Abstract
Protease-activated receptor (PAR)2 has been implicated in mediating allergic airway inflammation. We investigate the role of PAR2 in lung inflammation and neutrophil and eosinophil recruitment into the lungs in a mouse model of short-term acute allergic inflammation. Allergic lung inflammation was induced in sensitized BALB/c mice through intranasal instillations of ovalbumin (OVA), and mice were pretreated with the PAR2 antagonist ENMD1068 or with the PAR2-activating peptide (PAR2-AP) 1 hour before each OVA challenge. Bronchoalveolar lavage fluid (BALF) was collected, and the lungs, trachea and lymph nodes were removed after the last challenge to analyze the airway inflammation. PAR2 blockade reduced OVA-induced eosinophil and neutrophil counts, CXCL1, CCL5, amphiregulin, and interleukin (IL)-6 and 13 levels. Moreover, PAR2 blockade reduced OVA-induced PAR2 expression in cells present in BALF 2 hour after OVA challenge, and PAR2-AP acted synergistically with OVA promoting eosinophil recruitment into BALF and increased IL-4 and IL-13 levels in lymph nodes. Conversely, PAR2 blockade increased IL-10 levels when compared with OVA-treated mice. Our results provide evidence for a mechanism by which PAR2 meditates acute lung inflammation triggered by multiple exposures to allergen through a modulatory role on cytokine production and vascular permeability implicated in the lung diseases such as asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Eosinophils appear to play an important role in the pathogenesis of allergic diseases. Typically, eosinophils are tissue dwelling cells, and in allergic disorders, an increased number of activated eosinophils is found in the submucosa and mucosa of affected tissues (Vu et al. 1991). These cells are recruited from blood vessels into the allergy inflamed tissue via the local production of eotaxin-1/C-C motif chemokine 11 (CCL11), leukotriene (LT)B4, and interleukin (IL)-5 (Faccioli et al. 1996, 1991; Pope et al. 2005), produced by airway epithelial cells, macrophages, eosinophils, and mast cells (Cook et al. 1998; Martin et al. 1984). Neutrophils are prominent in acute inflammation, recruited from blood vessels into tissues in response to chemoattractants released at local sites of injury which directly act on their surface (Kobayashi 2008). In the inflammatory environment, neutrophil and eosinophil accumulation play a role in the progression of tissue damage through the release of proteases contained in their azurophil granules, lysosomal enzymes, reactive oxygen species, and cationic proteins, promoting lesions in surrounding tissues and contributing to the severity of inflammatory diseases, as well as playing an important role in the pathogenesis and severity of allergic and inflammatory diseases (Hosoki et al. 2016; Korkmaz et al. 2010; Rothenberg and Hogan 2006; Weiss 1989). Thus, elucidating mechanisms underlying eosinophil and neutrophil recruitment in vivo may aid in the development of novel strategies for the treatment of inflammatory and allergic diseases.
Protease-activated receptors (PARs) are a unique family of four G protein-coupled receptors (PAR 1-4), first described by Coughlin et al. in the early 1990s as the proteolytic receptor for thrombin (Vu et al. 1991), and activated through the proteolytic cleavage of a specific site in the N-terminus of the receptor, revealing a tethered ligand that binds to and activates the receptor (Hollenberg and Compton 2002). PARs can also may be activated independently of proteolytic unmasking of the tethered ligand sequence, through small synthetic peptides corresponding to the first five or six amino acids of the tethered ligand sequence (activator ligand), such as for PAR2 the human sequence SLIGKV-NH2, and the more potent murine variant, SLIGRL-NH2 (Hollenberg and Compton 2002). As opposite, blockade of PAR2 at activator ligand site, can be achieved through the use of selective antagonists, such as ENMD1068, a piperazine derivative, resulting in the inhibition of PAR2-induced calcium signaling on human and murine cells (Kelso et al. 2006).The role of proteinases and PAR activation in the development of inflammation has been widely investigated, and their activation has been implicated in the regulation of inflammatory events, including vascular endothelial cell activity (Hollenberg and Compton 2002; Vergnolle et al. 2002), in vivo leukocyte recruitment (Gomides et al. 2012) and cytokine release (Asokananthan et al. 2002).
PAR2 is widely expressed in airway cells such as endothelial and vascular smooth muscle cells, bronchial smooth muscle cells, airway epithelial cells and others (Adams et al. 2011; Bolton et al. 2003; Cocks and Moffatt 2001; Compton et al. 2000; Schmidlin et al. 2001), playing a major role in the pathophysiology of allergic airway inflammation (Ebeling et al. 2007). In this study, we investigated the effects of PAR2 blockade on lung inflammation and neutrophil and eosinophil recruitment into the lungs of mice in a mouse model of short-term acute allergic lung inflammation.
2 Materials and methods
2.1 Animals
Female BALB/c mice (8–10 weeks old, 18–25 g) were obtained from the Animal Care Facilities of the UFMG and housed in a temperature-controlled room with free access to commercial chow and water. All experimental procedures were subject to evaluation and were approved by the local Animal Ethics Committee (CETEA, certificate number 348 /2014).
