Abstract
In the present study, we tested the hypothesis whether flight-related traits such as wing area, flight-muscle ratio, wing loading and dispersal yield evidence of geographical variation in nine wild-collected as well as laboratory-reared (at 21°C) latitudinal populations of Drosophila melanogaster from the Indian subcontinent. We observed positive clinal variation in the wing–thorax ratio, wing aspect ratio and wing area, along a latitudinal gradient for both the sexes. In contrast, geographical changes in three parameters of flight ability, i.e. flight-muscle ratio, wing loading and dispersal, showed negative correlation withlatitude. On the basis of isofemale line variability, we observed positive correlation of wing loading with flight-muscle ratio as well as dispersal behaviour in both the sexes. We also found positive correlation between duration of development and wing area. Interestingly, southern populations of D. melanogaster from warm and humid habitats exhibited higher flight-muscle ratio as well as the higher wing loading than northern populations which occur in cooler and drier climatic conditions. Laboratory tests for dispersal-related walking behaviour showed significantly higher values for southern populations compared with northern populations of D. melanogaster. Multiple regression analysis of geographical changes in flight-muscle ratio, wing loading as well as walking behaviour as a function of average temperature and relative humidity of the origin of populations in wild-collected flies have suggested adaptive changes in flight-related traits in response to steeper gradients of climatic factors in the Indian subcontinent. Finally, adaptive latitudinal variations in flight-related traits in D. melanogaster are consistent with results of other studies from different continents despite differences due to specific climatic conditions in the Indian subontinent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In insects, flight is crucial for finding resources as well as mates (Kammer and Heinrich 1978; Alexander 1983; Brodsky 1994). In temperate as well as tropical regions, insects have evolved morphological and physiological changes in flight-related traits due to genetic adaptation and/or phenotypic plasticity (James et al. 1995, 1997;Partridge and French 1996; Nunney and Cheung 1997). Several studies have shown genetic differences in body-size-related traits in geographical populations of diverse Drosophila species such as D. melanogaster, D. kikkawai, D. subobscura, D. serrata, D. birchii, etc. (Azevedo et al. 1998; Parkash et al. 1998; Gilchrist and Huey 2004; Hoffmann and Weeks 2007). For Drosophila species, mean values of body-size-related traits have evidenced linear increase with latitude on different continents such as Europe, Australia and America. Repeatability of body size clines in different continents and across different Drosophila species suggest the role of natural selection in maintaining such geographical variations in fitness-related-quantitative traits (Endler 1977; Coyne and Beecham 1987; Imasheva et al. 1994; Van’t Land et al. 1999). However, from the Indian subcontinent, so far only one species, D. kikkawai (Parkash et al. 1998) has been investigated for geographical variation in body-size-related traits. D. melanogaster is abundantly available across all the Indian subcontinent but geographical changes in flight-related traits have not been investigated so far.
Temperature is generally assumed to affect body-size-related traits in ectothermic organisms such as Drosophila species (Unwin and Corbet 1984; Greenewalt 1962; Brodsky 1994; Junge-Berberovic 1996). The effects of different growth temperatures on phenotypic plasticity of body-size-related traits have also been evidenced in D. melanogaster (James et al. 1995, 1997). Several studies have investigated mechanistic basis of flight ability of Drosophila species under variable thermal conditions in the laboratory (Unwin and Corbet 1984; Starmer and Wolf 1989; Dudley 2000; Frazier et al. 2008). These studies have shown that the amount of lift generated by a single wing stroke is positively related to wing area; and average lift is proportional to the square of wing beat frequency (Ellington 1984; Dudley 2000). Because the wing beat frequency and power output of small ectotherms, like Drosophila, declines with decreasing temperature (Curtsinger and Laurie-Ahlberg 1981; Unwin and Corbet 1984; Stevenson and Josephson 1990), the total lift generated by flies exposed to cold temperatures declines substantially. One way to compensate for these effects of low temperature on wing beat frequency could be to increase the relative size of the wings or to reduce the overall wing loading (Reed et al. 1942; Stalker 1980; Starmer and Wolf 1989; Azevedo et al. 1998). Some studies have shown that greater wing area could be advantageous in cold because flies can generate increased lift despite the decreased muscle power output that occurs at colder temperatures (Lehmann 1999; Frazier et al. 2008). If changes in wing loading represent adaptations of insect flight in nature, we expect similar changes in Indian populations of D. melanogaster which occur under tropical, subtropical and montane habitats along latitude on the Indian subcontinent but there are no data to support such arguments.
