Abstract
Synthesis of a functionalized tricyclo[6.2.0.02,6]decane derivative in enantiomerically pure form, the core structure present in the natural products kelsoene and poduran, is described. The key steps involve a stereocontrolled copper (I)-catalyzed intramolecular [2 + 2] photocycloaddition of a 1, 6-diene prepared from D-mannitol to form a substituted bicyclo[3.2.0]heptane derivative and a ring closing olefin metathesis involving the vicinal substituents on the five-membered ring of the bicyclo[3.2.0]heptane derivative.

An asymmetric synthesis of a functionalized tricyclo[6.2.0.02,6]decane derivative has been described. The key step involves an intramolecular Cu (I)-catalyzed [2 + 2] photocycloaddition of a 1,6-diene derived from D-mannitol to provide a bicyclo[3.2.0]heptane derivative. Annulation of a five-membered ring on to the five-membered ring of this derivative using RCM provided the title compound.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
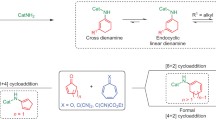
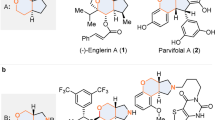
Kelsoene 1 and poduran 2 are two structurally related natural products that possess a common tricyclo[6.2.0.02,6]decane unit. Kelsoene[1] was isolated from a tropical marine sponge Cymbastela hooperi. Later, it was also found[2] in the liverworts Ptychanthus Striatus, Caypogeia muelleriana and Tritomaria quinquedentata. Poduran[3] was isolated from springtail Podura aquatica. The formidable task associated with the synthesis of kelsoene lies in the construction of the unique cis-anti-cis fused 5-5-4 tricarbocyclic structure with the C-7 substituent eclipsed to the cyclobutane methylenes. Kelsoene thus elicited considerable interest for its synthesis culminating in a number of elegant approaches.[4] Intermolecular [2 + 2] photoycloaddtion of an appropriately designed bicyclo[3.3.0]octenenone derivatives to make cis-anti-cis 5-5-4 system was the key step[4] a–f in most of the reported approaches.

An intramolecular copper (I) catalyzed [2 + 2] photocycloaddition[5] of cis-1, 2-disubstituted cyclopentane derivatives could have been the direct approach to the 5-5-4 ring system present in kelsoene. However, investigations by us[6] and Bach[7] revealed that such intramolecular [2 + 2] photocycloaddition of cis-1, 2-disubstituted cyclopentane derivatives led to cis-syn-cis 5-5-4 tricyclic systems rather than the cis-anti-cis 5-5-4 systems present in 1 and 2. This led us to consider a new approach involving annulation of a five-membered ring on to a bicyclo[3.2.0]heptane derivative 3 using the appropriately functionalized appendages R 2 and R 3 (scheme 1). The substituent R 1 can be employed to provide the iso-propenyl chain at C-7 of 1. The bicyclo[3.2.0]heptane derivative 3 should be available from Cu (I)-catalyzed [2 + 2] photocycloaddition of the 1, 6-diene 4. Thus our approach involving elaboration of 4-5 bicyclic system to 4-5-5 tricyclic system is fundamentally different than most of the reported approaches involving elaboration of 5-5 bicyclic system to the 5-5-4 tricyclic system. We herein present the results of this investigation.
2 Experimental
All reactions were carried out under an atmosphere of nitrogen. PE refers to the fraction of petroleum ether having bp 60–80 ∘C. EA refers to ethyl acetate. Organic extract was dried over anhydrous Na2 SO 4. Column chromatography was carried out with silica gel (100–120 mesh). Optical rotation values are given in 10−1 deg cm2 g −1 and measured using Jasco P-1020 digital polarimeter. Infrared spectra for liquids were recorded as thin films on Shimadzu FTIR-8300 instrument. NMR spectra were recorded at 500 MHz for 1H and 125 MHz for 13C on Bruker-Avance DPX500 instrument. 13C peaks assignment is based on DEPT experiment. High Resolution Mass spectra (HRMS) were measured in a QTOF I (quadrupole-hexapole-TOF) mass spectrometer with an orthogonal Z-spray-electrospray interface on Micro (Ya-263) mass spectrometer (Manchester, UK).