2.2 Drugs and reagents
Ovalbumin (OVA, grade V) and aluminum hydroxide gel were obtained from Sigma-Aldrich (St. Louis, MO). The anti-PAR2 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The PAR2 antagonist ENMD1068 (N1-3-methylbutyryl-N4-6-aminohexanoyl-piperazine), was purchased from Enzo Life Sciences (San Diego, CA, USA). The PAR2-activating peptide SLIGRL–NH2 (H–Ser–Leu–Ile–Gly–Arg–Leu–NH2), and PAR2 peptide control, LRGILS-NH2, were purchased from Tocris Bioscience (Bristol, UK). ENMD1068, SLIGRL–NH2, and LRGILS-NH2 were dissolved in phosphate-buffered saline (PBS, pH 7.4) and all samples were stored at −20°C until use. Ketamine (Vetnil™) was purchased from Louveira (Brazil), and xylazine (Kensol™) from König (Santana de Parnaíba, Brazil). Enzyme-linked immunosorbent assay (ELISA) kits for detection of the murine chemokines (C-X-C motif) ligand 1 (CXCL1) and (C-C motif) ligand 5 (CCL5), as well as the amphiregulin protein (AREG), were obtained from R&D Systems (Rock Hill, NJ, USA). ELISA kits for interleukin (IL)-4, IL-10, and IL-13 were obtained from and eBioscience (Thermo Fisher Scientific, USA), and the IL-6 ELISA kit was obtained from BD Biosciences (NJ, USA).
2.3 Sensitization
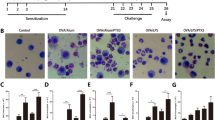
Mice were sensitized with an intraperitoneal (i.p.) injection (200 µL) of OVA (100 μg) in 2% aluminum hydroxide gel as previously described (Kurowska-Stolarska et al. 2008). Control mice were injected (200 μL, i.p.) 2% aluminum hydroxide gel alone (non-sensitized/naïve mice). The protocol used in this study is shown in figure 1.
Immunization scheme and experimental protocols (1–6). Sensitization was performed by one intraperitoneal (i.p.) injection of OVA (100 μg) in 2% aluminum hydroxide gel Al(OH)3. (A) On days 9, 10, and 11 post-sensitization, mice were challenged with intranasal instillation of PBS (20 μL/control) or OVA (10 µg/20 μL); 4–72 h after the last OVA challenge bronchoalveolar lavage fluid (1) was collected. (B) On days 9, 10, and 11 post-sensitization, mice were pretreated or not with the PAR2 antagonist ENMD1068 (0.1–1.0 mg/kg, i.p.) or PBS (i.p.) 11h before the intranasal instillation (i.n.) of PBS (20 μL), OVA (10 µg/20μL), 0.5–48 h after the last OVA challenge (according to each research protocol) leukocyte recruitment (2), inflammatory mediators (3), PAR2 expression (4) or airway vascular permeability (5) were evaluated. (C) On days 9, 10, and 11 post-sensitization, mice were pretreated or not with the PAR2-activating peptide SLIGRL–NH2 (PAR2-AP, 30 µg/20 μL, i.n.), or PAR2 peptide control LRGILS-NH2 (30 µg/20 μL, i.n.), or SLIGRL–NH2 + OVA, leukocyte recruitment and cytokine production were evaluated 48 h after the last treatment (6).
2.4 Leukocyte migration induced by OVA or PAR2-activating peptide to the lungs of sensitized mice
On days 9, 10, and 11 after sensitization, mice were anesthetized with i.p. ketamine (130 mg/kg) and xylazine (8.5 mg/kg) in a saline solution and challenged with intranasal instillation of PBS (20 μL), OVA (10 µg/20 μL), PAR2-activating peptide SLIGRL–NH2 (PAR2-AP, 30 µg/20 μL), or PAR2 peptide control LRGILS-NH2 (30 µg/20 μL). On day 11, at 4, 24, 48, or 72 h after the last challenge with PBS or OVA, mice were anesthetized by i.p. administration of a solution of ketamine and xylazine, and a catheter was inserted into the trachea of each animal to collect the bronchoalveolar lavage fluid (BALF), and the lung was washed with 2 mL PBS (2 × 1000 µL). A Neubauer chamber was used to determine thetotal leukocyte counts using Turk's reagent, and differential cell counts were performed on cytospin preparations stained with May-Grünwald solution, using standard morphological criteria to identify cell types, with 100 cells were counted per slide as previously described (Klein et al. 2001). In some experiments, OVA-sensitized mice were challenged with the intranasal instillation of PAR2-AP (30 µg/30 μL) 1 h before intranasal OVA instillation and leukocytes present in the BALF harvested 48 h after the last challenge.
2.5 Effects of PAR2 antagonist pretreatment on neutrophil and eosinophil recruitment induced by OVA
To investigate the contribution of PAR2 in OVA-induced leukocyte recruitment, the PAR2 antagonist, ENMD1068 (0.1–1.0 mg/kg) or PBS (100 μL), were administered i.p. 1 h before the intranasal instillation of OVA (10 μg/20 μL) or PBS on days 9–11 in sensitized and naïve mice, respectively, and leukocytes were assessed 48 h after the last challenge. In BALF, neutrophils and eosinophils were harvested at 0.5, 2, 4, 8, and 12 h after the last challenge.
2.6 Enzyme-linked immunosorbent assay (ELISA) for inflammatory mediators
Frozen supernatants obtained from lymph node and lung homogenates were used to detect IL-4 and IL-13 inflammatory mediators 48 h after the last OVA challenge. Briefly, axillary lymph nodes were collected in the RPMI culture medium, and the supernatant was used for cytokine quantification by ELISA following the manufacturer recommendations for each kit (Alves-Filho et al. 2006). In other experiments, BALF was used to determine the concentrations of CXCL1, CCL5, AREG, IL-6, and IL-10, at 0.5, 2, 4, 8, and 12 h after the last OVA challenge. To collect samples, the mice were anesthetized, BALF was collected and centrifuged at 400 × g for 10 min, and the supernatant was recovered and used for cytokine quantification by ELISA.