Several studies have suggested that flight ability of different insect taxa is likely to affect their dispersal and walking behaviour (Roff and Fairbairn 2001; Marden 1989). Dispersal is a life-history-related trait that affects distribution and abundance of a species (Dieckermann et al. 1999). Laboratory as well as wild strains of D. melanogaster have shown relationship between growth temperature and dispersive behaviour (Mikasa and Narise 1979). This study has shown higher dispersive ability in flies raised at higher temperature (30°C) as compared with flies reared at colder temperatures (15 or 20°C). If thermal conditions affect dispersal behaviour, we might expect geographical differences in dispersal of flies adapted to montane versus tropical habitats. Accordingly, we have examined possible relationships between flight ability (wing loading) and dispersal-related walking behaviour in geographical populations of D. melanogaster.
In the present study, we investigated the geographical variations in flight-related traits (wing length, thorax length, wing width and wing area) in wild-caught as well as laboratory-reared D. melanogaster. We estimated the wing loading as thorax volume / wing area in place of body weight / wing area so as to control the age and nutrition related changes in body weight. Further, we examined whether two flight-related traits (i.e. flight-muscle ratio and walking behaviour) are related to wing loading in geographical populations of D. melanogaster. Finally, we subjected the morphometric data on all the flight-related traits to multiple regression analysis as a function of average temperature(T ave) and relative humidity (RH) of the sites of origin of D. melanogaster populations to assess selection responses to local environmental conditions on the Indian subcontinent.
Materials and methods
Cultures
We investigated the nine Indian populations of D. melanogaster collected across the Indian subcontinent along a latitudinal range (8.29–32.16°N) involving a distance of ∼3000 km in a single trip in September–October 2009. Wild-caught female flies were used to set up isofemale (IF) lines (n = 20 per population) and were maintained on standard Drosophila culture medium (Sugar – Agar – yeast medium) at 21°C in temperature-controlled-growth chambers. For all isofemale line cultures, initial egg-laying period was limited to a few hours ( ∼6 h) and therefore crowding or density effect was minimized and laboratory growth conditions for all cultures were kept uniform. For geographical populations of D. melanogaster, climatic data on thermal variables were obtained from Indian Institute of Tropical Meteorology. Data on RH were obtained from climatological tables published by the Indian Meteorological Department, Govt. of India, New Delhi, and are shown in table 1.
Measurement of morphometric traits
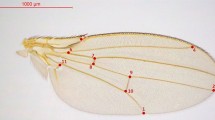
For wild-caught populations we measured body-size-related traits (wing length, thorax length and wing width) in 100 male as well as 100 female individuals per population. These metric traits were also measured in 5-day-old virgin laboratory-reared 100 males as well as 100 female flies of each of 20 laboratory-reared IF lines per population. Morphometric traits were measured using a micrometer in an Olympus Stereo-zoom microscope SZ-61 (www.olympus.com). Wing length (WL) was measured from the point of attachment with the thorax up to the tip of third longitudinal vein; thorax length (TL) was measured from the anterior margin of the thorax to the tip of postscutellum. Wing width (WW) was measured along the mid vertical line of the wing. Micrometer observations were transformed according to the magnifications and the data were expressed in millimetre. For body weight measurements, each group of 10 individuals (males and females separately) was weighed on a Sartorius microbalance (model CPA26P, 0.01 mg precision; www.sartorius.com). All the morphometric studies were done on virgin flies in a temperature-controlled room set at 25°C.
