Abstract
Allohexaploid wheat was derived from interspecific hybridization, followed by spontaneous chromosome doubling. Newly synthesized hexaploid wheat by crossing Triticum turgidum and Aegilops tauschii provides a classical model to understand the mechanisms of allohexaploidization in wheat. However, immediate chromosome level variation and microsatellite level variation of newly synthesized hexaploid wheat have been rarely reported. Here, unreduced gametes were applied to develop synthesized hexaploid wheat, NA0928, population by crossing T. turgidum ssp. dicoccum MY3478 and Ae. tauschii SY41, and further S0–S3 generations of NA0928 were assayed by sequential cytological and microsatellite techniques. We demonstrated that plentiful chromosomal structural changes and microsatellite variations emerged in the early generations of newly synthesized hexaploid wheat population NA0928, including aneuploidy with whole-chromosome loss or gain, aneuploidy with telosome formation, chromosome-specific repeated sequence elimination (indicated by fluorescence in situ hybridization) and microsatellite sequence elimination (indicated by sequencing), and many kinds of variations have not been previously reported. Additionally, we reported a new germplasm, T. turgidum accession MY3478 with excellent unreduced gametes trait, and then succeeded to transfer powdery mildew resistance from Ae. tauschii SY41 to synthesized allohexaploid wheat population NA0928, which would be valuable resistance resources for wheat improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allopolyploids, such as wheat, one of the most staple crops in the world, are widely believed to arise from interspecific or intergeneric hybridization, followed by chromosome doubling. Both tetraploid pasta wheat (Triticum turgidum L. ssp. durum, 2n = 4x = 28, genome AABB) and hexaploid bread wheat (T. aestivum L., 2n = 6x = 42, genome AABBDD) experienced allopolyploidization events that originated from interspecific hybridization of ancestors. Hybridization of the contributor of A genome, T. urartu, and the donor of B genome, a undiscovered Aegilops species closely related to Ae. speltoides, leads to the formation of allotetraploid wheat (T. turgidum ssp. dicoccoides) (Riley et al. 1958; Chapman et al. 1976; Dvořák 1976; Dvořák et al. 1993; Blake et al. 1999; Huang et al. 2002). Hybridization of early-domesticated T. turgidum and the ancestor of D genome, Ae. tauschii, contributes to the formation of hexaploid wheat (T. aestivum L.) (Kihara 1944; McFadden and Sears 1946; Nesbitt and Samuel 1996). It is widely considered that the fusion of unreduced gametes was the main pathway contributing to the polyploidization of wheat (Xu and Joppa 2000; Matsuoka and Nasuda 2004; Cai and Xu 2007; Jauhar 2007).
Intriguingly, unreduced gametes resulting from unreductional meiosis has been well described in the Triticeaetribe, especially in wheat polyhaploids (Jauhar 2007; Cai et al. 2010) and interspecific hybrids of tetraploid wheat with Ae. tauschii, rye, barley and some other related species (Wagenaar 1968a, 1968b; Stefani 1986; Fukuda and Sakamoto 1992a, 1992b; Xu and Dong 1992; Xu and Joppa 1995, 2000; Zhang et al. 2010). The formation of unreduced gametes was reported to be commonly distributed in the T. turgidum–Ae. tauschii F1 hybrids, and newly formed allohexaploid wheat were synthesized by unreduced gametes (Ozkan et al. 2001; Zhang et al. 2010).
Decades of research has shown that ‘genome shock’ (McClintock 1984) accompanies the newly formed amphidiploids, which has demonstrated a variety of genetic and epigenetic changes in the nascent hybrids (Comai 2000; Chen 2007; Feldman and Levy 2012). In newly formed allopolyploid wheat, genomic alterations could be triggered by allopolyploidization, including the loss of coding and noncoding DNA sequences, transposon activations, gene silencing or duplication and pseudogenization (Fukui 1996; Levy and Feldman 2004; Feldman and Levy 2009). Specifically, immediate nonrandom elimination of specific noncoding, low-copy and high-copy DNA sequences were discovered in the allopolyploidization of the Triticeae tribe (Feldman et al. 1997; Liu et al. 1998a, 1998b; Ozkan et al. 2001; Shaked et al. 2001; Han et al. 2003, 2005). In comparison with the parents, both natural and synthetic wheat allopolyploids exhibit 2–10% DNA elimination after allopolyploidization (Furuta et al. 1974; Eilam et al. 2008, 2010), while octoploid and hexaploid triticale display 9% and 28–30% DNA reduction, respectively (Boyko et al. 1984, 1988; Ma and Gustafson 2005).
Further, Mestiri et al. (2010) reported that synthetic allohexaploids exhibited parent-dependent meiotic irregularities and aneuploidy in the initial generations (S0–S2), while no other structural changes were observed in these synthetic allohexaploids. Highly variable frequencies (20–100%) of whole-chromosome aneuploidy were also observed in early generations (from selfed generation S1 to >S20) of allohexaploid wheat, however, spontaneous doubled stocks were not utilized (Zhang et al. 2013b). Additionally, Luo et al. (2012) reported that only one altered microsatellite was investigated in S1 generation of synthesized hexaploid wheat at a mutation rate of 6.74 × 10 −6. Despite the extensive research until now, no sequence and other types of chromosomal structural alterations were evidently assayed in the early generations of T. turgidum–Ae. tauschii hybrids in the spontaneous doubled condition.
