Abstract
The Schiff base ligand 1-(4-((2-hydroxybenzylidene)amino)phenyl)ethan-1-one, obtained from 4-aminoacetophenone and salicylaldehyde, and its complexes with Fe(III), Co(II), Ni(II) and Cu(II) have been synthesized. These complexes were characterized by FTIR spectroscopy, elemental, and SCXRD analysis. FTIR spectra of complexes show the bidentate coordination of metal ions with ligands where O and N are electron-donating sites of the azomethine group. The electronic absorption spectra of these complexes show the characteristics of absorption bands involved in the Fe, Co, and Ni complexes due to their π→π*, n→π* transitions. Further, the geometry of the complexes was deduced from the calculated magnetic moment values and single crystal XRD analysis.
Graphical abstract
A Schiff base derived from the condensation reaction between 2-aminoacetophenone and salicylaldehyde was involved in the formation of four different metal complexes, i.e., with Nickel, Cobalt, Iron, and Copper. The synthetic route to forming these complexes followed an easy process, and the complexes were obtained in good yields.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Schiff base is a unique class of ligands with different donor atoms exhibiting a fascinating coordination style towards numerous metal ions.1,2 The compatibility for the chelation of the Schiff bases towards the transition metal ions is applied in preparing their metal complexes. Schiff base metal complexes are well-known for their easy synthesis, stability, and wide application.3,4 Metal complexes containing Schiff base ligands have been studied for their interesting and important properties, such as their ability to bind with oxygen, photochromism, antibacterial and antifungal properties, and catalytic activity in olefins, hydrogenation, and complexing proficiency towards a few toxic metals.5,6
Schiff bases are very efficient as ligands. The presence of the lone pair of electrons on the nitrogen atom of the imine bond means that they can be donated to the appropriated metal ion. Many Schiff base ligands have a second or even third functional group, such as an OH (salicylaldehyde or its derivatives) and the nitrogen atoms from heterocyclic rings, e.g. pyridine or imidazole rings. This electron donation, in conjunction with a functional group, implies that a vast number of transition metal complexes could be prepared. There are various examples of the activity of Schiff base complexes in homogeneous as well as heterogeneous catalysis.7,8 N, O donor atom Schiff base complexes were intensively studied for catalytic and biological properties.9 Transition metal complexes (apart from Pd) with Schiff base have been widely used in applications such as in the polymer industry, dye industry, medicinal chemistry, agrochemical, and biological activities.10 Promising catalytic activity shown by these transition metal complexes has encouraged the researchers to develop cheaper catalysts for coupling reactions compared to the ones with Pd.
Cobalt, iron, and ruthenium complexes of Schiff bases obtained from hydroxy benzaldehyde are used as catalysts in the oxidation of cyclohexane to cyclohexanol and cyclohexanone in the presence of H2O2.11 Ni(II) complexes with Bidentate (N-N) ligands become an efficient catalyst precursor for olefin oligomerization in the presence of an activator.12 A wide variety of Co(II) complexes are known to bind oxygen reversibly and are, therefore, frequently studied as model compounds for natural oxygen carriers. They are used in O2 storage, as well as in organic synthesis due to their catalytic properties under mild conditions.13 Exploratory studies on employing the first-row transition metal complexes as precatalysts in coupling activity is an ongoing research topic because of the several benefits like low cost and abundance of metal, ease of synthesis, facile scale-up, etc.14 Contributing to this subject of research, we have attempted in this work to synthesize a few Schiff base complexes of Fe, Co, Ni, and Cu ions. The scope of our research study is to throw more light on the chelation behavior of the Schiff base ligand towards the chosen metal ions.
2 Experimental
2.1 Materials and reagents
All chemicals were of analytical reagent (AR) grade and used without any further purification. 4-aminoacetophenone, salicylaldehyde, nickel acetate tetrahydrate, copper acetate monohydrate, cobalt acetate tetrahydrate, and ferric chloride hexahydrate were purchased from Merck India and used as received. Ethyl alcohol, methanol, diethyl ether, and dichloromethane (DCM) used in the study were purchased from Sigma-Aldrich.
2.2 Instrumentation
The C, H, and N contents of the ligand and metal complexes were determined using microanalysis Thermoflash EA1112 series elemental analyzer. FTIR results were obtained on a Bruker-Alpha ECO-ATR FTIR spectrophotometer. The spectra were obtained as KBr pellets. Electronic spectra of ligands and complexes were measured on Analytik Jena SPECORD S600 UV–Vis spectrophotometer in the 200–800 nm range. The magnetic susceptibilities of the complexes were recorded at room temperature on a Sherwood UK magnetic balance, Hg[Co(SCN)4] was used as a calibrant. Molecular mass was determined using a Waters Q-ToF micro mass spectrometer with an ESI source. The 1H NMR spectrum of the ligand was recorded in Bruker AV 400 instrument using TMS as an internal standard. Thermogravimetric measurements were performed on (EXSTAR-6000) using nitrogen as the carrier gas (flow rate: 50 mL/min). The heating rate was 10 °C/min.