2.7 Western blot analysis of leukocyte PAR2 expression in OVA-injected mice
PAR2 expression was evaluated in leukocytes recovered from collected BALF obtained at 2 or 4 h after intranasal instillation of OVA. The fluid recovered from the animals was pooled and centrifuged at 1300×g. Next, 0.3 mL of the lysis buffer containing 1% Triton X-100 and 0.5% sodium dodecyl sulfate, plus a cocktail of protease inhibitors (SigmaFAST), was added to the cell pellets. The lysate was centrifuged at 8000 g at room temperature, and the protein concentration was spectrophotometrically determined using the Bradford method. 30 µg of protein was transferred to a nitrocellulose membrane. The blots were blocked with 3% bovine serum albumin in TBS and 0.1% Tween-20 at 4°C overnight. The following primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were used: goat anti-PAR2 polyclonal immunoglobulin (Ig)G (1:1000) and mouse monoclonal anti-β-actin (1:3000), and goat anti-mouse IgG-horseradish peroxidase (HRP) (1:8000) and rabbit anti-goat IgG-HRP (1:8000)were used as secondary antibodies. Immunocomplexes were detected using a chemiluminescent reaction (Luminata Western HRP Substrates, Millipore™), followed by densitometric analyses using the Image Quant software package. β-actin was used as a housekeeping control.
2.8 Effects of PAR2 antagonist ENMD1068 on airway vascular permeability in OVA-injected mice
To determine the impact of PAR2 blockade on OVA-induced vascular permeability, we measured the vascular extravasation of Evans blue dye into the lung and trachea parenchyma as a direct estimate of vascular permeability, as previously described (Lundberg et al. 1983), and the results are presented as the amount of Evans blue (pg) present in 100 mg of tissue.
2.9 Statistical analysis
Results are presented as the mean ± standard deviation (SD). Statistical analyses were performed using SigmaStat® 3.5 software (San Diego, CA, USA) and GraphPad Prism version 5.0 (Windows 7, GraphPad Software, San Diego, CA, USA). Comparisons between groups were performed using One-way ANOVA, and differences between groups were assessed using Newman–Keuls post-test. P <0.05 was considered significant.
3 Results
3.1 PAR2 mediates allergen-induced eosinophil recruitment and cytokine production
Intranasal instillation of OVA induced time-dependent eosinophil recruitment to the lungs of OVA-sensitized mice, peaking 48 h after the intranasal OVA challenge (figures 1A [1] and 2A). In contrast, eosinophil recruitment was not detected in mice injected with the adjuvant aluminum hydroxide alone, followed by the intranasal challenge of PBS or OVA (figure 2A). OVA instillation in the sensitized mice induced the recruitment of neutrophils and eosinophils at 4 h after intranasal challenge in BAL fluid (figure 1A [1]; table 1), and a significant increase in total cells was observed at 48 and 72 h after the last challenge when compared to PBS-instilled mice (sensitized and naive mice). Eosinophil recruitment after OVA challenge was indistinguishable at all the evaluated times, which peaked after 48 h, and pretreatment with the PAR2 antagonist ENMD1068 significantly inhibited eosinophil recruitment and total cells recovered in BALF at 48 h after intranasal instillation of OVA (figures 1B [2] and 2B; table 2).
Effects of PAR2 antagonist ENMD1068 on eosinophil recruitment by OVA. OVA-sensitized and non-sensitized/naïve mice were challenged by intranasal (i.n.) instillations of PBS or OVA (10 µg), and cells present in BAL fluid were harvested at 4, 24, 48 or 72 h after last challenge. Data are expressed as mean ± standard deviation (SD) of 4–6 mice/group (A). Ovalbumin-sensitized mice were instilled with PBS, OVA, or pretreated with ENMD1068 (0.1–1.0mg/kg) 1 h before i.n. instillation of OVA. Infiltrating eosinophils were measured in BALF 48h after the last intranasal allergen challenge in mice. Data are expressed as mean ± SD of 5–6 mice/group (B). One-way ANOVA followed by Newman–Keuls post hoc test was used for comparison of eosinophil recruitment among the groups. In all analyses, statistical differences were considered significant at p<0.05. (A) *p<0.001 OVA-treated mice and immunized with Al(OH)3 instilled with OVA (▼) versus others groups. (B) *p<0.001 versus PBS-treated mice, #p<0.001 versus OVA-treated mice.
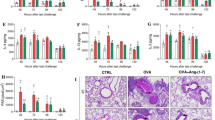
Once ENMD1068 inhibited eosinophil recruitment induced by OVA in the lungs of sensitized mice, we examined whether PAR2-AP promoted the eosinophil recruitment (figure 1C). As shown in Figs. 3A–D, SLIGRL-NH2 promoted leukocyte and neutrophil recruitment, acted synergistically with OVA to promote eosinophil recruitment into the lungs of sensitized mice at 48 h after allergen challenge, as well as increased IL-4 and IL-13 levels as measured in lymph nodes, when compared with the control group (figure 3E and F). Higher IL-13 level was observed in lung homogenates at 48 h after i.n. OVA challenge, whereas PAR2 antagonist treatment strongly reduced these levels (figure 3H). For IL-4, no significant differences were observed between the experimental groups (figure 3G).