To have a better understanding of the immediate chromosome level and microsatellite level consequences of newly synthesized hexaploid wheat mediated by unreduced gametes, we conducted a detailed cytological and microsatellite assay on S0–S3 generations of T. turgidum MY3478–Ae. Tauschii SY41 combination. Plentiful chromosomal structural changes occurred during the spontaneous-double process and in the early generations of the hybrids. Dynamic microsatellite loci were then tracked and confirmed by changed number of repeat sequence.
Materials and methods
Plant material and experimental scheme
T. turgidum L. ssp. dicoccum accession MY3478 (2 n = 4 x = 28, BBAA), T. turgidum L. ssp. durum accession Langdon, T. turgidum L. ssp. carthlicum accession PS5 and Ae. tauschii Coss. (2 n = 2 x = 14, DD) accession SY41 were provided by Dr Lihui Li from the Institute of Crop Science, Chinese Academy of Agricultural Sciences. MY3478, Langdon and PS5 were pollinated by SY41 to form triploid F1 hybrids, respectively, according to the scheme shown in figure 1. No embryo rescue or hormone treatment was applied on the F1 seeds. Synthesized hexaploid wheat population NA0928 was obtained by selfing the MY3478/SY41 triploid F1 hybrid plants. S0 plants carrying different chromosome numbers were preserved to form separate lines.
The seeds of F1 and NA0928 population were sown every year in the field of College of Agronomy, Northwest A&F University, located at the Yangling city of Shaanxi province, China.
Morphology and disease-resistance evaluation
Seedling resistance to powdery mildew race E09 of MY3478, SY41 and the synthesized hexaploid wheat population NA0928 were evaluated in the incubator of Northwest A&F University. Adult-plant resistance to powdery mildew of MY3478, SY41 and NA0928 lines were evaluated in the field of Northwest A&F University under natural conditions.
Cytological analysis
Fresh root tips were collected from germinating seeds or from seedlings of the field in late March, and treated with ice-cold water for 20–24 h, and then fixed in Carnoy’s fluid. To record the somatic chromosome number, 1% iron acetocarmine method was used according to Li et al. (2014). For meiotic behaviour analysis, young spikes were collected, fixed in Carnoy’s fluid for 48 h at room temperature and then pollen mother cells were stained and squashed in 1% acetocarmine, as described by Li et al. (2014). At least one spike per plant was analysed. Photographs were captured with an Olympus BX-43 microscope (Olympus Corporation, Tokyo, Japan) equipped with a Photometrics SenSys CCD camera.
Fluorescence in situ hybridization (FISH) and genomic in situ hybridization (GISH)
Actively growing roots were excised from germinating progenies of S1 plants, treated and fixed as described above, then stored in 70% ethanol (v/v). Chromosome spread preparations and FISH were performed as previously described by Li et al. (2014).
Clone 45S was labelled with biotin-16-dUTP (Roche Diagnostics GmbH, Mannheim, Germany, cat. no. 11745816910) by the nick translation method, further detected using Avidin Fluoseacin (Invitrogen, USA, cat. no. A-821). Clone pSc119.2 was labelled with digoxigenin-11-dUTP (Roche Diagnostics GmbH, Mannheim, Germany, cat. no. 11745816910) and detected by antidigoxigenin–rhodamine (Roche Diagnostics GmbH, Mannheim, Germany, cat. no. 11207750910).
For multicolour GISH, genomic DNA of T. urartu was labelled by nick translation with biotin-16-dUTP and Ae. tauschii was labelled by digoxigenin-11-dUTP, with genomic DNA of Ae. speltoides as a blocker.
Chromosome spreads were examined with an Olympus BX53 fluorescence microscope and digital images were captured using cellSens Standard 1.8 software (Olympus Corporation, Tokyo, Japan) with a DP80 microscope digital camera (Olympus Corporation, Tokyo, Japan) and processed with Adobe Photoshop CS 6.0.
Genomic DNA isolation and microsatellite marker analysis
Young leaf samples of NA0928 lines were collected and used for DNA extraction using CTAB method of Li et al. (2015). Primer sequences of simple sequence repeat (SSR) markers were obtained from Graingene (http://wheat.pw.usda.gov/ggpages/SSRclub/GeneticPhysical/). A total of 16 microsatellite markers showing polymorphism between 2 parents of NA0928 (MY3478 and SY41) were employed to screen the NA0928 population. Polymerase chain reactions (PCR) were performed according to Li et al. (2015) in a 20 μL volume with 2.5 nM of each dNTP, 50–100 ng of template DNA, 5 nM of each primer, 0.5 U Ex Taq polymerase (TaKaRa, Dalian, China). PCR products were then detected by 8% polyacrylamide gels using silver staining and photographing.
Cloning and sequencing
Acrylamide gel electrophoresis (8%) showing the target fragments of microsatellite Xgwm159 were collected and washed by 100 μL ddH 2O for overnight, and then supernatant was used to perform the second PCR reaction. The re-PCR products were then confirmed by 8% polyacrylamide gel and the remaining portions were cloned into pMD18-T vector using cloning kit from TaKaRa Biotechnology (Dalian, China). Positive clones were picked up and then sequenced by AUGCT company (Beijing, China). Sequence analysis was conducted with the DNAMAN 8.0 Demo software (Lynnon Biosoft, San Ramon, California, USA).
Results
Production of new synthetic allohexaploid wheat was mediated by unreduced gametes formation
F1 interspecific hybrids (n = 3 x = 21, ABD) were successfully obtained by crossing Ae. tauschii accession SY41 as male parent with MY3478, Langdon (LDN) or PS5 as female parent without embryo rescue and colchicine treatment. Given that tetraploid wheat Langdon and PS5 have been reported to be the most excellent accessions with unreduced gametes traits, the seed-set of these three combinations were compared. In MY3478 × SY41, a total of 117 seeds were harvested from 422 spikelets, which means a high seed-set rate at 0.277 seed per spikelet. In Langdon × SY41 and PS5 × SY41, seed-set of F1 plant was 0.296 seed per spikelet and 0.222 seed per spikelet, respectively (table 1). Therefore, a high percentage of unreduced gametes formation was indicated in MY3478 × SY41 F1 hybrids, and MY3478 turned out to be an excellent accession as Langdon or PS5.