2.1 Ethyl 2-(1-(1,4-dioxaspiro[4.5]decan-2-yl)allyl)-3-hydroxy-4-methylpent-4-enoate (6)
To a magnetically stirred solution of diisopropylamine (2.0 mL, 14.92 mmol) in anhydrous THF (20 mL) cooled to −20∘C was added dropwise n-BuLi (7 mL, 11.1 mmol, 1.6 M in THF). After stirring for 20 min. at this temperature, the solution was cooled to −78∘C and a solution of the ester 5[8] (1 g, 3.7 mmol) in THF (10 mL) was added dropwise. The reaction mixture was then slowly warmed to −30∘C and stirred at that temperature for 30 min. The temperature of the reaction mixture was again brought to −78∘C and to it HMPA (1 mL) followed by methacrolien (0.9 mL, 11.2 mmol) was added dropwise. It was allowed to stir for 15 min at −78∘C. After quenching with saturated aqueous NH4Cl solution, the reaction mixture was extracted with diethyl ether (3 ×10 mL). The organic extract was washed with brine (10 mL) and dried. The residual material after evaporation of the solvent in vacuum was chromatographed (1:9 Et2O/PE) to afford an inseparable mixture containing all the four possible diastereoisomers of the hydroxy-ester 6 (700 mg, 56%) as a light yellow oil: R f : 0.6 [EA/PE (3:7)]; [\(\alpha ]_{\mathrm {D}}^{\mathrm {26}} =+ 11.84\) (c 0.625, CHCl3); IR (KBr) ν max 3473, 2937, 1730, 1641 cm −1; 1H NMR (500 MHz, CDCl3) δ 1.16–1.22 (t, 3H, J=7.0 Hz), (triplets of all the isomers merged together), 1.35 (brs, 2H), 1.52 (brs, 4H), 1.56 (brs, 4H), 1.73 (s, 6H), 2.57–2.89 (m, 2H), 3.17–3.66 (m, 1H), 4.26–4.42 (m, 1H), 4.42 (m, 5H), 4.84–5.06 (m, 2H), 5.13 (m, 1H), 5.27 (m, 1H), 5.58–6.04 (m, 1H); 13C NMR (125 MHz, CDCl3) δ (for the major isomer from the mixture) 14.1 (CH3), 17.8 (CH3), 23.9 (CH2), 25.2 (2xCH2), 34.7 (2xCH2), 46.8 (CH), 52.8 (CH), 60.6 (OCH2), 66.6 (OCH2), 72.7 (OCH), 75.1 (OCH), 109.8 (C), 113.7 (CH2), 119.5 (CH2), 135.3 (CH), 145.2 (C), 172.8 (CO); HRMS (ESI) calcd for C19 H 30 O 5Na (M + Na) +, 361.1991; found, 361.1994.
2.2 (1S, 2R, 3R, 4S, 5S)-Ethyl 2-hydroxy-1-methyl-4- (1,4-dioxaspiro[4.5]decan-2-yl)bicyclo[3.2.0]heptane-3-carboxylate (7)
A solution of the diene 6 (700 mg, 2.07 mmol) in anhydrous diethyl ether (250 mL) was poured into a pyrex cell. The ethereal solution was then degassed by bubbling Ar through it for 30 min. Freshly-prepared cuprous triflate (10 mg) was added to the reaction mixture. The reaction mixture was then irradiated internally under a positive pressure of Ar with a Hanovia 450 W medium pressure mercury vapour lamp through a water cooled quartz immersion well for about 4 h. After completion (TLC), the reaction mixture was poured into ice cold ammonia solution (10 mL, 35%) in a separatory funnel. After thoroughly shaking, the blue coloured aqueous layer was separated. The organic layer was washed with brine and concentrated in vacuum and the residual material was purified through column chromatography using [Et2O/PE (1:9)] as the eluent to afford the hydroxy-ester 7 as light yellow oil (200 mg, 51%); R f : 0.5 [EA/PE (3:7)]; [\(\alpha ]_{\mathrm {D}}^{\mathrm {26}} -11.26\) (c 1.4, CHCl3); IR (KBr) ν max 2953, 2862, 1712, 1620 cm −1; 1H NMR (300 MHz, CDCl3) δ 1.07 (s, 3H), 1.25 (t, 3H, J=7.2 Hz), 1.33 (brs, 2H), 1.50–1.55 (m, 10H), 2.10–2.29 (m, 4H), 2.52 (m, 1H), 2.86 (dd, 1H, J=4.8, 10.4 Hz), 3.26 (brs, 1H), 3.40 (t, 1H, J=7.6 Hz), 3.9–3.96 (m, 2H), 4.16 (q, 2H, J=7.0 Hz): 13C NMR (75 MHz, CDCl3) δ 14.2 (CH3), 23.5 (CH2), 23.8 (CH2), 23.9 (CH2), 25.2 (CH2), 25.2 (CH2), 25.6 (CH3), 34.7 (CH2), 36.1 (CH2), 43.5 (CH), 49.1 (C), 51.2 (CH), 52.6 (CH), 60.7 (CH2), 67.6 (CH2), 75.6 (CH), 80.6 (CH), 109.2 (C), 174.0 (CO); HRMS (ESI) m/z calcd for C19 H 30 O 5Na (M + Na) +, 361.1991; found : 361.1994.