2.3 Synthesis of Schiff base ligand (L)
4-aminoacetophenone (0.135 g, 1 mmol) and salicylaldehyde (0.122 g, 1 mmol) were dissolved in ethanol and heated to 60 °C in a 50 mL round bottom flask. The reaction mixture was then refluxed for 3 h, followed by filtration of obtained product, washing with diethyl ether, re-crystallization using ethanol, and drying. Yield: 80.40%; Color: Yellow; C15H13NO2: Anal. Found: C, 75.94; H, 5.40; N, 5.05% Calc.: C, 75.90; H, 5.48; N, 5.85%. Mol. Wt.: 239.00: M+ peak: 239.09 m/z. FTIR (KBr) (ν/cm−1): 3333 (O–H), 1411–1490 (νC=C aromatic), 1577 (νC=N), 1279 (νC–O), 1112–1166 (νC–N), 3046.22 (νC–H); 1H NMR (δ, ppm in CDCl3): 12.87 (s, 1H); 8.63 (s, 1H); 8.04 (d, 2H); 8.02 (d, 2H); 7.46–6.94 (m, 4H); 2.60 (s, 3H) (Scheme 1).
2.4 Synthesis of metal complexes
2.4.1 Synthesis of complex C-1
The metal complex C-1 was prepared by the addition of a hot solution of the ferric chloride (0.270 g, 1 mmol) in a 50% v/v mixture of ethanol in water (5 mL) to the hot solution of the synthesized Schiff base (0.478 g, 2 mmol) in the same solvent system (5 mL). The mixture was refluxed with continuous stirring for 5 h. The reaction mixture was filtered, washed with methanol, and dried. Yield: 84.50%; Color: Dark purple; C30H24ClFeN2O4: Anal. Found: C, 59.92; H, 4.35; N, 4.45% Calc.: C, 59.83; H, 4.33; N, 4.63%. Mol. Wt.: 568.10: M+ peak: 568.1 m/z FTIR (KBr) (ν/cm−1): 3351.84 (O–H), 1425–1475 (νC=C aromatic), 1596 (νC=N), 1279 (νC–O), 1112–1166 (νC–N), 3061.86 (νC–H) (Scheme 2).
2.4.2 Synthesis of complex C-2
The metal complex C-2 was prepared by the addition of a hot solution of the cobalt acetate tetrahydrate (0.249 g, 1 mmol) in a 50% v/v mixture of ethanol in water (5 mL) to the hot solution of the synthesized Schiff base (0.478 g, 2 mmol) in the same solvent system (5 mL). The mixture was refluxed with continuous stirring for 5 h. The reaction mixture was filtered, washed with methanol, and dried. Yield: 68.60%; Color: Orange; C30H24CoN2O4: Anal. Found: C, 64.31; H, 4.75; N, 4.56% Calc.: C, 64.43; H, 4.90; N, 4.70%. Mol. Wt.: 535.90: M+ peak: 535.90 m/z FTIR (KBr) (ν/cm−1): 3421.85 (O–H), 1356–1437 (νC=C aromatic), 1556.55 (νC=N), 1264 (νC–O), 3063 (νC–H) (Scheme 2).
2.4.3 Synthesis of complex C-3
Nickel complex C-3 was prepared by the addition of a hot solution of the nickel acetate tetrahydrate (0.248 g, 1 mmol) in a 50% v/v mixture of ethanol in water (5 mL) to the hot solution of the synthesized Schiff base (0.478 g, 2 mmol) in the same solvent system (5 mL). The mixture was refluxed with continuous stirring for 5 h. The reaction mixture was filtered, washed with diethyl ether and dried. Yield: 78.0; Color: Bright green; C30H24N2NiO4: Anal. Found: C, 67.00; H, 4.81; N, 5.11% Calc.: C, 67.07; H, 4.88; N, 5.21%. Mol. Wt.: 535.85: M+ peak: 535.85 m/z FTIR (KBr) (ν/cm−1): 3366 (O–H), 1419–1445 (νC=C aromatic), 1588 (νC=N), 1279 (νC–O), 1124–1178 (νC–N), 3056.73 (ν C−H) (Scheme 2).