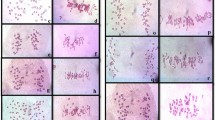
First division restitution (FDR) and single-division meiosis (SDM) have been specifically described in the T. turgidum–Ae. tauschii F1 hybrids and considered to be the main pathways of unreduced gametes formation (Islam and Shepherd 1980; Xu and Dong 1992; Xu and Joppa 1995; Matsuoka and Nasuda 2004). Thus, the meiotic cell division of MY3478 × SY41’s F1 plants was characterized. As shown in figure 2, all the F1 plants were triploids with 21 chromosomes. However, a large number of cells undergoing the presegregation of sister chromatids were observed in the F1 hybrids of this study, even though some other cells remained in metaphase of first meiotic division. Hence, the presegregation of sister chromatids could also frequently occurred in the F1 interspecific hybrids and lead to the formation of unreduced gametes.
Meiotic analysis of F1 plants of T. turgidum MY3478–Ae. tauschii SY41 and mitotic analysis of derived S0 plants. (a) F1 plant showed 21 univalents at metaphase of first meiotic division. (b) Preseparated sister chromatids (arrow indicated) were observed in pollen mother cells of F1 plant, while other cells stayed at metaphase of first meiotic division. (c) One pollen mother cell displayed one trivalent and would result in random segregation later. (d–g) Four types of chromosome constitution in S0 generation of NA0928. Arrows indicate the observed telosomes.
To further demonstrate the production of unreduced gametes, cytological observation was carried out to check the somatic chromosome number in the progenies of F1 interspecific plants, i.e., the S0 generation seeds named NA0928. A variety of chromosome constitutions were assayed, including euploid plants with completely doubled genomes (2 n = 42), as well as aneuploidy carrying 35 or 34 chromosomes (figure 2). Intriguingly, two plants displaying 43 chromosomes at mitotic root tips were recorded as well. As shown in table 2, 36.4% euploidy plants appeared in the S0 generation, which indicated around 0.10 euploidy seed per spikelet. Therefore, unreduced euploid gametes containing the entire chromosome number of parent and unreduced aneuploid gametes carrying less or more chromosome (8) could be both produced.
S0 plants with different chromosome numbers were implanted separately and selfed to develop isolated S1 lines later. The synthetic NA0928 lines displayed a conspicuous overall phenotype similar to other synthesized hexaploid wheats. One typical trait is tough tenacious glumes, which was inherited from their male parent Ae. tauschii, as shown in figure 3. The kernels of euploid lines were plump and a little bigger than the parents. Further, the resistance results showed that NA0928 lines were highly resistant to the tested isolates of powdery mildew at adult-plant stage and immune to the E09 race of powdery mildew at seedling stage (figure 3). Considering that SY41 displayed high resistance to powdery mildew, while MY3478 did not display, NA0928 lines might inherit powdery mildew resistance donated by SY41.
Morphologic traits of T. turgidum MY3478, Ae. tauschii SY41 and derived synthesized hexaploid wheat NA0928. (a) Spikes of MY3478, SY41 and S1 generation of NA0928. (b) Adult plant resistance of MY3478, SY41 and S2 generation of NA0928 for powdery mildew and stripe rust. (c) The kernels of MY3478, SY41 and S1 generation of NA0928. 1, MY3478; 2, SY41; 3, S1 generation of NA0928.
Chromosome level dynamics in the early generation of newly synthesized hexaploid wheat population, NA0928
It has been demonstrated that meiotic irregularity and aneuploidy accompanied the newly synthesized allohexaploid wheat in a progenitor-dependent manner (Mestiri et al. 2010), while no other chromosomal changes have been reported to date. To detect whether other chromosome alterations could have occurred in the early generations of synthesized N0928 lines, mitotic analysis was conducted on the S0, S1 and S2 progenies, and meiotic analysis was performed on S1 progenies as well.
Consistent with previous research, aneuploidy occurrence indeed accompanied the S0, S1 and S2 generations. Specifically, 63.6% aneuploidy plants appeared in the S0 generation. In 18 S1 progenies of two separate S0 plants possessing 42 chromosomes, eight plants with 42 chromosome and 10 plants carrying 41 or 40 chromosomes were observed, which indicated 55.6% aneuploidy frequency. In 11 S2 progenies of S1 plants possessing 42 chromosomes, 27.3% aneuploidy occurred. As to the S2 progenies of S1 plants with 41 or 40 chromosomes, 100% aneuploidy and 85.7% aneuploidy emerged, respectively (table 2).
Further, one plant containing four telosomes was observed in the S0 generation (figure 2), which suggested that chromosome aberration could occur during the allohexaploidization. One plant with a telosome was also observed in S2 progenies of S1 plant carrying 41 chromosomes (figure 4g). As meiotic analysis on S1 plant carrying 41 chromosomes, most of the pollen mother cells showed 20 II + 1 I or 19 II + 3 I in metaphase of the first meiotic division, and cells with a lagging chromosome were also observed in anaphase of the first meiotic division, which might result in the breakage of the lagging chromosome (figure 4c).