2.3 (1S, 2R, 3R, 4S, 5S)-Ethyl 1-methyl-4-(1,4-dioxaspiro [4.5]decan-2-yl)-2-(tosyloxy)bicyclo[3.2.0]heptane-3-carboxylate (8)
A magnetically stirred solution of the alcohol 7 (390 mg, 1.15 mmol) in dichloromethane (20 mL) along with triethyl amine (0.6 mL, 4.6 mmol), DMAP (cat), and TsCl (1.09 g, 5.75 mmol) was refluxed for 12 h. After completion of the reaction (TLC) it was cooled to r.t. The resulting suspension was diluted with diethyl ether (150 mL), stirred for 30 min. and the precipitated solid was removed by filtration. The filtrate was then washed sequentially with 10% aqueous copper sulphate (2 ×5 mL), 10% aqueous sodium hydrogen carbonate (2 ×5 mL), brine (5 mL), dried and concentrated in vacuo. The resiudual material was then purified through column chromatography using [Et2O/PE (1 :9)] as the eluent to afford the compound 8 (400 mg, 71%) as light yellow oil. R f : 0.6 [EA/PE (3:7)]; [\(\alpha ]_{\mathrm {D}}^{\mathrm {25}} -37.7\) (c 0.88, CHCl3); IR (KBr) ν max 2937, 2862, 1732, 1599, 1448 cm −1; 1H NMR (500 MHz, CDCl3) δ 1.12 (s, 3H), 1.14 (t, 3H, J=7.5 Hz), 1.34–1.39 (brs, 2H), 1.48–1.54 (m, 8H), 2.07–2.11 (m, 2H), 2.16–2.20 (m, 1H), 2.26–2.30 (m, 2H), 2.41 (s, 3H), 2.61 (dddd, 1H, J=3.6, 6.8, 10.6 Hz), 3.01 (dd, 1H, J=6.0, 11.5 Hz), 3.42 (t, 1H, J=7.5 Hz), 3.71–3.74 (m, 1H), 3.92 (t, 1H, J=7.0 Hz), 4.01–4.07 (m, 1H), 4.16 (td, 1H, J=3.0, 7.5 Hz), 4.83 (d, 1H, J=6.0 Hz), 7.29 (d, 2H, J=8.5 Hz), 7.73 (d, 2H, J=8.0 Hz): 13C NMR (125 MHz, CDCl3) δ 14.0 (CH3), 21.6 (CH3), 21.9 (CH2), 23.8 (CH2), 24.0 (CH2), 24.8 (CH3), 25.2 (CH2), 25.8 (CH2), 34.7 (C), 36.0 (CH2), 42.8 (CH), 49.2 (CH), 52.9 (CH), 60.9 (CH2), 67.3 (CH2), 74.6 (CH), 90.3 (CH), 109.2 (C), 127.8 (CH), 129.7 (CH), 134.4 (C), 144.6 (C), 169.7 (CO); HRMS (ESI) m/z calcd for C26 H 36 O 7SNa (M + Na) +, 515.2079; found : 515.2078.
2.4 (1S, 4S, 5S)-Ethyl 1-methyl-4-(1,4-dioxaspiro[4.5] decan-2-yl)bicyclo[3.2.0]hept-2-ene-3-carboxylate (10)
A solution of the tosylate 8 (400 mg, 0.813 mmol) in toluene (5 mL) and DBU (0.2 mL) was refluxed at 110 ∘C for 1 h and excess toluene was removed. The residual material was dissolved in ether. The ethereal layer was washed with water (10 mL) and brine (10 mL) and dried and the solvent was evaporated. Column chromatography [Et2O/PE (1: 19)] of the residual material afforded the unsaturated ester 10 (200 mg, 77%) as yellow liquid; R f : 0.7 [EA/PE (1:9]; [\(\alpha ]_{\mathrm {D}}^{\mathrm {25}}= -36.02\) (c 1.25, CHCl3); IR (KBr) ν max 2933, 2860, 1714, 1622 cm−1; 1H NMR (500 MHz, CDCl3) δ 1.23 (s, 3H), 1.31 (t, 3H, J=7.5 Hz), 1.40–1.64 (m, 10H), 1.95–2.05 (m, 3H), 2.09–2.16 (m, 1H), 2.58 (dd, 1H, J=8.0, 3.0 Hz), 2.78 (s, 1H), 3.55 (t, 1H, J=7.5 Hz), 3.98 (t, 1H, J=7.5 Hz), 4.16–4.26 (m, 2H), 4.32 (td, 1H, J=3.0, 7.0 Hz), 6.76 (s, 1H); 13C NMR (125 MHz, CDCl3) δ 14.4 (CH3), 22.4 (CH3), 23.8 (CH2), 24.1 (CH2), 24.2 (CH2), 25.4 (CH2), 33.5 (CH2), 34.4 (CH2), 36.2 (CH2), 42.5 (CH), 52.5 (C), 55.5 (CH), 60.4 (CH2), 67.8 (CH2), 74.1 (CH), 109.5 (C), 134.7 (C), 152.4 (CH), 165.8 (CO); HRMS (ESI) calcd for C19 H 28ONa (M + Na) +: 343.1885 , found:343.1888.
2.5 ((1S, 4S, 5S)-1-Methyl-4-(1,4-dioxaspiro[4.5]decan- 2-yl)bicyclo[3.2.0]hept-2-en-3-yl)methanol l (11)
To a magnetically stirred solution of the compound 10 (2 g, 6.25 mmol) in diethyl ether (50 mL), cooled to 0 ∘C, was added LiAlH4 (475 mg, 12.5 mmol). Stirring was continued for 1 h. It was then quenched by sequential addition of 0.5 mL H2O, 0.5 mL 15% NaOH solution and 1.5 mL H2O. The clear ethereal solution obtained after decanation was concentrated and the residual material was purified through coloumn chromatography (3:7 Et2O/PE) to afford the alcohol 11 (1.3 g, 76%) as a viscous liquid; R f : 0.5 [EA/PE (3:7)]; [\(\alpha ]_{\mathrm {D}}^{\mathrm {25}}\) 54.79 (c 5.75, CHCl3); IR(KBr) ν max 3406, 3375, 2935, 2887, 2858, 1446 cm −1; 1H NMR (300 MHz, CDCl3) δ 1.10 (s, 3H), 1.37 (brs, 2H), 1.59–1.61 (m, 8H), 1.68–1.74 (m, 1H), 1.89 (t, 1H, J = 7.9 Hz), 2.03–2.04 (m, 1H), 2.13–2.17 (m, 1H), 2.22–2.27 (m, 1H), 2.44 (brs, 1H), 2.80 (brs, 1H), 3.45–3.52 (m, 1H), 3.88 (t, 1H, J=6.9 Hz), 4.15–4.20 (m, 1H), 4.25 (brs, 2H), 5.61(s, 1H); 13C NMR (75 MHz, CDCl3) δ 23.2 (CH2), 24.0 (CH2), 24.1 (CH2), 24.9 (CH3), 25.2 (CH2), 33.5 (CH2), 34.5 (CH2), 36.1 (CH2), 44.1 (CH), 51.3 (C), 55.5 (CH), 61.3 (CH2), 66.7 (CH2), 76.1 (CH), 109.4 (C), 137.0 (CH), 141.5 (C); HRMS (ESI) m/z calcd for C17 H 26 O 3Na (M + Na) + 301.1780; found : 301.1781.