Effects of PAR2 antagonist ENMD1068 or PAR2-activating peptide (PAR2-AP) on the leukocyte recruitment and cytokine production induced by OVA. On days 9, 10, and 11 post-sensitization, OVA-sensitized mice were pretreated with PBS (i.p.) or PAR2 antagonist ENMD1068 (0.5 mg/kg, i.p.) 1 h before i.n. instillation with PBS (20 μL), or OVA (10 µg/20 μL), or PAR2-activating peptide SLIGRL–NH2 (PAR2-AP, 30 µg/20 μL), or PAR2 peptide control LRGILS-NH2 (30 µg/20 μL), or SLIGRL–NH2 + OVA. 48 h after the last i.n. instillation BAL fluid was collected and leukocytes were harvested: (A) total cells, (B) mononuclear cells, (C) neutrophils and (D) eosinophils. Lymph nodes (E and F) and lungs (G and H) also were removed and used for determination of IL-4 and IL-13 using a commercially available ELISA kit, the cytokine levels correspond to an average obtained from triplicates for each animal representative of 2 independent experiments. (A–H) Data are expressed as the mean ± SD of 3–5 mice/group. One-way ANOVA followed by Newman–Keuls post hoc test was used for comparison among the groups. In all analyses, statistical differences were considered significant at p<0.05. ns indicates not significant (p > 0.05) whereas *p<0.001 versus the PBS-treated mice, #p<0.001 versus the OVA-treated mice, and &p<0.001 versus the SLIGRL-NH2 treated mice.
3.2 PAR2 mediates allergen-induced neutrophil recruitment
Given that neutrophils contribute to the pathophysiology of inflammatory diseases and have demonstrated a role for PAR2 in OVA-induced lung inflammation in sensitized mice, we investigated whether the blockade of PAR2 would interfere with the recruitment of BAL neutrophils in OVA-instilled mice (figure 1B [3]). OVA induced time-dependent neutrophil and eosinophil recruitment into the lung of sensitized mice after the intranasal OVA challenge, and concerning neutrophils were dropped to baseline levels after 12 h. Pretreatment of OVA-sensitized mice with ENMD1068, before OVA instillation, potently inhibited neutrophil and eosinophil recruitment at 2, 4, 8 and 12 h following the intranasal OVA challenge (figure 4A and B).
Time-course effects on the recruitment of neutrophils and eosinophils, and cytokine/chemokine production in lungs of OVA-instilled mice treated with PAR2 antagonist ENMD1068. Mice were immunized with OVA and pretreated with ENMD1068 (0.5 mg/kg) 1 h before the intranasal instillation of OVA (10 μg/20 μL) or PBS9, 10, 11 days after, and BAL fluid was collected and neutrophil (A) and eosinophil (B) recruitment assessed at 0.5, 2, 4, 8 and 12 h after last challenge. Data are expressed as the mean ± SD of 4–5 mice/group. The cells were removed by centrifugation, and the supernatants were used for determination of cytokines and chemokines using a commercially available ELISA kit. The cytokine levels correspond to an average obtained from triplicates for each animal representative of 2 independent experiments expressed as the mean ± SD of 3–4 mice/group (C–G). One-way ANOVA followed by Newman–Keuls post hoc test was used to assess comparisons among the groups. In all analyses, statistical differences were considered significant at p<0.05. *p<0.05 versus the PBS + PBS group #p<0.05 versus the PBS + OVA-treated mice (A–G).
3.3 PAR2 modulates pro- and anti-inflammatory cytokine production in BALF
Considering that the PAR2 antagonist ENMD1068 inhibited neutrophil and eosinophil recruitment in BALF of sensitized mice following the OVA challenge, and chemokines and pro-inflammatory cytokines CXCL1, CCL5, AREG and IL-6 play a major role in mediating the recruitment of neutrophils and eosinophils following an allergen challenge, we examined whether PAR2 plays a role in pulmonary allergic inflammation by modulating the release of cytokines and chemokines (figure 1B [3]). As shown in figure 4, in OVA-sensitized animals, the OVA challenge induced a significant increase in the levels of CXCL1, CCL5, AREG, and IL-6 in BALF, with maximal levels detected at 2 and 4 h, when compared with the PBS control, and ENMD1068 significantly inhibited this effect, specifically 2 h after the last OVA challenge (figure 4C–F). Interestingly, CXCL1 production, as well as their inhibition by the PAR2 antagonist ENMD1068, persisted for 12 h, while CCL5 and AREG returned to baseline levels by 8 h. Consistent with the ability of ENMD1068 to inhibit chemokines and pro-inflammatory cytokine release, PAR2 blockade by ENMD1068 induced an increase in the levels of the anti-inflammatory cytokine IL-10, detected in BALF of sensitized mice 4 h after intranasal OVA (figure 4G).
3.4 PAR2 protein expression increases after antigen challenge
PAR2 was highly and significantly expressed in mice 2 and 4 h after the intranasal OVA challenge when compared with PBS-instilled mice (figures 1B [4] and 5). Furthermore, the PAR2 antagonist ENMD1068 abrogated PAR2 expression at 2 h, but not 4 h after OVA challenge (figure 5).