Meiotic analysis of S1 plants of NA0928 and mitotic analysis of derived S2 plants. (a) S1 plant showed 21 bivalents at metaphase of first meiotic division. (b) S1 plant displayed 20 bivalents and one univalent at metaphase of first meiotic division. (c) One lagged chromosome observed at anaphase of first meiotic division (indicated by arrow). (d–g) Four types of chromosome constitutions in S2 progenies of S1 plant with 41 chromosomes. (d) S2 plant with 42 chromosomes; (e) S2 plant with 41 chromosomes; (f) S2 plant with 40 chromosomes; (g) S2 plant with 40 chromosomes including one telosome (indicated by an arrow).
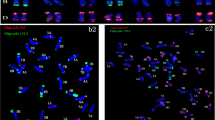
Inspired by the pSc119.2 probe’s ability to distinguish the B genome chromosomes, and 45S probe to recognize nucleolus organizer region (NOR) regions in wheat chromosomes, FISH and multicolour GISH were applied to karyotype the S2 generation plants. Only one plant carrying the altered 1B chromosome was assayed, i.e. the pSc119.2 signal loss in the terminal region of 1B’s long arm (figure 5b). NOR regions are located on the 1B, 6B and 5D chromosomes. No obvious NOR regions elimination was observed and most of the plants carried six NORs. Besides, whole-chromosome loss was tracked in some plants. Chromosomes 2B and 5B were missing in a plant with 40 chromosomes (figure 5c), and a B genome chromosome was lost in a plant with 41 chromosomes (figure 5d).
FISH and multicolour GISH analyses of S2 generation plants. (a) S2 plant carried 42 chromosomes. (b) An altered 1B chromosome (chromosome in white frame) was captured in S2 plant with 41 chromosomes. (c) one 2B and one 5B chromose were missing in S2 plant carried 40 chromosomes. (d) Multicolour GISH showed one chromosome of B genome was missing. (a–c) 4\(^{\prime }\),6-diamidino-2-phenylindole (DAPI), blue fluorescence; pSc119.2, red fluorescence; 45S and pSc119.2, green fluorescence. (d) A genome, red fluorescence; B genome, blue fluorescence; D genome, red fluorescence.
Thus, plentiful chromosome variations could emerge in the early generations of newly synthesized hexaploid wheat NA0928, such as aneuploidy with whole-chromosome loss or gain, aneuploidy with telosome formation and chromosome-specific repeated sequence elimination.
DNA repeat sequence rearrangements were characterized by microsatellites
For analysis of DNA structural changes during the allopolyploidization process of synthetic allohexaploids at the microsatellite-level, a total of 16 microsatellites that display polymorphism between MY3478 and SY41 were applied in this study (table 3). All of them were employed to screen the S0 and S1 generations, while only Xgwm159 was further applied for S2 and S3 generations. From S0 to S3 generations, the plants carrying 41, 42 or 43 chromosomes were used to assay microsatellite variations.
In S0 generation, five plants with 42 or 43 chromosomes were screened and four markers showing microsatellite variations were observed, including Xgwm159, Xwmc181, Xwmc36 and Xcfa2141, as shown in figure 6. At least two microsatellite gains were detected in the Xgwm159 allele (the longer variant and the shorter variant are noted below), and one microsatellite gain in Xwmc181 allele, two microsatellite gains in the Xcfa2141 allele, while one microsatellite loss was detected in Xwmc36 allele. In the S1 generation, microsatellite assays were conducted in 18 plants containing 41 or 42 chromosomes. However, microsatellite variations were detected at loci Xgwm159 and Xwmc36, but not at loci Xwmc181 or Xcfa2141. Similar to the S0 generation, two microsatellite gains of Xgwm159 and one microsatellite loss of Xwmc36 were still observed.
Microsatellite assay of S0–S3 generations of synthesized hexaploid wheat. (a) Five microsatellite assay (Xgwm159, Xwmc181, Xwmc36, Xcfa2141 and Xcfd12) on parental lines and S0 plants with 42 or 43 chromosomes. 1, MY3478; 2, SY41; 3–7, S0 plants. (b) S1 plants, S0 parents and original parental lines were assayed by Xgwm159 and Xwmc36(different generations are separated by red lines). 1, MY3478; 2, SY41. (c–d) S2–S3 plants were assayed by Xgwm159. Microsatellite variations are indicated by arrows (different generations are separated by red lines).
Since the variations in locus Xgwm159 were so active and apparent, S2 and S3 progenies were further screened by Xgwm159. As shown in figure 6, the longer variant of Xgwm159 locus was spreading in all S2 lines, when there was no mutation present in the last generation. However, the shorter variant could not be detected in the S2 lines, because the investigated S2 lines were not derived from the S0 progenitor carrying the mutation. The result could still suggest that no de novo mutation of the second variant came out, while de novo mutation of the first variant reemerged in the S2 generation. In the S3 generation, the lines from S3-1 to S3-4 originate from the same progenitor, while lines from S3-5 to S3-8 were derived from another progenitor. Apparently, the lower variant of Xgwm159 was detectable in lines from S3-1 to S3-4, and the variant of Xgwm159 seemed to be stable in the S3-4 line, while only one plant with the first variant was assayed. In addition, the first variant was detectable in most of the lines from S3-5 to S3-8, while the first variant was not. As indicated, the divergence of the lines became more and more distinguishable from the microsatellite level. Moreover, the mutation rates were calculated in the S0 and S1 generations by quantifying the number of observed mutations in the proportion of the number of possible mutation alleles. In S0 generation, nine variants were captured in a total of 284 alleles, i.e. the mutation rate was 3.17 × 10−2. In S1 generation, 12 variants were recorded among 1356 alleles and the mutation rate was around 8.85 × 10 −3. Hence, the microsatellite loci were quite changeable among the early generations of NA0928.