Assessment of wing loading
For estimation of wing loading, two methods have been followed in different studies, i.e. wing loading ( ρ w1) = body weight / wing area; ρ w (2) = thoracic volume /wing area (Stalker 1980; Azevedo et al. 1998). For wild-caught individuals, variations in body weight due to age are difficult to control in females but variations are not significant in males of D. melanogaster. However, we controlled age-related effects by following Stalker (1980) and used thorax volume in place of body weight for wild-caught as well as laboratory-reared male and female individuals of D. melanogaster. The thorax volume was calculated as thorax length × thorax width × thorax depth. This was done because thorax volume and body weight showed positive linear correlation (see figure 3), and was necessitated due to possible and uncontrolled variations in body weight due to age as well as nutrition.
Estimation of morphometric traits
We followed standard methods to estimate wing area, wing thorax-length ratio, wing aspect ratio and wing-load index (Stalker 1980; Azevedo et al. 1998; Van’t Land et al. 1999). The wing area was estimated as wing length × wing width in mm 2. Wing aspect ratio is a metric character which provides information about wing shape and was measured as the ratio wing length2/ wing area (Azevedo et al. 1998). Further, wing-load index was calculated as the ratio of thorax volume / wing area (Stalker 1980). Another flight-related trait, flight-muscle ratio, was calculated as the ratio thorax dry mass / total body dry mass (Frazier et al. 2008). To determine flight-muscle ratio, we randomly selected 10 IF lines of nine latitudinal populations of D. melanogaster. For each fly, the abdomen, head and thorax were separated from leg and wing and each part was weighed separately (before and after drying at 60°C for 24 h) with an electronic microbalance (Sartorius CPA26P, 0.01 mg precision; www.sartorius.com). This was followed for 100 virgin male and female flies of wild-caught and 10 randomly chosen IF lines of laboratory-reared individuals of nine latitudinal populations of D. melanogaster. The data on thoracic dry mass and total body dry mass were used for estimation of flight-muscle ratio.
Assessment of developmental time
For randomly chosen 20 IF lines for each of nine latitudinal populations of D. melanogaster, developmental time was recorded as the time between average egg-laying time and the midpoint of the interval (8 h) in which flies emerged. Finally, the developmental time was calculated as the weighted mean eclosion time. The flies were grown at 21°C, and when the first flies were about to eclose the culture vials were checked for newly emerged adults every 8 h.
Assessment of dispersal-related walking behaviour
For wild-caught and laboratory-reared flies of each population, we measured dispersal index by following the method suggested by Mikasa and Narise (1979). The setup includes a set of four migration tubes (see figure 1, pp. 218, Mikasa and Narise 1979). Fifty pairs of flies (virgin and 5-day-old in case of laboratory-reared flies) were introduced in the central food vial and kept for 24 h. Thereafter, three new tubes with fresh Drosophila culture medium were connected with the central tube and flies were allowed to disperse for 6 h in a temperature controlled room at 25°C. The dispersal activity was calculated as the per cent of the dispersed flies in connected tubes to total flies after 6 h.
Geographical variation in body-size-related traits wing length/thorax length ratio (A&D, positive cline), wing aspect ratio (B&E, positive cline), and wing area (C&F, positive cline) in males (A–C) and females (D–F) of wild-caught and laboratory-reared individuals (20 IF lines × 10 individuals of each isofemale line (IF)) of nine latitudinal populations of D. melanogaster from the Indian subcontinent.