Western blot analysis of PAR2 protein expression in BALF of OVA-instilled mice treated with the PAR2 antagonist ENMD1068. Mice were immunized with OVA and pretreated with ENMD1068 (0.5 mg/kg) 1 h before i.n. instillation of OVA (10 μg/20 μL) or PBS 9, 10, 11 days after, and BALF was collected at 2 or 4 h after last challenge. The cells were centrifuged and pooled, and protein expression was determined by Western Blot. (A) Quantitative analysis and (B) representative gel of PAR2 and β-actin proteins. This gel is representative of three experiments with similar outcomes. The results were normalized by the β-actin contained in each sample and expressed as the mean ± SD of 5 mice/group. One-way ANOVA followed by Newman–Keuls post hoc test was used to assess comparison among the groups. In all analyses, statistical differences were considered significant at p<0.05. *p<0.05 when compared to PBS group, #p<0.05 when compared to OVA-treated mice.
3.5 PAR2 blockade impairs OVA-induced airway inflammation
To evaluate the impact of the pharmacological blockade of PAR2 on OVA-induced airway inflammation, we measured the vascular leakage of Evans blue dye into the lung and tracheal tissues 2 h after the last OVA challenge (figure 1B [5]). Pretreatment of sensitized mice with ENDM1068 (0.5 mg/kg) 1 h before OVA instillation, inhibited the OVA-induced increase in vascular permeability as demonstrated by Evans blue extravasation into the lung parenchyma (figure 6A), and confirmed with pulmonary macroscopic analysis (figure 6B-D). Conversely, PAR2 blockade failed to reduce the OVA-induced increased permeability in the trachea, as shown in figure 6E and confirmed at the macroscopic level (figure 6F–H).
Effects of PAR2 antagonist ENMD1068 on vascular permeability in airways of OVA-instilled mice. Mice were immunized with OVA and pretreated with ENMD1068 (0.5 mg/kg) 1 h before the i.n. instillation of OVA (10μg) or PBS 9, 10, 11 days after. Mice were intravenously injected with 0.2 mL of 2% Evans blue dye in saline, 1h before the last OVA instillation and 2 h after, lungs and trachea were removed, weighed, dried at 37°C for 48 h and Evans blue extracted by formamide. The amount of Evans blue was colorimetrically measured using spectrometry at 620 nm. (A and E) The results are presented as the amount (pg) of Evans blue present in 100 mg of tissue and are expressed as the mean ± SD of 3–5 mice/group. One-way ANOVA followed by Newman–Keuls post hoc test was used to assess comparison among groups. In all analyses, statistical differences were considered significant at p<0.05. *p<0.05 when compared to PBS group, and #p<0.05 when compared to OVA-treated mice. (B, C, D; F, G, H) Photomicrographs of lungs and trachea were captured before weighed and dried.
4 Discussion
It is well established that lung diseases are characterized by initial alterations induced by allergens, typically involving eosinophil and neutrophil accumulation that leads to an inflammatory environment. These cells play a pivotal role in the progression of airway inflammatory diseases, through the release of granule proteases, among other effects that amplify the inflammatory response and the underlying tissue damage (Rothenberg and Hogan 2006; Weiss 1989). Thus, it is hypothesized that drugs that block neutrophil or eosinophil recruitment to the inflamed tissues and/or their activation may be important as new therapeutic strategies for the treatment of inflammatory diseases. Several experimental studies support the role of PAR2 in the development of allergen-induced airway inflammation, specifically on the mechanisms of leukocyte recruitment or activation in experimental airway inflammation and in human asthma (Asaduzzaman et al. 2015; Cocks and Moffatt 2001; Davidson et al. 2013; de Boer et al. 2014; Schmidlin et al. 2001). However, to date, the role of PAR2 in mediating OVA-induced leukocyte recruitment in airway acute inflammation and the underlying mechanisms are not well understood. In this study, we investigated the relevance of PAR2 on neutrophil and eosinophil recruitment into the lungs of mice in a mouse model of short-term acute allergic inflammation.
We demonstrated that pharmacological blockade of PAR2 abolished neutrophil and eosinophil migration evoked by i.n. instillation of OVA in sensitized mice and impaired the development of allergen-induced acute lung inflammation. These effects occurred at least partially through a modulatory role on cytokine production in cells present in the BALF and vascular permeability in the lung. PAR2 has been extensively studied with a focus on understanding its role in mechanisms of recruitment and activation of leukocytes in allergic inflammation (Asaduzzaman et al. 2015; Davidson et al. 2013; de Boer et al. 2014; Matos et al. 2014), and previous studies have demonstrated that PAR2 and endogenous serine proteases play a role in neutrophil and eosinophil recruitment in allergic inflammation (de Boer et al. 2014; Matos et al. 2014, 2013; Saw et al. 2012). However, to the best of our knowledge, this is the first study demonstrating that PAR2 plays a key role in the initial hours of airway inflammation following acute allergen exposure, with a synergic effect in eosinophil migration in BALF as well IL-4 and IL-13 production by lymph nodes, played between PAR2 activation and the inflammatory mechanisms triggered by allergen challenge.