2.6 Ethyl 2-((1S,2S,4S,5S)-1-methyl-3-methylene-4-(1,4- dioxaspiro[4.5]decan-2-yl)bicyclo[3.2.0]heptan-2-yl) acetate (12)
A mixture of the allylic alcohol 11 (200 mg, 0.72 mmol), triethyl orthoacetate (4.7 mL, 2.16 mmol), propionic acid (0.2 mL) and xylene (5 mL) was heated in a sealed tube at 140 ∘C for 3 h. Triethyl orthoacetate was distilled out from the reaction mixture and the residual material was purified by column chromatography [Et2O/PE(1:24)] to afford the ester 12 (150 mg, 60%) as a viscous liquid; R f : 0.5 [EA/PE (1:9)]; [\(\alpha ]_{\mathrm {D}}^{\mathrm {25}} -18.67\) (c 2.87, CHCl3); IR (KBr) ν max 2935, 2862, 1735, 1651, 1448 cm −1; 1H NMR (500 MHz, CDCl3) δ 1.08 (s, 3H), 1.24 (t, 3H, J=3.5 Hz), 1.51–1.61 (m, 10H), 1.69–1.74 (m, 1H), 1.84–1.90 (m, 1H), 2.19–2.32 (m, 3H), 2.37–2.43 (m, 3H), 2.83 (t, 1H, J=8.0 Hz), 3.61 (t, 1H, J=7.2 Hz), 3.95 (dd, 1H, J=6.5, 7.7 Hz), 4.03 (dd, 1H, J=6.7, 13.2 Hz), 4.08–4.15 (m, 2H), 4.95 (d, 2H, J=5.0 Hz): 13C NMR (125 MHz, CDCl3) δ 14.4 (CH3), 21.7 (CH3), 23.0 (CH2), 23.9 (CH2), 24.1 (CH2), 25.3 (CH2), 32.2 (CH2), 35.2 (CH2), 35.3 (CH2), 36.1 (CH2), 36.6 (CH2), 46.1 (C), 46.9 (CH), 51.5 (CH), 55.9 (CH), 60.3 (CH2), 68.0 (CH2), 78.1 (CH), 109.4 (C), 109.5 (CH2), 157.1 (C), 173.1 (CO); HRMS (ESI) m/z calcd for C21 H 32 O 4Na (M + Na) + 371.2198; found : 371.2195.
2.7 2-((1S,2S,4S,5S)-1-Methyl-3-methylene-4-(1,4-dioxaspiro[4.5]decan-2-yl)bicyclo[3.2.0]heptan-2-yl) ethanol (13)
To a suspension of LiAlH4 (20 mg, 0.517 mmol) in dry ether (5 mL) at 0 ∘C was added a solution of the ester 12 (90 mg, 0.258 mmol) in dry ether (5 mL). After 5 min at 0 ∘C, the reaction mixture was allowed to stir at rt for 1 h. It was quenched with sequential addition of H2O (0.05 mL), 15% aqueous NaOH (0.05 mL) and H2O (0.15 mL). The resulting suspension was filtered and the precipitated solid material was washed with Et2O (3 ×5 mL). The combined filtrate and the washings were dried, concentrated and the residual material was purified by column chromatography (3:7 Et2O/PE) to afford the hydroxy compound 13 (70 mg, 88%) as colorless oil; R f : 0.5 [EA/PE (2:8)]; [\(\alpha ]_{\mathrm {D}}^{\mathrm {25}} - 4.51\)(c 0.88, CHCl3); IR (KBr) ν max 3410, 3400, 3383, 3365, 2935, 2862 cm −1; 1H NMR (400MHz, CDCl3) δ 1.11 (s, 3H), 1.32–1.44 (m, 2H), 1.53–1.58 (m, 8H), 1.61–1.72 (m, 2H), 1.74–1.83 (m, 3H), 2.15–2.28 (m, 2H), 2.34–2.42 (m, 3H), 3.57–3.73 (m, 3H), 3.94–3.97 (m, 1H), 4.00 (dd, 1H, J=6.8, 13.4 Hz), 4.94 (s, 1H), 5.00 (s, 1H): 13C NMR (100 MHz, CDCl3) δ 21.5 (CH3), 23.3 (CH2), 24.0 (CH2), 24.1 (CH2), 25.3 (CH2), 32.2 (CH2), 32.3(CH2), 35.2 (CH2), 36.7 (CH2), 46.0 (CH), 47.1 (C), 51.8 (CH), 56.4 (CH), 62.2 (CH2), 68.2 (CH2), 78.3 (CH), 109.4 (C), 109.5 (CH2), 157.3 (C); HRMS (ESI) m/z calcd for C19 H 30 O 3Na (M + Na) + 329.2093; found : 329.2097.