To further demonstrate the DNA structural changes from the sequence level, the variants of Xgwm159 were cloned and sequenced. As shown in figure 7, the product sizes of MY3478 and SY41 were 180 bp (GenBank number KP400627) and 218 bp (GenBank number KP400626), respectively, which could also be distinguished by several single-nucleotide polymorphisms (SNPs), while four types of variants were collected in NA0928 lines, including 218 bp, 210 bp, 192 bp and 180 bp, despite some rare SNPs. After the alignment of the DNA sequence, 210 bp (GenBank number KP400628) and 192 bp (GenBank number KP400629) turned out to be the novel variants of Xgwm159 in NA0928 lines. After comparing with MY3478 and SY41’s sequences, the novel variants were both proved to result from the SY41’s Xgwm159 locus by deleting three and 13 GT repeats, respectively. According to the dynamics of Xgwm159 locus from S0–S3 generations, DNA repeat sequences were suggested to be preferentially rearranged in the early generations of newly synthesized allohexaploid wheat NA0928.
Detection and sequence alignment of four variations of Xgwm159 locus. (a) PCR products of Xgwm159 were used for sequencing. 1, MY3478; 2, SY41; 3–6, NA0928 plants. PCR products of parents are indicated by arrows, while PCR products of NA0928 plants are indicated by arrowheads. (b) Sequence alignment of variations of Xgwm159 locus. (c) Raw sequence electropherogram data files were generated in sequencing platform. The repeat units of Xgwm159 were framed in black boxes.
Discussion
To give a better knowledge of immediate chromosome level and microsatellite level consequences of newly synthesized hexaploid wheat, we synthesized hexaploid NA0928 population by crossing T. turgidum MY3478 and Ae. tauschii SY41, and further assayed S0–S3 generations of NA0928 by detailed cytological and microsatellites methods. Intriguingly, we found that plentiful chromosome or microsatellite variations in the early generations. Aneuploidy with whole-chromosome loss or gain, aneuploidy with telosome formation, chromosome-specific repeated sequence elimination (indicated by FISH) and microsatellite sequence elimination (indicated by sequencing) were accompanying the alloploidization of NA0928, while previously many kinds of variations were not been fully discovered.
Given that unreduced gametes have been widely applied to synthesize hexaploid wheat (Fukuda and Sakamoto 1992a, 1992b; Xu and Dong 1992; Zhang et al. 2010, 2011), here, we report a new germplasm, T. turgidum accession MY3478 with excellent spontaneous double trait, F1 hybrids of MY3478 and Ae. Tauschii could produce 0.277 seed per spikelet. Both FDR and SDM have been well-documented in F1 hybrids of T. turgidum and Ae. tauschii, and considered to be main pathways leading to the formation of unreduced gametes (Islam and Shepherd 1980; Xu and Dong 1992; Xu and Joppa 1995; Matsuoka and Nasuda 2004). However, no detailed description of the process had been reported previously, until Cai et al. (2010) demonstrated that amphitelical orientation of univalent in LDN polyhaploid and the interspecific hybrid contribute to the onset of unreduced meiosis. Later, Oleszczuk and Lukaszewski (2014) illustrated that separation of sister chromatids of univalent could generate dyads in F1 hybrids of T. turgidum L. and rye. Similarly, preseparation of sister chromatids were also detected in our F1 hybrids of T. turgidum L. MY3478 and Ae. tauschii SY41, while most of other pollen mother cells remained in metaphase of first meiotic division. Hence, the preseparation of sister chromatids might be a characteristic in unreduced gametes production.
To exploit the processes and mechanisms of allopolyploids proliferation and adaptation to new habitats, previous studies have shown that allopolyploidization of newly formed allopolyploid wheat could induce extensive genomic, genetic or epigenetic changes at both chromosome and molecular levels, such as chromosomal aneuploidy accompanying the nascent allopolyploid wheat (Mestiri et al. 2010; Zhang et al. 2013b), elimination of coding and specific noncoding DNA sequences (Feldman et al. 1997; Liu et al. 1998a, 1998b; Ozkan et al. 2001; Shaked et al. 2001; Han et al. 2003, 2005; Salina et al. 2004), and gene silencing by intergenomic suppression (Galili and Feldman 1984; Galili et al. 1986; Zhang et al. 2014). However, compared with allotetraploid Brassica (Xiong et al. 2011), Tragopogon (Chester et al. 2012) and synthetic allotetraploid wheat (Zhang et al. 2013a), no gross chromosome structural alterations including chromosome rearrangements, chromosome breakage/fusion, and loss of other repeat sequence were reported in newly formed hexaploid wheat. Mestiri et al. (2010) and Zhang et al. (2013b) both concluded that prominent numerical changes in the chromosome-level variation was associated with nascent allohexaploidization in wheat. In contrast, extensive structural changes including formation of telosome, elimination of chromosome-specific repeated sequence (indicated by FISH) were absolutely identified in our study. Moreover, no variation of the PCR-based markers (SSRs, TE-based, ESTs) was detected in the first three generations of synthetic allohexaploid wheat (Mestiri et al. 2010), and only one changed microsatellite was investigated in S1 generation of another synthesized allohexaploid wheat (Luo et al. 2012), while plentiful variants of microsatellite length were present from S0–S3 generations in our study. The microsatellite mutation rates of this study were quite higher than that of common wheat (Raquin et al. 2008), durum wheat (Thuillet et al. 2002) and some other resynthesized hexaploid wheats (Luo et al. 2012). Thus, a diversity of variations was indicated in our newly synthesized allohexaploid wheat. It is reasonable to speculate that different accession of T. turgidum L. and Ae. tauschii might have a diverse influence on the derived allohexaploid wheat.