Several allergens have previously been used to investigate the effects of PAR2 in an allergic environment (Arizmendi et al. 2011; de Boer et al. 2014). Our study focused on the importance of PAR2 activation in the production of inflammatory mediators and the recruitment of neutrophils and eosinophils in an experimental model of OVA-induced acute asthma following multiple exposures to allergens. It is well documented that neutrophil accumulation in the airways is correlated with severe forms of chronic asthma, exhibiting exacerbation of the acute phase of the disease (Dejager et al. 2015; Fahy 2009) and characterized by the release of cytokines, proteases, oxygen metabolites, and lipid mediators. CXCL1 and CCL5 chemokines play a major role in OVA-induced allergic asthma models, and CXCL1 is increased in OVA-induced allergic asthma models (Chiu et al. 2021; Hanashiro et al. 2019; Hou et al. 2021; Yi et al. 2020). Thus, it has been hypothesized that drugs that inhibit the release of these chemokines may be useful in the treatment of allergic asthma. Importantly, other studies suggest that PAR2 activation on vascular smooth muscle cells increases the expression of CXCL1, while PAR2 deficiency significantly reduced levels of this chemokine in the circulation and macrophage content in atherosclerotic lesions (Jones et al. 2018), Furthermore, we recently demonstrated that pharmacological blockade of PAR2 impairs CXCL1 chemokine release in the BAL fluid of LPS-instilled mice (Almeida et al. 2020). Here, we demonstrated that PAR2 blockade impaired the release of AREG, an epidermal growth factor produced and released by eosinophils and mast cells in response to various inflammatory stimuli (Matsumoto et al. 2009; Wang et al. 2005), in the lungs of OVA-instilled mice, and increased levels of the anti-inflammatory cytokine IL-10 in the BALF of OVA-instilled mice. AREG plays a role in pulmonary remodeling in allergic diseases, resulting in the proliferation of primary human pulmonary fibroblasts (Wang et al. 2005), and has previously been associated with airway remodeling in chronic allergic disorders (Isgrò et al. 2013; Morimoto et al. 2018).
Some of the major factors controlling allergy are T helper (Th) 2 polarization (Leung et al. 2015) and the commitment of immune tolerance by immune regulatory cells, such as regulatory B cells and their cytokines, such as IL-10 (Braza et al. 2014; Lykken et al. 2015). In this study, a significant increase in neutrophils and eosinophils was observed in BALF after the last allergen challenge, and PAR2 blockade partially inhibited neutrophil and eosinophil recruitment, impairing the release of the pro-inflammatory cytokines CXCL1, IL-6, and CCL5 in BALF 2 h after the last OVA challenge, as well as IL-13 in lung homogenate 48 h after the last challenge. We speculate that PAR2 blockade may play a role in both neutrophil recruitment and Th2 inflammation in allergic airway disease, increasing the release of the anti-inflammatory cytokine IL-10, suggesting that PAR2 blockade may be useful in airway inflammatory diseases acting on the onset of acute lung inflammation, at least in part due to their ability to inhibit the production of pro-inflammatory cytokines, such as the chemokine CXCL1, triggered after allergen exposure. Our results are consistent with previous studies in short-term models of allergic airway inflammation, demonstrating an impairment in eosinophil infiltration after exposure to allergens in Par2-/- mice as compared to wild-type mice and in mice intranasally pretreated with anti-PAR2 monoclonal antibody (Asaduzzaman et al. 2015; Davidson et al. 2013; de Boer et al. 2014). A recent study by Nadeem et al. demonstrated that the number of neutrophils recovered from BALF as well as pro-inflammatory cytokines and chemokines, followed by dust mite extract instillation, is attenuated by ENMD1068 (5 mg/kg) treatment before allergen administration (Nadeem et al. 2019). Our results are in line with recent evidence suggesting that human peripheral B cells express PAR2, and activation of PAR2 inhibits the expression of IL-10 in B cells (Xue et al. 2017), suggesting that an increase in this cytokine may play a regulatory role in acute airway allergic inflammation following pharmacological PAR2 blockade. Interestingly, allergic patients produce lower levels of IL-10 following allergen exposure, and restoring IL-10 secretion from dendritic cells is one of the objectives of allergen-specific immunotherapy (Schülke 2018). Furthermore, PAR2 blockade partially inhibited PAR2 expression in cells collected from BALF, and partially restored microvascular leakage in lungs obtained from OVA-instilled mice.
Taken together, our results suggested that the influx of neutrophils and eosinophils into the lung in OVA-instilled mice, plasma exudation, and cytokine/chemokine production in the lung, may be at least partially dependent on the local release of PAR2-agonist proteases such as mast cell tryptase and neutrophil elastase by leukocytes. Given that these effects are essential to reduce the tissue damage induced by allergen exposure, PAR2-based therapy may be a potential pharmacological approach for the treatment of allergic lung diseases such as asthma.