Statistical analysis
For statistical analysis of various morphometric traits, we used Statistica software (Statistica 1997). For wild-caught flies, we used means ± SE values of 100 female or 100 male individuals per population. For each of the 20 IF lines per population, we used means ± SE values of 100 male and 100 female flies of each isofemale line. For laboratory-reared flies of various populations, trait variability of different flight-related traits (wing area, wing loading, flight-muscle ratio and dispersal index) were assessed on the basis of nested analysis of variance so as to partition effects due to populations and IF lines for male flies or female individuals of nine geographical populations. Based on isofemale line data, we calculated correlation (r ± SE) between traits such as thorax volume and body weight of laboratory-reared 10 IF lines of one southern (Trivandrum) and one northern population (Manali). Similarly, correlations were estimated between wing loading and flight-muscle ratio, and finally with dispersal index. Thus, for all the quantitative traits, we treated data on male and female individuals separately. Finally, we used multiple regression analysis of trait variability as a simultaneous function of T ave and RH of the sites of origin of nine wild-caught geographical populations of D. melanogaster(y = a + b 1 T ave + b 2 ⋅ RH).
Results
We have illustrated geographical variation for three body-size-related traits (wing and thorax length, wing aspect ratio, and wing area) in both the sexes of wild-caught and laboratory-reared individuals of nine populations of D. melanogaster in figure 1. For each trait population mean values have evidenced clinal increase along latitude, i.e. trait values increase in populations from south to north. This is confirmed by regression analysis. For these three body-size traits, slope values did not vary between sexes for wild-caught populations (figure 1). Likewise for laboratory-reared populations of D. melanogaster slope values did not vary between the sexes. However, there are significant differences in slope values between wild versus laboratory-reared populations of D. melanogaster. Similar results on slope values of wing loading are evident in wild and laboratory populations of D. melanogaster (figure 2, B&E). In contrast, we found significant differences in slope values between sexes as well as wild versus laboratory populations for two traits i.e. flight-muscle ratio and dispersal (figure 2, A, C, D&F). We found negative slopes for flight-muscle ratio, wing loading as well as dispersal (figure 2). Further, based on the results of nested analysis of variation (IF lines: random factor and nested into populations) for duration of development, wing area, wing loading, flight-muscle ratio and dispersal, we found significant genetic differentiation between, as well as within populations of male and female individuals of laboratory-reared (IF lines) nine latitudinal populations of D. melanogaster. For all the body-size-related traits (table 2), the F values were highly significant (P < 0.001; table 2).
Geographical variation in two flight-related traits flight-muscle ratio (A & D) and wing loading (B & E, negative clines), and one behaviour-related trait dispersal (C & F, negative cline) in males (A–C) and females (D–F) of wild-caught and laboratory-reared individuals (20 IF lines × 10 individuals of each IF) of nine latitudinal populations of D. melanogaster from the Indian subcontinent.
Correlation between thorax volume and body weight
In the present study, we calculated wing load as thorax volume / wing area in place of body weight / wing area because body weight is subjected to changes due to age as well as nutrition. This was confirmed by correlation analysis of thorax volume and body weight in 10 randomly selected isofemale lines of one northmost and one southmost population of D. melanogaster. The males and females are shown separately in figure 3, A&B. A significant positive correlation is evident suggesting that for calculation of wing load, thorax volume data can represent body weight.
Relationship between wing area and duration of development
We observed significant changes in duration of development for laboratory-reared male and female individuals of nine geographical populations of D. melanogaster (table 2). Such a cline was significant and positive along latitude. For tropical populations of D. melanogaster, we found shorter duration of development as compared with longer duration of development for northern populations. We found significant positive correlation between duration of development and wing area for 10 IF lines of one southernmost (Trivandrum) and one northernmost (Manali) populations of D. melanogaster (figure 3, C&D). Thus, our results show correlated changes in a life history trait and a body size trait.
Trait correlation with wing loading
We found negative clines for wing loading, flight-muscle ratio as well as dispersal in males as well as females of wild-caught as well as laboratory-reared populations of D. melanogaster (figure 1). To test whether these traits are correlated, we used IF line data (n = 40) for these traits in one northernmost (Manali) and one southernmost (Trivandrum) populations of D. melanogaster and the results are shown in figure 4. There are sex-specific differences for both the traits, i.e. male flies showed higher trait values than females. Further, southern population showed 50% higher trait value for wing load, flight-muscle ratio as well as dispersal compared with northern population. Finally, significant positive correlations are evident between wing load and flight-muscle ratio, and between wing load and dispersal in both the sexes as well as populations (figure 4).