2.8 1-((2S,4S)-1-Methyl-3-methylene-4-(1,4-dioxaspiro[4.5]decan-2-yl)bicyclo[3.2.0]heptan-2-yl)but-3-en-2-one (14)
To a magnetically stirred suspension of DMP (145 mg, 0.342 mmol) in CH2Cl2 (5 mL) at 0 ∘C, the alcohol 13 (70 mg, 0.23 mmol) in CH2Cl2 (5 mL) was added dropwise. The reaction mixture was stirred for 30 min and was quenched with 10% Na2 S 2 O 3 solution (0.5 mL) doped with NaHCO3. The organic layer was dried and concentrated to give an unstable aldehyde (65 mg, 94%). Without further purification and characterization this material was used in the next step.
To a magnetically stirred solution of this aldehyde (65 mg, 0.213 mmol) in dry THF (5 mL) at 0 ∘C was added vinyl magnesium bromide (1.0 M solution in THF, 0.42 mL, 0.42 mmol), dropwise. The reaction mixture was stirred at that temperature for 1 h. The temperature of the reaction mixture was slowly raised to rt and it was quenched with saturated NH4Cl solution (0.1 mL) and diluted with ether (10 mL). The organic layer was washed with brine, dried, and evaporated to dryness to afford the corresponding alcohol (60 mg, 85%) which was immediately used in the next reaction without characterization. To a magnetically stirred suspension of DMP (114 mg, 0.27 mmol) in CH2Cl2 (5 mL) at rt, the alcohol (60 mg, 0.18 mmol) in CH2Cl2 (5 mL) was added dropwise. The reaction mixture was stirred for 30 min and was quenched with 10% Na2 S 2 O 3 solution (0.5 mL) doped with NaHCO3. The organic layer was dried, concentrated and purified by column chromatography [Et2O/PE (1:9)] to give the ketone 14 (47 mg, 79%) as colourless oil; R f : 0.5 [EA/PE (1:9)]; [\(\alpha ]_{\mathrm {D}}^{\mathrm {25}} - 7.02\) (c 5.0, CHCl3); IR (KBr) ν max l2931, 2856, 1728, 1695, 1610, 1448 cm −1; 1H NMR (500 MHz, CDCl3) δ 1.04 (s, 3H), 1.55–1.61 (m, 10H), 1.88–1.94 (m, 2H), 2.21–2.29 (m, 2H), 2.44–2.45 (m, 2H), 2.67 (d, 2H, J = 8.5 Hz), 3.03 (t, 1H, J=7.5 Hz), 3.59 (t, 1H, J=7.7 Hz), 4.00 (t, 1H, J=7.0 Hz), 4.09 (t, 1H, J=6.5 Hz), 4.87 (s, 1H), 4.93 (s, 1H), 5.80 (d, 1H, J=10.5 Hz), 6.22 (d, 1H, J=18 Hz), 6.38 (dd, 1H, J=7.0, 17.5 Hz); 13C NMR (75MHz, CDCl3) δ 22.0 (CH3), 23.2 (CH2), 24.1 (CH2), 24.2 (CH2), 25.3 (CH2), 32.3 (CH2), 35.2 (CH2), 36.8 (CH2), 41.0 (CH2), 45.7 (CH), 47.1 (C), 50.3 (CH ), 55.6 (CH), 68.3 (CH2), 78.3 (CH ), 108.8 (CH2), 109.7 (C), 127.9 (CH2), 137.1 (CH), 158.0 (C), 200.3 (CO); HRMS (ESI) m/z calcd for C21 H 30 O 3Na (M + Na) + 353.2093; found :353.2094.
2.9 Ring closing metathesis of the enone 14. Synthesis of the tricyclic enone (15)
To a solution of the ketone 14 (47 mg, 0.142 mmol) in degassed anhydrous toluene, Grubbs II catalyst (6 mg, 5 mol %) was added and the reaction mixture was refluxed at 110 ∘C for 3 h. The solvent was evaporated and the residual mass was chromatographed [Et2O/PE (4:6)] to give the enone 15 (30 mg, 79%) as colourless oil; R f : 0.5 [EA/PE (1:1)] ; [\(\alpha ]_{\mathrm {D}}^{\mathrm {25}}+\)31.09 (c 0.60, CHCl3); IR (KBr) ν max 3010, 2935, 2860, 1707, 1624, 1448 cm −1; 1H NMR (500 MHz, CDCl3) δ 0.90 (s, 3H), 1.36–1.38 (m, 2H), 1.55–1.60 (m, 8H), 1.90–1.97 (m, 3H), 2.22 (dd, 1H, J=3.0, 18.0 Hz), 2.29–2.37 (m, 1H), 2.45 (dd, 1H, J=6.5, 18.0 Hz), 2.47 (d, 1H, J=6.5 Hz), 2.52–2.55 (m, 1H), 3.47–3.49 (m, 1H), 3.58 (t, 1H, J=7.5 Hz), 4.08 (dd, 1H, J=6.0, 7.5 Hz), 4.23 (dd, 1H, J=6.0, 12.5 Hz), 5.81 (s, 1H); 13C NMR (125 MHz, CDCl3) δ 21.8 (CH3), 23.8 (CH2), 24.0 (CH2), 24.7 (CH2), 25.2 (CH2), 31.5 (CH2), 34.6 (CH2), 36.4 (CH2), 37.3 (CH2), 44.7 (C), 48.9 (CH), 51.0 (CH), 54.1 (CH), 67.9 (CH2), 75.4 (CH), 109.7 (C), 123.8 (CH), 190.2 (C), 211.6 (CO); HRMS (ESI) m/z calcd for C19 H 26 O 3H (M + H) + 303.1955; found : 303.1952.