From S0 to S3 generations, two novel variants originating from the SY41’s Xgwm159 locus by deleting three and 13 GT repeats were assayed. Similarly, Luo et al. (2012) detected the novel Xwmc312 allele in S1 generation of SynDH1 line by deleting seven GA repeats from the LDN allele. Microsatellite genesis is a complex evolutionary dynamic process, while the mechanism of SSR change in repeat number is suggested to be determined by mutation rate (Ellegren 2004; Pearson et al. 2005). Specifically, replication slippage, unequal crossing over and gene conversion are generally thought to lead to the length changes in microsatellite (Levinson and Gutman 1987; Berg et al. 2003). Similar to Jiangtao Luo’s demonstration on Xwmc312 allele, the Xgwm159 locus variants of current study might also result from DNA polymerase slippage.
Devastating diseases such as stripe rust and powdery mildew are worldwide threats to wheat, which could lead to loss of yield and decrease in grain quality (Fu et al. 2009; Wicker et al. 2013). As a result, researchers and breeders are endeavouring to search and transfer novel and effective sources of resistance from relative species of common wheat. By applying spontaneous double trait of some tetraploid wheat, Zhang et al. (2011) reported a synthesized doubled haploid method for allopolyploid wheat, which was immensely useful for researchers and breeders to conduct crop improvement and basic research. Similarly, Zhu et al. (2005) succeeded to transfer Pm33 from T. carthlicum accession PS5 into common wheat by utilizing meiotic restitution. Thus, unreduced gametes can be efficiently applied to exploit the genetic variation in a related species. Here, we show that the newly synthesized allohexaploid wheat population NA0928 was highly resistant to the tested isolates of powdery mildew and thereby inherited powdery mildew resistance donated by Ae. tauschii SY41. It is apparent that these synthesized allohexaploid wheat will be a valuable bridge resources for resistance gene transfer. Thus, this study not only demonstrated the chromosome and microsatellite level variations accompanying the newly synthesized allohexaploid wheat mediated by unreduced gametes, but also contributes to potential resistance resources for wheat improvement.
References
Berg I., Neumann R., Cederberg H., Rannug U. and Jeffreys A. J. 2003 Two modes of germline instability at human minisatellite MS1 (locus D1S7): complex rearrangements and paradoxical hyperdeletion. Am. J. Hum. Genet. 72, 1436–1447.
Blake N. K., Lehfeldt B. R., Lavin M. and Talbert L. E. 1999 Phylogenetic reconstruction based on low copy DNA sequence data in an allopolyploid: the B genome of wheat. Genome 42, 351–360.
Boyko E., Badaev N., Maximov N. and Zelenin A. 1984 Does DNA content change in the course of triticale breeding. Cereal Res. Commun. 12, 99–100.
Boyko E., Badaev N., Maximov N. and Zelenin A. 1988 Regularities of genome formation and organization in cereals. 1. DNA quantitative changes in the process of allopolyploidization. Genetika 24, 89–97.
Cai X. and Xu S. S. 2007 Meiosis-driven genome variation in plants. Curr. Genomics 8, 151–161.
Cai X., Xu S. S. and Zhu X. 2010 Mechanism of haploidy-dependent unreductional meiotic cell division in polyploid wheat. Chromosoma 119, 275–285.
Chapman V., Miller T. and Riley R. 1976 Equivalence of the A genome of bread wheat and that of Triticum urartu. Genet. Res. 27, 69–76.
Chen Z. J. 2007 Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu. Rev. Plant. Biol. 58, 377–406.
Chester M., Gallagher J. P., Symonds V. V., da Silva A. V. C., Mavrodiev E. V., Leitch A. R. et al. 2012 Extensive chromosomal variation in a recently formed natural allopolyploid species, Tragopogon miscellus (Asteraceae). Proc. Natl. Acad. Sci. USA 109, 1176–1181.
Comai L. 2000 Genetic and epigenetic interactions in allopolyploid plants. Plant Mol. Biol. 43, 387–399.
Dvořák J. 1976 The relationship between the genome of Triticum urartu and the A and B genomes of Triticum aestivum. Can. J. Genet. Cytol. 18, 371–377.
Dvořák J., Terlizzi P. D., Zhang H. -B. and Resta P. 1993 The evolution of polyploid wheats: identification of the A genome donor species. Genome 36, 21–31.
Eilam T., Anikster Y., Millet E., Manisterski J. and Feldman M. 2008 Nuclear DNA amount and genome downsizing in natural and synthetic allopolyploids of the genera Aegilops and Triticum. Genome 51, 616–627.
Eilam T., Anikster Y., Millet E., Manisterski J. and Feldman M. 2010 Genome size in diploids, allopolyploids, and autopolyploids of mediterranean Triticeae. J. Bot. 2010, 12. article id 341380.
Ellegren H. 2004 Microsatellites: simple sequences with complex evolution. Nat. Rev. Genet. 5, 435–445.
Feldman M. and Levy A. A. 2009 Genome evolution in allopolyploid wheat—a revolutionary reprogramming followed by gradual changes. J. Genet. Genomics 36, 511–518.
Feldman M. and Levy A. A. 2012 Genome evolution due to allopolyploidization in wheat. Genetics 192, 763–774.
Feldman M., Liu B., Segal G., Abbo S., Levy A. A. and Vega J. M. 1997 Rapid elimination of low-copy DNA sequences in polyploid wheat: a possible mechanism for differentiation of homoeologous chromosomes. Genetics 147, 1381–1387.