Abbreviations
- PAR2:
-
Protease-activated receptor 2
- PAR2-AP:
-
PAR2-activating peptide
- OVA:
-
Ovalbumin
- BALF:
-
Bronchoalveolar lavage fluid
- AREG:
-
Amphiregulin protein
- CCL5:
-
Chemokine (C-C motif) ligand 5
- CXCL1:
-
Chemokine (C-X-C motif) ligand 1
- ENMD1068 (PAR2 antagonist):
-
N1-3-methylbutyryl-N4-6-aminohexanoyl-piperazine)
- SLIGRL–NH2 (PAR2-activating peptide):
-
Ser-Leu-Ile-Gly-Arg-Leu-NH2
- LRGILS-NH2 (peptide control):
-
Leu-Arg-Gly-Ile-Leu-Ser-NH2
- i.n.:
-
Intranasal
- i.p.:
-
Intraperitoneal
- IL:
-
Interleukin
- ELISA:
-
Enzyme-linked immunosorbent assay
- Par2 -/- :
-
PAR2 knockout mice
References
Adams MN, Ramachandran R, Yau MK, Suen JY, Fairlie DP, et al. 2011 Structure, function and pathophysiology of protease activated receptors. Pharmacol. Ther. 130 248–282
Almeida AD, Silva IS, Fernandes-Braga W, LimaFilho ACM, Florentino RM, et al. 2020 A role for mast cells and mast cell tryptase in driving neutrophil recruitment in LPS-induced lung inflammation via protease-activated receptor 2 in mice. Inflamm. Res. 69 1059–1070
Alves-Filho JC, de Freitas A, Russo M and Cunha FQ 2006 Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit. Care Med. 34 461–470
Arizmendi NG, Abel M, Mihara K, Davidson C, Polley D, et al. 2011 Mucosal allergic sensitization to cockroach allergens is dependent on proteinase activity and proteinase-activated receptor-2 activation. J. Immunol. 186 3164–3172
Asaduzzaman M, Nadeem A, Arizmendi N, Davidson C, Nichols HL, et al. 2015 Functional inhibition of PAR2 alleviates allergen-induced airway hyperresponsiveness and inflammation. Clin. Exp. Allergy 45 1844–1855
Asokananthan N, Graham PT, Fink J, Knight DA, Bakker AJ, et al. 2002 Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J. Immunol. 168 3577–3585
Bolton S, McNulty C, Thomas R, Hewitt C and Wardlaw A 2003 Expression of and functional responses to protease-activated receptors on human eosinophils. J. Leukocyte Biol. 74 60–68
Braza F, Chesne J, Castagnet S, Magnan A and Brouard S 2014 Regulatory functions of B cells in allergic diseases. Allergy 69 1454–1463
Chiu MH, Hou TY, Fan CK, Chang JH, Lin CL et al. 2021 Catalpol exerts antiallergic effects in IgE/ovalbumin-activated mast cells and a murine model of ovalbumin-induced allergic asthma. International immunopharmacology 96
Cocks TM and Moffatt JD 2001 Protease-activated receptor-2 (PAR2) in the airways. Pulm. Pharmacol. Ther. 14 183–191
Compton SJ, Cairns JA, Palmer KJ, Al-Ani B, Hollenberg MD, et al. 2000 A polymorphic protease-activated receptor 2 (PAR2) displaying reduced sensitivity to trypsin and differential responses to PAR agonists. J. Biol. Chem. 275 39207–39212
Cook EB, Stahl JL, Lilly CM, Haley KJ, Sanchez H, et al. 1998 Epithelial cells are a major cellular source of the chemokine eotaxin in the guinea pig lung. Allergy Asthma Proc. 19 15–22
Davidson CE, Asaduzzaman M, Arizmendi NG, Polley D, Wu Y, et al. 2013 Proteinase-activated receptor-2 activation participates in allergic sensitization to house dust mite allergens in a murine model. Clin. Exp. Allergy 43 1274–1285
de Boer JD, Van’t Veer C, Stroo I, van der Meer AJ, de Vos AF, et al. 2014 Protease-activated receptor-2 deficient mice have reduced house dust mite-evoked allergic lung inflammation. Innate Immun. 20 618–625
Dejager L, Dendoncker K, Eggermont M, Souffriau J, Hauwermeiren FV, et al. 2015 Neutralizing TNFα restores glucocorticoid sensitivity in a mouse model of neutrophilic airway inflammation. Mucosal Immunol. 8 1212–1225
Ebeling C, Lam T, Gordon JR, Hollenberg MD and Vliagoftis H 2007 Proteinase-activated receptor-2 promotes allergic sensitization to an inhaled antigen through a TNF-mediated pathway. J. Immunol. 179 2910–2917
Faccioli LH, Mokwa VF, Silva CL, Rocha GM, Araujo JI et al. 1996 IL-5 drives eosinophils from bone marrow to blood and tissues in a guinea-pig model of visceral larva migrans syndrome. Mediators Inflamm. pp 24–31
Faccioli LH, Nourshargh S, Moqbel R, Williams FM, Sehmi R, et al. 1991 The accumulation of 111In-eosinophils induced by inflammatory mediators, in vivo. Immunology 73 222–227
Fahy JV 2009 Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc. Am. Thoracic Soc. 6 256–259
Gomides LF, Duarte ID, Ferreira RG, Perez AC, Francischi JN, et al. 2012 Proteinase-activated receptor-4 plays a major role in the recruitment of neutrophils induced by trypsin or carrageenan during pleurisy in mice. Pharmacology 89 275–282
Hanashiro J, Muraosa Y, Toyotome T, Hirose K, Watanabe A, et al. 2019 Schizophyllum commune induces IL-17-mediated neutrophilic airway inflammation in OVA-induced asthma model mice. Sci. Rep. 9 19321
Hollenberg MD and Compton SJ 2002 International Union of Pharmacology XXVIII. Proteinase-Activated Receptors. Pharmacol. Rev. 54 203–217
Hosoki K, Boldogh I and Sur S 2016 Neutrophil recruitment by allergens contribute to allergic sensitization and allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 16 45–50
Hou JY, Wu JR, Xu D, Chen YB, Shang DD et al. 2021 Integration of transcriptomics and system pharmacology to reveal the therapeutic mechanism underlying Qingfei Xiaoyan Wan to treat allergic asthma. J. Ethnopharmacol. 278
Isgrò M, Bianchetti L, Marini MA, Bellini A, Schmidt M, et al. 2013 The C-C motif chemokine ligands CCL5, CCL11, and CCL24 induce the migration of circulating fibrocytes from patients with severe asthma. Mucosal Immunol. 6 718–727