2.4.4 Synthesis of complex C-4
Copper complex C-4 was prepared by the addition of a hot solution of the copper acetate monohydrate (0.199 g, 1 mmol) in 50% v/v mixture of ethanol in DCM (5 mL) to the hot solution of the synthesized Schiff base (0.478 g, 2 mmol) in ethanol (5 mL). The mixture was refluxed with continuous stirring for 5 h. The formed product was filtered and washed with 50% ethanol-water mixture, diethyl ether, and dried. Yield: 80.50%; Color: Brown; C30H24CuN2O4: Anal. Found: C, 66.68; H, 4.40; N, 5.17% Calc.: C, 66.72; H, 4.48; N, 5.19%. Mol. Wt.: 539.00: M+ peak: 539.00 m/z FTIR (KBr) (ν/cm−1): 3446 (O–H), 1442–1531 (ν C=C aromatic), 1606 (νC=N), 1270 (νC–O), 1150–1181 (ν C−N), 2993.81 (ν C−H) (Scheme 2).
2.5 X-ray crystallography
Ligand and complex (C-4) crystals were obtained by a slow evaporation method using 50% DCM in ethanol as solvent. The X-ray diffraction studies for these crystals were performed on a Bruker APEX-II CCD diffractometer with Mo Kα radiation (λo = 0.71073 Å) at 296 K. The structure was solved using SHELXL-2007/2014 software and refined by full-matrix least square methods.
3 Results and Discussion
3.1 1H NMR and mass spectra
The 1H NMR (400 MHz) spectrum of ligand (L) was recorded in CDCl3 (Figure S1, Supplementary Information). The electron impact mass spectra of the Schiff base and metal complexes were recorded and investigated at 50 eV of electron energy. The electron impact mass spectra of the Schiff base ligand and metal complexes were recorded and investigated to confirm the molecular weight of the compounds. The important mass fragmentations of the ligands and complexes are shown in the spectra (Figures S7-S11, SI). The expected molecular weight and the observed molecular weight of the ligands and complexes are given in Table S2 (SI). From the table, it is confirmed that the observed molecular weight of the compounds matches the expected molecular weight of the compounds.
3.2 FTIR spectra
The synthesized ligand and complex molecules were examined by the FTIR analysis (Figures S2–S6 in Supplementary Information). The new bands in the range 580–672 cm−1 in complexes tentatively assign coordination of metal with oxygen atom ν (M–O).15 The bands which appear around 400–500 cm−1 in the spectra of the complexes may be assigned to the coordination of metal with a nitrogen atom.
3.3 Electronic absorption spectra
The electronic spectra of synthesized ligand and complexes were recorded in ethanol solvent (10−3 M) (Figure 1). Quartz cells with a 1 cm path length were employed in the 200–700 nm spectral range. The peak in the region 218 nm is assigned to the π→π* transitions of aromatic rings. The peak at 310–350 nm involves n→π* transition of the CH=N group.16 Iron complex shows bands at 210, 322, 511 nm (449–563 nm). In the cobalt complex, peaks at 208, 263, 296, 354 nm (460–514 nm) were observed. Similarly, the nickel complex shows two peaks at 215 and 321 nm, which are due to π→π*, n→π* transitions, respectively. The electronic spectrum of the Cu complex shows peaks at 213 and 315 nm, 402, 512 nm due to π→π* and n→π*, respectively.
3.4 Magnetic susceptibility measurements
The magnetic moment for iron complex C-1 was found to be 1.80 BM, which indicates that the complex is paramagnetic with one unpaired electron present (Table 1).
The observed magnetic moment supports the iron complex to be in low-spin Fe (III) state. Similarly, cobalt complex C-2 shows a magnetic moment of 1.73 BM with one unpaired electron, which indicates that the cobalt complex possesses a low spin Co (II) state. The almost zero magnetic moments of nickel complex C-3 clearly confirm the absence of unpaired electrons. It also affirms the diamagnetic nature of the complex with a possible square planar geometry.17 The magnetic moment of 1.65 BM supports the copper complex C-4 to be in a high spin Cu (II) state with tetrahedral geometry.
3.5 Thermogravimetric analysis
The purpose of the thermal study is to confirm the course of the degradation and the presence of the hydrated water molecules in the complexes. Complexes were subjected to thermal analysis in order to interpret the stability as well as the structural information. C-1, C-2, C-3, and C-4, which are the complex of ligand L, show typical TGA curves representing single-step dissociation of the complexes. Thermal degradations of synthesized complexes were studied in the temperature range from 50 to 700 °C. The thermogravimetric analysis data clearly indicates the ligand's decomposition in the temperature range of 180–450 °C. Removal of the ligand molecule proceeds in a single step; further complete decomposition was observed at ≥500 °C (Figure 2). The dissociation of the ligands is observed well near a temperature of 500 °C, indicating the stability of the complex formed.
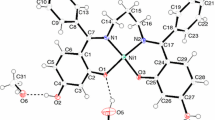
3.6 Structures of ligand L and complex C-4
Selected bond lengths and bond angles are listed in Table S1 (Supplementary Information). The molecular structures of L and C-4 are shown in (Figure 3 (a) and (b)).