Fu D., Uauy C., Distelfeld A., Blechl A., Epstein L., Chen X. et al. 2009 A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science 323, 1357–1360.
Fukuda K. and Sakamoto S. 1992a Cytological studies on unreduced male gamete formation in hybrids between tetraploid emmer wheats and Aegilops squarrosa L. Jpn. J. Breed. 42, 255–266.
Fukuda K. and Sakamoto S. 1992b Studies on unreduced gamete formation in hybrids between tetraploid wheats and Aegilops squarrosa L. Hereditas 116, 253–255.
Fukui K. 1996 Plant chromosomes at mitosis: laboratory methods, pp. 1–17. CRC Press, Boca Raton, USA.
Furuta Y., Nishikawa K. and Tanino T. 1974 Stability in DNA content of AB genome component of common wheat during the past seven thousand years. Jpn. J. Genet. 49, 179–187.
Galili G. and Feldman M. 1984 Intergenomic suppression of endosperm protein genes in common wheat. Can. J. Genet. Cytol. 26, 651–656.
Galili G., Levy A. A. and Feldman M. 1986 Gene-dosage compensation of endosperm proteins in hexaploid wheat Triticum aestivum. Proc. Natl. Acad. Sci. USA 83, 6524–6528.
Han F., Fedak G., Guo W. and Liu B. 2005 Rapid and repeatable elimination of a parental genome-specific DNA repeat (pGc1R-1a) in newly synthesized wheat allopolyploids. Genetics 170, 1239–1245.
Han F. P., Fedak G., Ouellet T. and Liu B. 2003 Rapid genomic changes in interspecific and intergeneric hybrids and allopolyploids of Triticeae. Genome 46, 716–723.
Huang S., Sirikhachornkit A., Su X., Faris J., Gill B., Haselkorn R. et al. 2002 Genes encoding plastid acetyl-CoA carboxylase and 3-phosphoglycerate kinase of the Triticum/Aegilops complex and the evolutionary history of polyploid wheat. Proc. Natl. Acad. Sci. USA 99, 8133–8138.
Islam A. K. M. R. and Shepherd K. W. 1980 Meiotic restitution in wheat–barley hybrids. Chromosoma 79, 363–372.
Jauhar P. P. 2007 Meiotic restitution in wheat polyhaploids (amphihaploids): a potent evolutionary force. J. Hered. 98, 188–193.
Kihara H. 1944 Discovery of the DD-analyser, one of the ancestors of Triticum vulgare. Agric. Hortic. 19, 13–14.
Levinson G. and Gutman G. A. 1987 High frequencies of short frameshifts in poly-CA/TG tandem repeats borne by bacteriophage M13 in Escherichia coli K-12. Nucleic Acids Res. 15, 5323–5338.
Levy A. A. and Feldman M. 2004 Genetic and epigenetic reprogramming of the wheat genome upon allopolyploidization. Biol. J. Linn. Soc. 82, 607–613.
Li H., Han Y., Guo X., Xue F., Wang C. and Ji W. 2015 Genetic effect of locus B2 inhibiting awning in double-ditelosomic 6B of Triticum durum DR147. Genet. Resour. Crop Evol. 62, 407–418.
Li H., Wang C., Fu S., Guo X., Yang B., Chen C. et al. 2014 Development and discrimination of 12 double ditelosomics in tetraploid wheat cultivar DR147. Genome 57, 89–95.
Liu B., Vega J. and Feldman M. 1998a Rapid genomic changes in newly synthesized amphiploids of Triticum and Aegilops. II. Changes in low-copy coding DNA sequences. Genome 41, 535–542.
Liu B., Vega J., Segal G., Abbo S., Rodova M. and Feldman M. 1998b Rapid genomic changes in newly synthesized amphiploids of Triticum and Aegilops. I. Changes in low-copy noncoding DNA sequences. Genome 41, 272–277.
Luo J., Hao M., Zhang L., Chen J., Zhang L., Yuan Z. et al. 2012 Microsatellite mutation rate during allohexaploidization of newly resynthesized wheat. Int. J. Mol. Sci. 13, 12533–12543.
Ma X. -F. and Gustafson J. 2005 Genome evolution of allopolyploids: a process of cytological and genetic diploidization. Cytogenet. Genome Res. 109, 236–249.
Matsuoka Y. and Nasuda S. 2004 Durum wheat as a candidate for the unknown female progenitor of bread wheat: an empirical study with a highly fertile F1 hybrid with Aegilops tauschii Coss. Theor. Appl. Genet. 109, 1710–1717.
McClintock B. 1984 The significance of responses of the genome to challenge. Science 226, 792–801.
McFadden E. S. and Sears E. R. 1946 The origin of Triticum spelta and its free-threshing hexaploid relatives. J. Hered. 37, 107–116.
Mestiri I., Chagué V., Tanguy A. M., Huneau C., Huteau V., Belcram H. et al. 2010 Newly synthesized wheat allohexaploids display progenitor-dependent meiotic stability and aneuploidy but structural genomic additivity. New Phytol. 186, 86–101.
Nesbitt M. and Samuel D. 1996 From staple crop to extinction? The archaeology and history of the Hulled wheats. In Proceedings of the first international workshop on hulled wheats, pp. 41–100. Castelvecchio Pascoli, Tuscany, Italy.
Oleszczuk S. and Lukaszewski A. J. 2014 The origin of unusual chromosome constitutions among newly formed allopolyploids. Am. J. Bot. 101, 318–326.