Positive correlation between wing loading and flight-muscle ratio (A&B), and for wing loading and dispersal index (C & D) of laboratory-reared 10 IF lines for male and female flies (10 replicates of 50 flies of each IF line) of one southernmost (Trivandrum) and one northernmost (Manali) population of D. melanogaster.
Analysis of climatic associations of body-size-related traits
To assess whether geographical variation in body-size-related traits reflect adaptations to local climatic conditions, we considered multiple regression analysis of trait variability as a simultaneous function of T ave and RH and the results are shown in table 3. For each body-size-related trait, we found significant slope value which did not vary between sexes and were significantly higher for T ave as well as RH (table 3). For all the traits, we found significantly higher R 2, i.e. coefficient of genetic determination ( > 0.75). Thus, geographical variation in all the body-size-related traits reflect adaptation to climatic conditions of the origin of populations of D. melanogaster.
Discussion
In the present study, analysis of body-size-related traits in Indian populations of D. melanogaster has shown significant positive clines along latitude for wild as well as laboratory-reared populations. The persistence of clines in the laboratory-reared populations evidence genetic differentiation of body-size-related traits. The steeper clinal variation in wild populations signifies the role of variable climatic conditions along latitude which contribute to greater phenotypic variation in D. melanogaster. We observed negative association of wing load, flight-muscle ratio and dispersal with latitude. Thus tropical populations of D. melanogaster have evolved significantly higher flight as well as dispersal ability consistent with warmer and humid conditions. In contrast, subtropical populations of D. melanogaster from north Indian localities have evidenced lower wing-load, lower flight-muscle ratio and possibly lower wing beat frequency associated with adaptations under colder climatic conditions. We may suggest that steeper gradients in climatic conditions (T ave and RH) along latitude on the Indian subcontinent may provide strong selection pressures to cause clinal variation in body-size-related traits in geographical populations of D. melanogaster.
For body-size-related traits, our results on clinal variations in Indian populations of D. melanogaster are interesting in several respects when compared with D. melanogaster populations from other continents. First, for body-size-related traits (wing length, thorax length, wing-thorax ratio and wing-area) we found a strong positive correlation with latitude for both wild-caught and laboratory-reared populations of D. melanogaster. These results are in concurrence with previous reports on Australian and South American populations of D. melanogaster (James et al. 1995, 1997; Van’t Land et al. 1999). However, our results on wing-aspect ratio are different from previous studies. For South American populations of D. melanogaster, wing aspect ratio is much shallower (Van’t Land et al. 1999). However, for Australian populations of D. melanogaster, wing aspect ratio was lower in wild-caught than laboratory-reared flies (Azevedo et al. 1998). In contrast, for Indian populations of D. melanogaster, we found higher values of wing-aspect ratio as well as wing–thorax ratio in wild-caught as compared with laboratory-reared flies. These results suggest that evolutionary changes in wing shape are significant in D. melanogaster flies from the Indian subcontinent.
Second, for body size in D. melanogaster, previously reported clines are based on populations collected from the southern hemisphere, i.e., South America (Chile 18°5′S to 41°5′S, Van’t Land et al. 1999) and Australia (16°5′S to 42°8′S, James et al. 1995, 1997; Azevedo et al. 1998). In contrast, for D. melanogaster populations from the northern hemisphere, morphometric traits were investigated in extreme northern populations such as Eurasia (25°9′N to 44°6′N, Imasheva et al. 1994) and North America (25°9′N to 44°6′N; Coyne and Beecham 1987). Thus, analysis of body-size-related traits for D. melanogasteraround equator has received lesser attention so far. In the present study, we considered populations from 8°29′N to 32°16′N which have evidenced steeper clines consistent with significant differences in climatic conditions prevailing in the tropics versus subtropics. Our results based on common garden experiment have evidenced genetic clines, i.e., increase in body-size-related traits with increase in latitude. The repeatability of clines in D. melanogaster from the Indian subcontinent has suggested the role of strong selection pressure for body-size-related traits.