2.10 Hydrogenation of the enone 15. Synthesis of the tricyclic ketone (16)
A solution of the cyclopentenone derivative 15 (30 mg, 0.09 mmol) in methanol (2 mL) was stirred under hydrogen atmosphere for 2 h in the presence of 10% Pd/C (5 mg). The reaction mixture was filtered through an alumina bed. The filtrate was concentrated to afford the saturated ketone 16 (25 mg, 83%) as a white solid m.p. 84–86 ∘C; [\(\alpha ]_{\mathrm {D}}^{\mathrm {25}}\) 13.05 (c 1.00, CHCl3); IR (KBr) ν max 2933, 2860, 1737, 1731, 1697, 1616 cm −1; 1H NMR (500 MHz, CDCl3) δ 1.12 (s, 3H), 1.41–1.63 (m, 10H), 1.77–1.81 (m, 1H), 1.94–2.00 (m, 1H), 2.10–2.13 (m, 2H), 2.15-2.28 (m, 5H), 2.30–2.40 (m, 1H), 2.56 (dd, 1H, J=7.0, 11.5 Hz), 3.13 (t, 1H, J=7.5 Hz), 3.52 (t, 1H, J=7.5 Hz), 3.97 (t, 1H, J=7.5 Hz), 4.04 (dd, 1H, J=6.2, 12.2 Hz); 13C NMR (125 MHz, CDCl3) δ 21.8 (CH2), 23.1 (CH3), 23.9 (CH2), 24.1 (CH2), 25.3 (CH2), 34.2 (CH2), 34.9 (CH2), 36.5 (CH2), 39.8 (CH2), 40.2 (CH2), 46.2 (CH), 46.8 (CH), 47.1 (C), 51.1 (CH), 52.4 (CH), 68.4 (CH2), 75.8 (CH), 110.0 (C), 220.6 (CO); HRMS (ESI) m/z calcd for C19 H 28 O 3Na (M + Na) + 327.1936; found : 327.1937.
2.11 Synthesis of the ester (17)
A solution of the ketal 16 (32 mg, 0.10 mmol) in 80% aqueous acetic acid (0.5 mL) was heated at 50 ∘C for 4 h. The resulting solution was concentrated under reduced pressure to afford the corresponding diol (16 mg, 69%) as a viscous liquid. To a magnetically stirred ice-cold solution of this diol (16 mg, 0.07 mmol) in THF/water (2:1) (0.5 mL) was added NaIO4 (8 mg, 0.035 mmol). The reaction mixture was allowed to stir at 0 ∘C for 30 min. The precipitated white solid was filtered off. The precipitated solid was washed thoroughly with diethyl ether. The combined filtrate and the washing was washed with brine, dried, and evaporated to dryness in vacuum to afford the corresponding aldehyde (13 mg, 71%). To a magnetically stirred solution of this aldehyde (13 mg, 0.06 mmol) in dry acetone (0.5 mL) was added dropwise Jones reagent (0.05 mL) at 0 ∘C and was allowed to stir for 10 min at that temperature. The reaction mixture was extracted with diethyl ether (2 ×3 mL). The ethereal extract was washed with brine, dried, and the solvent was evaporated to afford the acid (10 mg, 71%). A solution of the carboxylic acid (10 mg, 0.04 mmol) in diethyl ether (2 mL) was treated with ethereal diazomethane for 15 min. Removal of ether followed by column chromatography (Et2O/PE 1:9) gave the ester 17 (8 mg, 75%) as colorless oil; R f : 0.5 [EA/PE (1:9)] ; [\(\alpha ]_{\mathrm {D}}^{\mathrm {25}} -25.76\) (c 0.75, CHCl3); IR (KBr) ν max 2947, 1731, 1716, 1704 cm −1; 1H NMR (400 MHz, CDCl3) δ 1.21 (s, 3H), 1.82–1.89 (m, 1H), 1.91–1.93 (m, 1H), 2.14 (dd, 2H, J=9.2, 9.6 Hz), 2.20–2.23 (m, 1H), 2.28–2.31(m, 1H), 2.36 (d, 1H, J=8.4 Hz), 2.40 (d, 1H, J=8.4 Hz), 2.57 (q, 1H, J=8.4 Hz), 2.67– 2.70 (m, 1H), 2.98 (dd, 1H, J=3.6, 8.4 Hz), 3.46–3.51 (m, 1H), 3.61 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 21.0 (CH2), 23.1 (CH3), 33.6 (CH2), 40.0 (CH2), 40.9 (CH2), 46.1 (CH), 48.2 (C), 48.9 (CH), 51.7 (CH), 53.1 (CH ), 54.8 (CH3), 175.2 (CO), 219.4 (CO); HRMS (ESI) m/z calcd for C13H18O3Na (M + Na)+ 245.11554; found: 245.1155
2.12 Synthesis of the tricyclo[6.2.0.0 2,6]decane (18)
The ester 17 (8 mg, 0.04 mmol) was treated with 2% methanolic NaOMe solution (0.1 mL) at 50 ∘C for 8 h. The reaction mixture on acidification with 10% aqueous HCl was extracted with diethyl ether (2 ×3 mL). Removal of the solvent from the dried ether extract afforded the epimerized acid (5 mg) which was treated with ethereal diazomethane. Removal of ether gave the ester 18 (4 mg, 50%) as colourless oil; R f : 0.5[EA/PE (1:9)] ; [\(\alpha ]_{\mathrm {D}}^{\mathrm {25}} -36.10\) (c 0.12, CHCl3); IR (KBr) ν max; 2921, 2854, 1731, 1701, 1683, 1650 cm −1, 1H NMR (400 MHz, CDCl3) δ 1.21 (s, 3H), 1.84–1.91 (m, 4H), 1.93–2.07 (m, 1H), 2.20–2.27 (m, 3H), 2.30–2.37 (m, 1H), 2.51 (dd, J=7.2, 8.4 Hz, 1H), 2.67–2.71 (m, 2H), 3.66 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 16.07 (CH2), 23.3 (CH3), 31.6 (CH2), 40.1 (CH2), 43.0 (CH), 43.7 (CH2), 46.1 (C), 46.7 (CH), 47.2 (CH), 51.8 (CH), 52.8(CH3), 173.5 (CO), 218.9 (CO); HRMS (ESI) m/z calcd for C13 H 18 O 3Na (M + Na) + 245.1154; found: 245.1152.