Ozkan H., Levy A. A. and Feldman M. 2001 Allopolyploidy-induced rapid genome evolution in the wheat (Aegilops–Triticum) group. Plant Cell 13, 1735–1747.
Pearson C. E., Edamura K. N. and Cleary J. D. 2005 Repeat instability: mechanisms of dynamic mutations. Nat. Rev. Genet. 6, 729–742.
Raquin A. L., Depaulis F., Lambert A., Galic N., Brabant P. and Goldringer I. 2008 Experimental estimation of mutation rates in a wheat population with a gene genealogy approach. Genetics 179, 2195–2211.
Riley R., Unrau J. and Chapman V. 1958 Evidence on the origin of the B genome of wheat. J. Hered. 49, 91–98.
Salina E., Numerova O., Ozkan H. and Feldman M. 2004 Alterations in subtelomeric tandem repeats during early stages of allopolyploidy in wheat. Genome 47, 860–867.
Shaked H., Kashkush K., Ozkan H., Feldman M. and Levy A. A. 2001 Sequence elimination and cytosine methylation are rapid and reproducible responses of the genome to wide hybridization and allopolyploidy in wheat. Plant Cell 13, 1749–1759.
Stefani A. 1986 Unreduced gametes in the F1 hybrid of Triticum durum Desf. × Haynaldia villosa Schur. Z. Pflanzenzuchtg. 96, 8–14.
Thuillet A. C., Bru D., David J., Roumet P., Santoni S., Sourdille P. and Bataillon T. 2002 Direct estimation of mutation rate for 10 microsatellite loci in durum wheat, Triticum turgidum (L.) Thell. ssp durum desf. Molec. Biol. Evol. 19, 122–125.
Wagenaar E. B. 1968a Meiotic restitution and the origin of polyploidy. I. Influence of genotype on polyploid seedset in a Triticum crassum × T. turgidum hybrid. Genome 10, 836–843.
Wagenaar E. B. 1968b Meiotic restitution and the origin of polyploidy. II. Prolonged duration of metaphase I as causal factor of restitution induction. Genome 10, 844–852.
Wicker T., Oberhaensli S., Parlange F., Buchmann J. P., Shatalina M., Roffler S. et al. 2013 The wheat powdery mildew genome shows the unique evolution of an obligate biotroph. Nat. Genet. 45, 1092–1096.
Xiong Z., Gaeta R. T. and Pires J. C. 2011 Homoeologous shuffling and chromosome compensation maintain genome balance in resynthesized allopolyploid Brassica napus. Proc. Natl. Acad. Sci. USA 108, 7908–7913.
Xu S. and Dong Y. 1992 Fertility and meiotic mechanisms of hybrids between chromosome autoduplication tetraploid wheats and Aegilops species. Genome 35, 379–384.
Xu S. J. and Joppa L. R. 1995 Mechanisms and inheritance of first division restitution in hybrids of wheat, rye, and Aegilops squarrosa. Genome 38, 607–615.
Xu S. J. and Joppa L. R. 2000 First-division restitution in hybrids of Langdon durum disomic substitution lines with rye and Aegilops squarrosa. Plant Breed. 119, 233–241.
Zhang H., Bian Y., Gou X., Dong Y., Rustgi S., Zhang B. et al. 2013a Intrinsic karyotype stability and gene copy number variations may have laid the foundation for tetraploid wheat formation. Proc. Natl. Acad. Sci. USA 110, 19466–19471.
Zhang H., Bian Y., Gou X., Zhu B., Xu C., Qi B. et al. 2013b Persistent whole-chromosome aneuploidy is generally associated with nascent allohexaploid wheat. Proc. Natl. Acad. Sci. USA 110, 3447–3452.
Zhang H., Zhu B., Qi B., Gou X., Dong Y., Xu C. et al. 2014 Evolution of the BBAA component of bread wheat during its history at the allohexaploid level. Plant Cell 26, 2761–2776.
Zhang L. -Q., Liu D. -C., Zheng Y. -L., Yan Z. -H., Dai S. -F., Li Y. -F. et al. 2010 Frequent occurrence of unreduced gametes in Triticum turgidum–Aegilops tauschii hybrids. Euphytica 172, 285–294.
Zhang L., Luo J., Chen W., Hao M., Liu B., Yan Z. et al. 2011 Synthesizing double haploid hexaploid wheat populations based on a spontaneous alloploidization process. J. Genet. Genomics 38, 89–94.
Zhu Z., Zhou R., Kong X., Dong Y. and Jia J. 2005 Microsatellite markers linked to 2 powdery mildew resistance genes introgressed from Triticum carthlicum accession PS5 into common wheat. Genome 48, 585–590.
Acknowledgements
This research is supported by the National Basic Research 973 Program of China (grant no. 2011CB944601), the Key Technologies R&D Programme of China (grant no. 2013BAD01B02-6), Zhongying Tang Breeding Fund of Northwest A&F University and Foundation of China Scholarship Council. We thank Dr Patrick McGuire and Dr Chad Jorgensen (University of California, Davis) for helpful suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Umesh C. Lavania
[Li H., Wang Y., Guo X., Du Y., Wang C. and Ji W. 2016 Chromosomal structural changes and microsatellite variations in newly synthesized hexaploid wheat mediated by unreduced gametes. J. Genet. 95, xx–xx]
Hao Li and Yajuan Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
LI, H., WANG, Y., GUO, X. et al. Chromosomal structural changes and microsatellite variations in newly synthesized hexaploid wheat mediated by unreduced gametes. J Genet 95, 819–830 (2016). https://doi.org/10.1007/s12041-016-0704-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-016-0704-4