Jones SM, Mann A, Conrad K, Saum K, Hall DE, et al. 2018 PAR2 (protease-activated receptor 2) deficiency attenuates atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 38 1271–1282
Kelso EB, Lockhart JC, Hembrough T, Dunning L, Plevin R, et al. 2006 Therapeutic promise of proteinase-activated receptor-2 antagonism in joint inflammation. J Pharmacol. Exp. Ther. 316 1017–1024
Klein A, Talvani A, Silva PM, Martins MA, Wells TN, et al. 2001 Stem cell factor-induced leukotriene B4 production cooperates with eotaxin to mediate the recruitment of eosinophils during allergic pleurisy in mice. J. Immunol. 167 524–531
Kobayashi Y 2008 The role of chemokines in neutrophil biology. Front. Biosci. 13 2400–2407
Korkmaz B, Horwitz M, Jenne D and Gauthier F 2010 Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 62 726–759
Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, et al. 2008 IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J. Immunol. 181 4780–4790
Leung J, Beukema KR and Shen AH 2015 Allergic mechanisms of Eosinophilic oesophagitis. Best Pract. Res. Clin Gastroenterol. 29 709–720
Lundberg JM, Martling CR, Saria A, Folkers K and Rosell S 1983 Cigarette smoke-induced airway oedema due to activation of capsaicin-sensitive vagal afferents and substance P release. Neuroscience 10 1361–1368
Lykken JM, Candando KM and Tedder TF 2015 Regulatory B10 cell development and function. Int. Immunol. 27 471–477
Martin TR, Altman LC, Albert RK and Henderson WR 1984 Leukotriene B4 production by the human alveolar macrophage: a potential mechanism for amplifying inflammation in the lung. Am. Rev. Respir. Dis. 129 106–111
Matos NA, Silva JF, Damasceno KA, Cassali GD, Lemos VS, et al. 2014 Proteinase-activated receptor 2 blockade impairs CCL11- or allergen-induced eosinophil recruitment in experimental pleurisy. Eur. J. Pharmacol. 740 627–633
Matos NA, Silva JF, Matsui TC, Damasceno KA, Duarte ID, et al. 2013 Mast cell tryptase induces eosinophil recruitment in the pleural cavity of mice via proteinase-activated receptor 2. Inflammation 36 1260–1267
Matsumoto K, Fukuda S, Nakamura Y and Saito H 2009 Amphiregulin production by human eosinophils. Int. Arch. Allergy Immunol. 149 39–44
Morimoto Y, Hirahara K, Kiuchi M, Wada T, Ichikawa T, et al. 2018 Amphiregulin-producing pathogenic memory T helper 2 cells instruct eosinophils to secrete osteopontin and facilitate airway fibrosis. Immunity 49 134-150.e6
Nadeem A, Al-Harbi NO, Ahmad SF, Ibrahim KE, Alotaibi MR, et al. 2019 Protease activated receptor-2 mediated upregulation of IL-17 receptor signaling on airway epithelial cells is responsible for neutrophilic infiltration during acute exposure of house dust mite allergens in mice. Chem. Biol. Interact. 304 52–60
Pope SM, Zimmermann N, Stringer KF, Karow ML and Rothenberg ME 2005 The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J. Immunol. 175 5341–5350
Rothenberg M and Hogan S 2006 The eosinophil. Annu. Rev. Immunol. 24 147–174
Saw S, Kale S and Arora N 2012 Serine protease inhibitor attenuates ovalbumin induced inflammation in mouse model of allergic airway disease. PLoS One 7 e41107
Schmidlin F, Amadesi S, Vidil R, Trevisani M, Martinet N, et al. 2001 Expression and function of proteinase-activated receptor 2 in human bronchial smooth muscle. Am. J. Respir. Crit. Care Med. 164 1276–1281
Schülke S 2018 Induction of interleukin-10 producing dendritic cells as a tool to suppress allergen-specific T helper 2 responses. Front. Immunol. 9 455
Vergnolle N, Derian CK, D’Andrea MR, Steinhoff M and Andrade-Gordon P 2002 Characterization of thrombin-induced leukocyte rolling and adherence: a potential proinflammatory role for proteinase-activated receptor-4. J. Immunol. 169 1467–1473
Vu TK, Hung DT, Wheaton VI and Coughlin SR 1991 Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 64 1057–1068
Wang SW, Oh CK, Cho SH, Hu G, Martin R, et al. 2005 Amphiregulin expression in human mast cells and its effect on the primary human lung fibroblasts. J. Allergy Clin. Immunol. 115 287–294
Weiss S 1989 Tissue destruction by neutrophils. N. Engl. J. Med. 320 365–376
Xue JM, Yang LT, Yang G, Geng XR, Liu ZQ, et al. 2017 Protease-activated receptor-2 suppresses interleukin (IL)-10 expression in B cells via upregulating Bcl2L12 in patients with allergic rhinitis. Allergy 72 1704–1712
Yi L, Cui J, Wang W, Tang W, Teng F et al. 2020 Formononetin attenuates airway inflammation and oxidative stress in murine allergic asthma. Fron. Pharmacol. 11 533841
Acknowledgements
This work was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG/Brazil, grants n° APQ-00632-14 and PPM-00593-16) and by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP/Brazil, grant n° 2013/08216-2). N.A.M. would like to thank CNPq/Brazil and CAPES/Brazil for the scholarship and financial support. We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Dipankar Nandi.
Corresponding editor: Dipankar Nandi
Rights and permissions
About this article
Cite this article
de Matos, N.A., Lima, O.C.O., da Silva, J.F. et al. Blockade of protease-activated receptor 2 attenuates allergen-mediated acute lung inflammation and leukocyte recruitment in mice. J Biosci 47, 2 (2022). https://doi.org/10.1007/s12038-021-00239-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-021-00239-2