Third, the regression for body-size-related traits in wild-caught D. melanogaster with latitude are steeper than those observed for laboratory-reared populations. These differences are consistent with effect of local temperature for wild-caught flies as compared with constant temperature of development for laboratory-reared flies. For wild flies, the mean trait values of body-size-related traits are significantly lower in the southern populations than northern populations, and such results match with climatic conditions of localities of collection as shown in table 1. Few previous studies on D. melanogaster have considered wild data on body-size-related traits (Coyne and Beecham 1987; Imasheva et al. 1994). Our results on body size clines in wild flies concur with Eurasian populations of D. melanogaster (Imasheva et al. 1994). In contrast, wing size variations showed an opposite trend in D. melanogaster populations from the east coast of north America, i.e. latitudinal cline in wing length was shallower in wild-caught as compared with laboratory-reared populations (Coyne and Beecham 1987). The possible explanation could be that these North American populations were collected in different years as well as seasons which might have modified the variation in body size. However, similarity between our results on wild-caught populations of D. melanogaster and those of Eurasian populations could result due to sampling of wild-caught flies in the same month as well as year.
Fourth, for Indian populations of D. melanogaster, wefound a cline for developmental time along latitude. Similarly, clinal variation for duration of development has alsobeen reported for Australian populations of D. melanogaster (James et al. 1995). In contrast, South American populationsof D. melanogaster have not shown geographical variationsfor development time (Van’t Land et al. 1999). Further, correlated changes in body size and development time are evident in Australian populations of D. melanogaster(James etal. 1995), but not in South American populations (Van’t Land et al. 1999). In the present work, changes in wing area and duration of development are positively correlated, i.e., tropical populations (Trivandrum) evidenced shorter duration of development as well as smaller wing area while subtropical population (Manali) showed longer duration of development and larger wing area. These results are consistent with artificial selection of development time in D. melanogaster which showed positive correlations with adult body size (Zwann etal. 1995; Nunney 1996). Thus, climatic conditions of the origin of populations of D. melanogaster from Indian subcontinent are likely to result in correlated selection response on body size and developmental time.
Fifth, in several insect taxa, variations in flight-muscle ratio are directly linked to flight capability, e.g. insect taxa showing winged and wingless morphs differ in their flight-muscle ratio (Marden 1989). Some studies on the mechanics of flight in D. melanogaster have investigated the possible role of flight-muscle ratio but geographical variation in flight-muscle ratio has received lesser attention so far(Dudley 2000; Frazier et al. 2008). We found negative clines for flight-muscle ratio in wild as well as laboratory populations of D. melanogaster, i.e. southern tropical populations evidenced higher flight-muscle ratio as compared with northern subtropical populations. We also found significant positive correlation between wing loading and flight-muscle ratio which suggest that both these flight-related traits are likely under correlated selection and are consistent with cogradient selection hypothesis. The adaptive significance for flight ability can be argued on the basis of generation of higher wing beat frequency through higher flight-muscle ratio as well as higher wing loading for tropical flies and vice versa for northern flies from colder habitats. Both these traits have evidenced significant positive correlations with dispersal, i.e. higher flight ability could be associated with greater dispersal ability in D. melanogaster from warmer and humid conditions of the tropics while the reverse seems to be case for D. melanogaster flies from colder and drier northern localities on the Indian subcontinent.
