;19:75–83.
Zhao J, Wang D, Ballard-Croft C, et al. Hybrid extracorporeal membrane oxygenation using avalon elite double lumen Cannula ensures adequate heart/brain oxygen supply. Ann Thorac Surg. 2017;104:847–53.
Biscotti M, Bacchetta M. The "sport model": extracorporeal membrane oxygenation using the subclavian artery. Ann Thorac Surg. 2014;98:1487–9.
Brasseur A, Scolletta S, Lorusso R, Taccone FS. Hybrid extracorporeal membrane oxygenation. J Thorac Dis. 2018;10:S707–15.
Mazzeffi M, Kon Z, Menaker J, et al. Large dual-lumen extracorporeal membrane oxygenation cannulas are associated with more intracranial hemorrhage. ASAIO J. 2019. https://doi.org/10.1097/MAT.0000000000000917.
O'Brien C, Monteagudo J, Schad C, Cheung E, Middlesworth W. Centrifugal pumps and hemolysis in pediatric extracorporeal membrane oxygenation (ECMO) patients: An analysis of Extracorporeal Life Support Organization (ELSO) registry data. J Pediatr Surg. 2017;52:975–8.
Camboni D, Akay B, Sassalos P, et al. Use of venovenous extracorporeal membrane oxygenation and an atrial septostomy for pulmonary and right ventricular failure. Ann Thorac Surg. 2011;91:144–9.
Javidfar J, Brodie D, Sonett J, Bacchetta M. Venovenous extracorporeal membrane oxygenation using a single cannula in patients with pulmonary hypertension and atrial septal defects. J Thorac Cardiovasc Surg. 2012;143:982–4.
Kon ZN, Pasrija C, Shah A, Griffith BP, Garcia JP. Venovenous extracorporeal membrane oxygenation with atrial septostomy as a bridge to lung transplantation. Ann Thorac Surg. 2016;101:1166–9.
Broman LM, Malfertheiner MV, Montisci A, Pappalardo F. Weaning from veno-venous extracorporeal membrane oxygenation: how I do it. J Thorac Dis. 2018;10:S692–7.
Tignanelli CJ, Weinberg A, Napolitano LM. Optimal methods to secure extracorporeal membrane oxygenation bicaval dual-lumen cannulae: What works? ASAIO J. 2019;65:628–30.
Rubino A, Vuylsteke A, Jenkins DP, Fowles JA, Hockings L, Valchanov K. Direct complications of the Avalon bicaval dual-lumen cannula in respiratory extracorporeal membrane oxygenation (ECMO): Single-center experience. Int J Artif Organs. 2014;37:741–7.
Subramanian S, Vafaeezadeh M, Parrish AR, McMullan DM. Comparison of wire-reinforced and non-wire-reinforced dual-lumen catheters for venovenous ECMO in neonates and infants. ASAIO J. 2013;59:81–5.
Shaheen A, Tanaka D, Cavarocchi NC, Hirose H. Veno-Venous Extracorporeal Membrane Oxygenation (V V ECMO): Indications, preprocedural considerations, and technique. J Card Surg. 2016;31:248–52.
Ngai CW, Ng PY, Sin WC. Bicaval dual lumen cannula in adult veno-venous extracorporeal membrane oxygenation—clinical pearls for safe cannulation. J Thorac Dis. 2018;10:S624–8.
Griffee MJ, Tonna JE, McKellar SH, Zimmerman JM. Echocardiographic guidance and troubleshooting for venovenous extracorporeal membrane oxygenation using the dual-lumen bicaval cannula. J Cardiothorac Vasc Anesth. 2018;32:370–8.
Chimot L, Marqué S, Gros A, et al. Avalon© bicaval dual lumen cannula for venovenous extracorporeal membrane oxygenation: survey of cannula use in France. ASAIO J. 2013;59:157–61.
Fallon SC, Shekerdemian LS, Olutoye OO, et al. Initial experience with single-vessel cannulation for venovenous extracorporeal membrane oxygenation in pediatric respiratory failure. Pediatr Crit Care Med. 2013;14:366–73.
Jarboe MD, Gadepalli SK, Church JT, et al. Avalon catheters in pediatric patients requiring ECMO: Placement and migration problems. J Pediatr Surg. 2018;53:159–62.
Kanji HD, Chouldechova A, Harvey C, O'dea E, Faulkner G, Peek G. Safety and outcomes of mobile ecmo using a bicaval dual-stage venous catheter. ASAIO J. 2017;63:351–5.
Lorusso R, Gelsomino S, Parise O, et al. Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: Findings from the extracorporeal life support organization database. Crit Care Med. 2017;45:1389–97.
Heller A, Dollerschell J, Burk J, Haines H, Kozinn J. Safety of intensivist-led bedside decannulation of internal jugular bi-caval dual-lumen veno-venous extracorporeal membrane oxygenation cannulas and report of technique. Anaesthesiol Intensive Ther. 2016;48:211–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Zwischenberger has the following to disclose: he receives royalties for a patent licensed to Avalon/Maquet for the Avalon Elite double lumen cannula, he is the recipient of grant funding from the NIH, he is a consultant on the Cytosorbents, Inc. Cardiac Advisory Board, and he is a partner in WZ-Biotech. Vanessa M. Bazan has nothing to disclose. Evan M. Taylor has nothing to disclose. Dr. Gunn has nothing to disclose.
Funding
No funding was received for this review article
Informed Consent
Not Required for a Review Article
Ethical Clearance
Not Required for a Review Article
Human and Animal Rights Statement
Not Required for a Review Article
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bazan, V.M., Taylor, E.M., Gunn, T.M. et al. Overview of the bicaval dual lumen cannula. Indian J Thorac Cardiovasc Surg 37 (Suppl 2), 232–240 (2021). https://doi.org/10.1007/s12055-020-00932-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-020-00932-1