3 Results and Discussion
Initially we focused on the construction of a bicyclo[3.2.0]heptane analogous to 3. Reaction of the lithium enolate generated from the known unsaturated ester 5 (prepared from D-mannitol following the known procedure[8]) with methacrolein provided the hydroxy-ester 6 along with the other three possible diastereoisomers in 1:3:8:1.6 (from the intensities of the CO peaks in 13C NMR) in 56% yield (scheme 2). A solution of this mixture in diethyl ether was irradiated in presence of cuprous triflate as catalyst with a medium pressure mercury vapour lamp. Chromatographic purification of the product mixture afforded the adduct 7 in 51% yield. For assignment of stereochemistry and for carrying forward the synthesis towards the target, the hydroxy-ester 7 was transformed to the tosylate 8 in 71% yield. The relative stereochemistry of the three contiguous stereocenters at C-2, C-3 and C-4 could be established by comparison of the observed coupling constants of these protons with those reported[9] for cis- and trans-disubstituted cyclopentane derivatives. 1H NMR spectrum of the compound 7 revealed that the C-3 proton appeared at δ 3.01 as a doublet of doublet with J=6.0 and 11.5 Hz while the C-2 proton appeared as a doublet at δ 4.83 with the coupling constant 6.0 Hz. The vicinal protons trans to each other are known to display a higher coupling constant than that with the cis- protons. Thus the C-3 proton is cis to the adjacent C-2 proton but trans to the C-4 proton.
That the ketal unit occupies an exo positition in the bicyclo[3.2.0]heptane derivative 7 was established in the following way. It is well established[10] that photocycloaddition of a 1, 6-diene having an alkyl substituent at the allylic carbon proceeds through a copper (I)-diene complex in which the alkyl group occupies an exo position. Thus it is expected that photocycloaddition of the diene would take place through the copper (I)-complex 9 to produce adduct with alkyl group occupying an exo-position. Thus the structure of the photoadduct was tentatively assigned as 7. This structural assignment was confirmed through single crystal X-ray structure of the tricyclic compound prepared from 7 as shown in scheme 3.
The tosylate 8 was treated with DBU in toluene under reflux to give the unsaturated ester 10 in 77% yield. LiAlH4 reduction of 10 provided the allylic alcohol 11 in 76% yield. Ortho-ester Claisen rearrangement of the allylic alcohol 10 afforded the unsaturated ester 12 as the major product in 60% yield. The ester was then reduced to the alcohol 13. Oxidation of the hydroxy-compound 13 with DMP afforded the corresponding aldehyde which without purification and characterization was transformed to the enone 14 in 63% yield following a sequence involving addition of vinyl magnesium bromide and DMP oxidation of the resulting carbinol. Ring closing metathesis[11] of the dienone 14 was accomplished with Grubbs’ 2 nd generation catalyst (G II) to afford the enone 15 in 79% yield. Hydrogenation of the enone 15 over 10% Pd/C gave the tricyclic ketone 16 as a white crystalline solid, M.p. 84–86 ∘C in 83% yield. Hydrogen was added from the face opposite to the bulky ketal unit to give rise to the cis-anti-cis configuration. The structure of this compound was established by single crystal X-ray analysis (figure 1). With the establishment of the structure of the tricyclic compound 16, the structure of the photoadduct 7 was also confirmed.
The tricycle 16 was then transformed to the ester 17 using a standard sequence involving deketalization with 80% aqueous acetic acid, cleavage of the liberated diol with sodium meta-periodate, oxidation of the resulting aldehyde to the corresponding carboxylic acid followed by its treatment with diazomethane. Inspection of the structure of the tricycylic keto-ester 17 revealed that all the stereocentres in it except the C-7 centre had the correct stereochemistry required for the synthesis of kelsoene. An inversion of the C-7 substituent was now required. The methyl ester 17 epimerized smoothly when treated with 2% NaOMe in MeOH at 50∘C to produce the more stable[4]e tricyclic structure 18 in 50% yield. Disappearance of the dd (J = 3.6, 8.4 Hz) at δ 2.57 (q, J = 8.4Hz) of the unepimerized compound 17 indicated its epimerization to 18. The compound 18 possesses the fully functionalized tricyclic structure with cis-anti-cis 5-5-4 ring system having the C-7 substituent with the desired stereochemistry for elaboration to 1 and 2.
4 Conclusion
We have developed a route for the construction of the tricyclo[6.2.0.0 2,6]decane ring system 2,6 present in kelsoene and poduran in enantiomerically pure form. The key steps involve a copper (I)-catalyzed intramolecular [ 2+2] photocycloaddition of a diene prepared from D-mannitol to form a bicyclo[3.2.0]heptane derivative. Annulation of a five-membered ring on to it was achieved through ring closing olefin metathesis. The tricyclic system thus formed has the desired stereochemistry at the five stereocenters and is functionalized for elaboration to the natural products.