Finally, some studies have implicated temperature as the selection agent for evolutionary changes in the body-size-related traits. For Australian and South American populations of D. melanogaster, latitude is highly correlated with T max, T min and T ave, but not with relative humidity and rainfall (Van’t Land et al. 1999). In contrast, latitude is highly correlated with T ave and RH on the Indian subcontinent. Therefore, as expected our results on regression analysis for variation in body-size-related traits with climatic variables showed significant relationship with T ave, as well as RH. The role of humidity variations for body-size trait could be tested through artificial laboratory selection experiments whereas the previous studies have considered only thermal selection of body size in D. melanogaster (Partrdige and French 1996). Our results suggest that geographical changes in body-size-related traits are likely to involve selection pressure due to changes in thermal as well as humidity conditions on the Indian subcontinent.
In conclusion, we found large amount of phenotypic differentiation in body-size-related traits in wild-caught populations of D. melanogaster. The persistence of latitudinal clines for wing–thorax ratio, wing aspect ratio and wing-area evidenced the differences in genetic constitution of D. melanogaster populations. We found steeper clines for body-size-related traits in wild-caught as compared laboratory-reared populations. Interestingly, we observed negative clines for wing loading, flight-muscle ratio and dispersal. Thus, southern populations of D. melanogaster showed significantly higher trait values for flight-muscle ratio, wing loading as well as dispersal as compared with northern populations. Our results suggest correlated selection response for body size and duration of development. Further, we found that adaptive changes in body-size-related traits of D. melanogaster populations are consistent with climatic variables (T ave and RH) along a latitudinal gradient on the Indian subcontinent.
References
Alexander R. M. 1983 Animal mechanics. Blackwell Scientific Publications, London, UK.
Azevedo R. B. R., James A. C., McCabe J. and Partridge L. 1998 Latitudinal variation of wing: thorax size ratio and wing-aspect ratio in Drosophila melanogaster. Evolution 52, 1353–1362.
Brodsky A. K. 1994 The evolution of insect flight. Oxford University Press, Oxford, UK.
Coyne J. A. and Beecham E. 1987 Heritability of two morphological characters within and among natural populations of Drosophila melanogaster. Genetics 117, 727–737.
Curtsinger J. W. and Laurie-Ahlberg C. C. 1981 Genetic variability of flight metabolism in Drosophila melanogaster. I. Characterization of power output during tethered flight. Genetics 98, 549–564.
Dieckermann N., O’Hara B. and Weisser W. 1999 The evolutionary ecology of dispersal. Trends Ecol. Evol. 14, 88–90.
Dudley R. 2000 The biomechanics of insect flight: form, function, evolution. Princeton University Press, Princeton, USA.
Ellington C. P. 1984 The aerodynamics of hovering insect flight. VI. Lift and power requirements. Phil. Trans. R. Soc. London B 305, 145–181.
Endler J. A. 1977 Geographic variation, speciation and clines. Princeton University Press, Princeton, USA.
Frazier M. R., Harrison J. F., Kirkton S. D. and Roberts S. P. 2008 Cold rearing improves cold-flight performance in Drosophila via changes in wing morphology. J. Exp. Biol. 211, 2116–2122.
Gilchrist G. W. and Huey R. B. 2004 Plastic and genetic variation in wing loading as a function of temperature within and among parallel clines in Drosophila subobscura. Integrative and Comp. Biol. 44, 461–470.
Greenewalt C. H. 1962 Dimensional relationship for flying animals. Smithsonian Institution, Washington, USA.
Hoffmann A. A. and Weeks A. R. 2007 Climatic selection on genes and traits after 100 year-old invasion: a critical look at the temperate-tropical clines in Drosophila melanogaster from eastern Australia. Genetica 129, 133–147.
Imasheva A. G., Bubli O.A. and Lazebny O. E. 1994 Variation in wing length in Eurasian natural populations of Drosophila melanogaster. Heredity 72, 508–514.
James A. C., Azevedo R. B. R. and Partridge L. 1995 Cellular basis and developmental timing in a size cline of Drosophila melanogaster. Genetics 140, 659–666.
James A. C., Azevedo R. B. R. and Partridge L. 1997 Genetic and environmental responses to temperature of Drosophila melanogaster from a latitudinal cline. Genetics 146, 881–890.