4 Conclusions
In conclusion, efficient, cheap, and environmentally benign Schiff base transition metal (Fe, Co, Ni, and Cu) complexes have been synthesized and characterized by FTIR spectroscopy, elemental, and SCXRD analysis. FTIR spectrum confirms the bidentate coordination of metal ions with ligands where O, and N are at electron-donating sites of the azomethine group. The electronic absorption spectra show the specific band at 210, 322, and 511 nm (449–563 nm) corresponds to the Iron complex. In the cobalt complex, peaks appeared at 208, 263, 296, 354 nm (460–514 nm) and, the nickel complex shows two peaks at 215 and 321 nm, which are mainly due to π→π*, n→π* transitions, respectively. The thermal stability in the temperature range of 180–450 °C and complete decomposition at ≥500 °C shown by these complexes was excellent and can be explored further in various applications.
References
Gupta K C and Sutar A K 2008 Catalytic activities of Schiff base transition metal complexes Coord. Chem. Rev. 252 1420
Dhanaraj C J, Johnson J, Joseph J and Joseyphus R S 2013 Quinoxaline-based Schiff base transition metal complexes: review J. Coord. Chem. 66 1416
Abu-Dief A M and Mohamed I M A 2015 A review on versatile applications of transition metal complexes incorporating Schiff bases Beni-Suef Univ J. Basic Appl. Sci. 4 119
Alias M, Kassum H and Shakir C 2014 Synthesis, physical characterization and biological evaluation of Schiff base M(II) complexes J. Assoc. Arab Univ. Basic Appl. Sci. 15 28
Malik S, Ghosh S and Mitu L 2011 Complexes of some 3d-metals with a Schiff base derived from 5-acetamido-1, 3, 4-thiadiazole-2-sulphonamide and their biological activity J. Serb. Chem. Soc. 76 1387
Raman N, Johnson Raja S, Joseph J and Dhaveethu Raja J 2007 Synthesis, spectral characterization and dna cleav-age study of heterocyclic schiff base metal complexes J. Chil. Chem. Soc. 52 1138
Silva A R, Budarin V, Clark J H, Castro B de and Freire C 2005 Chiral manganese(III) Schiff base complexes anchored onto activated carbon as enantioselective heterogeneous catalysts for alkene epoxidation Carbon 43 2096
Zhang X and Llabrés i Xamena F X and Corma A, 2009 Gold(III)–metal organic framework bridges the gap between homogeneous and heterogeneous gold catalysts J. Catal. 265 155
Ejidike I P and Ajibade P A 2015 Transition metal complexes of symmetrical and asymmetrical Schiff bases as antibacterial, antifungal, antioxidant, and anticancer agents: progress and prospects Rev. Inorg. Chem. 35 191
Prakash A and Adhikari D 2011 Application of Schiff bases and their metal complexes-a review Int. J. Chem. Tech. Res. 3 1891
Tumer M, Akgun E, Toroglu S, Kayraldiz A and Donbak L 2008 Synthesis and characterization of Schiff base metal complexes: their antimicrobial, genotoxicity and electrochemical properties J. Coord. Chem. 61 2935
Das P and Linert W 2016 Schiff base-derived homo-geneous and heterogeneous palladium catalysts for the Suzuki-Miyaura reaction Coord. Chem. Rev. 311 1
Deligonul N, Tumer M and Serin S 2006 Synthesis, characterization, catalytic, electrochemical and thermal properties of tetradentate Schiff base complexes Trans. Met. Chem. 31 920
Cozzi P G 2004 Metal-Salen Schiff base complexes in catalysis: practical aspects Chem. Soc. Rev. 33 410
Kurup M R P, Varghese B, Sithambaresan M, Krishnan S, Sheeja S R and Suresh E 2011 Synthesis, spectral characterization and crystal structure of copper(II) com-plexes of 2-benzoylpyridine-N(4)-phenylsemicarbazone Polyhedron 30 70
Raman N, Raja J D and Sakthivel A 2007 Synthesis, spectral characterization of Schiff base transition metal complexes: DNA cleavage and antimicrobial activity studies J. Chem. Sci. 119 303
Cristóvão B 2011 Spectral, thermal and magnetic properties of Cu (II) and Ni (II) complexes with Schiff base ligands J. Serb. Chem. Soc. 76 1639
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ansari, R.M., Ramakrishna, D. & Bhat, B.R. Thermally stable complexes of Fe(III), Co(II), Ni(II) and Cu(II) with Schiff base derived from 4-aminoacetophenone and salicylaldehyde. J Chem Sci 134, 117 (2022). https://doi.org/10.1007/s12039-022-02113-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-022-02113-6