References
König G M and Wright A D 1997 J. Org. Chem. 62 3837
(a) Nabeta K, Yamamoto K, Hashimoto M, Koshino H, Funatsuki K and Katoh K 1998 Chem. Commun. 1485; (b) Warmers U, Wihstutz K, Bülow N, Fricke C and König W A 1998 Phytochemistry 49 1723; (c) Warmers U and König W A 1999 Phytochemistry 52 1519
Schulz S, Messer C and Dettner K 1997 Tetrahedron Lett. 38 2077
(a) Mehta G and Srinivas K 1999 Synlett 555; (b) Mehta G and Srinivas K 1999 Tetrahedron Lett. 40 4877; (c) Mehta G and Srinivas K 2001 Tetrahedron Lett. 42 2855; (d) Mehta G and Sreenivas K 2002 Tetraherdon Lett. 43 3319; (e) Razavian S F, Schulz S, Dix I and Jones P G 2001 Chem. Commun. 2154; (f) Piers E and Orellana A 2001 Synthesis 2138; (g) Bach T and Spiegel A 2002 Synlett 1305; (h) Zhang L and Koreeda M 2002 Org. Lett. 4 3755
(a) Salomon R G 1983 Tetrahedon 39 485; (b) Ghosh S 2004 In CRC Handbook of Organic Photochemistry and Photobiology W M Horspool and F Lenci F (eds.) (Bocaraton: CRC Press) Ch. 18 p1
(a) Ghosh S, Banerjee S P, Chowdhury K, Mukherjee M and Howard J A K 2001 Tetrahedron Lett. 42 5997; (b) Banerjee S P and Ghosh S 2003 J. Org. Chem. 68 3981
Bach T and Spiegel A 2002 Eur. J. Org. Chem. 645
Banerjee S, Ghosh S, Sinha S and Ghosh S 2005 J. Org. Chem. 70 4199
Liera J M and Fraser-Reid B 1989 J. Org. Chem. 54 5544
Salomon R G, Coughlin D J, Ghosh S and Zagorski M G 1982 J. Am. Chem. Soc. 104 998
(a) Grubbs R H, Miller S J and Fu G C 1995 Acc. Chem. Res. 28 446; (b) Frstner A 1997 Top. Catal. 4 285; (c) Schuster M and Blechert S 1997 Angew. Chem. Int. Ed. 36 2036; (d) Grubbs R H and Chang S 1998 Tetrahedron 54 4413; (e) Armstrong S K 1998 J. Chem. Soc. Perkin Trans. 1 371; (f) Fürstner A 2000 Angew. Chem. Int. Ed. 39 3012; (g) Kotha S and Sreenivasachary N 2001 Indian J. Chem. 40B 763; (h) Deiters A and Martin S F 2004 Chem. Rev. 104 2199; (i) Nicolaou K C, Bulger P G and Sarlah D 2005 Angew. Chem. Int. Ed. 44 4490; (j) Ghosh S, Ghosh S and Sarkar N 2006 J. Chem. Sci. 118 223; (k) Chattopadhyay S K, Karmakar S, Biswas T, Majumdar K C, Rahaman H and Roy B 2007 Tetrahedron 63 3919; (l) Vougioukalakis G C and Grubbs R Chem. Rev. 110 1746; (m) Kotha S and Dipak M K 2012 Terahedron 68 397
Acknowledgements
Financial support from Department of Science and Technology (DST), Government of India is gratefully acknowledged. SG thanks DST for award of JC Bose Fellowship. Financial support from DST for the National Single crystal X-ray Diffractometer facility at the department of Inorganic chemistry of this Institute is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Crystal data for compound 16: C19H28O3, M = 304.41, orthorhombic, a = 6.374(3) Å, b = 10.591(6) Å, c = 25.372(14) Å, α=90.00∘, β=90.00∘, γ=90.00∘, V = 1712.9(16) Å 3, T = 150(2) K, space group P2(1)2(1)2(1), Z = 4, 13228 reflections measured, 3556 independent reflections (Rint = 0.0439). The final R1 values were 0.0390 (I>2σ (I)). The final wR (F2) values were 0.1031 (I >2σ (I)). The final R1 values were 0.0463 (all data). The final wR (F2) values were 0.1160 (all data). The goodness of fit on F2 was 0.772. CCDC (CCDC no. – 904379) contain the supplementary crystallographic data for this paper. X-ray single crystal data were collected using MoKa (k = 0.7107 Å) radiation on a SMART APEX II diffractometer equipped with CCD area detector. Data collection, data reduction, structure solution/refinement were carried out using the software package of SMART APEX. The structures were solved by direct method and refined in a routine manner. Non-hydrogen atoms were treated anisotropically. The hydrogen atoms were geometrically fixed. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB21EZ, UK; fax: (+44) 1223 336 033; or deposit@ccdc.cam.ac.uk. The electronic supplementary material containing 1H NMR, 13C NMR and HRMS spectra of all the new compounds are available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
GHOSH, A., GHOSH, S. Asymmetric synthesis of a functionalized tricyclo[6.2.0.02,6]decane ring system present in kelsoene and poduran. J Chem Sci 126, 1875–1882 (2014). https://doi.org/10.1007/s12039-014-0739-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0739-0