Junge-Berberovic R. 1996 Effect of thermal environment on life histories of free living Drosophila melanogaster and D. subobscura. Oecologia 108, 262–272.
Kammer A. E. and Heinrich B. 1978 Insect flight metabolism. Adv. Insect Physiol. 13, 133–228.
Lehmann F. O. 1999 Ambient temperature affects free-flight performance in the fruit fly Drosophila melanogaster. J. Comp. Physiol. B 169, 165–171.
Marden J. H. 1989 Body building dragonflies: costs and benefits of maximizing flight muscle. Physiol. Zool. 62, 505–521.
Mikasa K. and Narise T. 1979 The Relation between dispersive behaviour and temperature in Drosophila melanogaster. I. Dispersal patterns. Jpn. J. Genet. 54, 217–228.
Nunney L. 1996 The response to selection for fast larval development in Drosophila melanogaster and its effect on adult weight: an example of a fitness trade-off. Evolution 50, 1193–1204.
Nunney L. and Cheung W. 1997 The effect of temperature on body size and fecundity in female Drosophila melanogaster, Evidence for adaptive plasticity. Evolution 51: 1529–1535.
Parkash R., Karan D. and Munjal A. K. 1998 Geographical divergence for quantitative traits in colonizing populations of Drosophila kikkawai from India. Hereditas 128, 201–205.
Partridge L. and French V. 1996 Thermal evolution of ectotherm body size: Why get big in the cold? In Animals and temperature: phenotypic and evolutionary adaptation (ed. I. A. Johnston and A. F. Bennett), pp. 265–292. Cambridge University Press, Cambridge, UK.
Reed S. C., Williams C. M. and Chadwick L. E. 1942 Frequency of wing-beat as a character for separating species, races and geographic varieties of Drosophila. Genetics 27, 349–361.
Roff D. A. and Fairbairn D. J. 2001 The genetic basis of dispersal and migration, and its consequences for the evolution of correlated traits. In Dispersal (ed. J. Colbert, E. Danchin, A. A. Dhondt and J. D. Nichols), pp. 191–202. Oxford University Press, Oxford, UK.
Stalker H. D. 1980 Chromosome studies in wild populations of Drosophila melanogaster. II. Relationship of inversion frequencies to latitude, season, wing-loading and flight activity. Genetics 95, 211–223.
Starmer W.T. and Wolf L. L. 1989 Causes of variation in wing loading among Drosophila species. Biol. J. Linn. Soc. 37, 247–261.
Stevenson R.D. and Josephson R.K. 1990 Effects of operating frequency and temperature on mechanical power output from moth flight muscle. J. Exp. Biol. 198, 61–78.
Unwin D. M. and Corbet S. A. 1984 Wingbeat frequency, temperature and body size in bees and flies. Physiol. Entomol. 9, 115–121.
Van’t Land J., Zwaan B. J., Van Putten W. F., Kamping A. and Van Delden W. 1999 Latitudinal variation in wild populations of Drosophila melanogaster: heritabilities and reaction norms. J. Evol. Biol. 12: 222–232.
Zwann B. J., Billsma R. and Hoekstra R. F. 1995 Artificial selection for developmental time in Drosophila melanogaster in relation to the evolution of ageing: direct and correlated responses. Evolution 49, 635–648.
Acknowledgements
We are indebted to anonymous reviewers for constructive comments that improved this manuscript. Financial assistance (F 41-823/2012/SR) from University Grants Commission, New Delhi is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
[Bhan V., Parkash R. and Aggarwal D. D. 2014 Effects of body-size variation on flight-related traits in latitudinal populations of Drosophila melanogaster. J. Genet. 93, xx–xx]
Rights and permissions
About this article
Cite this article
BHAN, V., PARKASH, R. & AGGARWAL, D.D. Effects of body-size variation on flight-related traits in latitudinal populations of Drosophila melanogaster . J Genet 93, 103–112 (2014). https://doi.org/10.1007/s12041-014-0344-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-014-0344-5