Abstract
The study was designed to investigate the pathogenesis of gastrointestinal (GI) impairment in Parkinson’s disease (PD). We utilized 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP, 20 mg/kg) and probenecid (250 mg/kg) to prepare a PD mice model. MPTP modeling was first confirmed. GI motility was measured using stool collection test and enteric plexus loss was also detected. Intestinal phosphorylated α-synuclein (p-α-syn), inflammation, and S100 were assessed using western blotting. Association between Toll-like receptor 2(TLR2) and GI function was validated by Pearson’s correlations. Immunofluorescence was applied to show co-localizations of intestinal p-α-syn, inflammation, and Schwann cells (SCs). CU-CPT22 (3 mg/kg, a TLR1/TLR2 inhibitor) was adopted then. Success in modeling, damaged GI neuron and function, and activated intestinal p-α-syn, inflammation, and SCs responses were observed in MPTP group, with TLR2 related to GI damage. Increased p-α-syn and inflammatory factors were shown in SCs of myenteron for MPTP mice. Recovered fecal water content and depression of inflammation, p-α-syn deposition, and SCs activity were noticed after TLR2 suppression. The study investigates a novel mechanism of PD GI autonomic dysfunction, demonstrating that p-α-syn accumulation and TLR2 signaling of SCs were involved in disrupted gut homeostasis and treatments targeting TLR2-mediated pathway might be a possible therapy for PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a progressively debilitating neurodegenerative disease affecting more than 1% over 65 populations. Principal pathological hallmarks of PD are loss of dopaminergic (DA) neurons in substantia nigra pars compacta (SNc) and concomitant abnormal phosphorylated α-synuclein (p-α-syn) named Lewy bodies (LB) aggregated in remaining DA neurons, which propagates through diverse regions in a staged/predicative fashion. In fact, apart from motor disturbances such as tremor, rigidity, and dyskinesia, most patients experience some level of non-motor impairments including constipation, hyposmia and sleep disturbances [1]. Epidemiological and histological studies demonstrated that the non-motor evidence, especially the gastrointestinal (GI) dysfunctions, exists decades before the onset of motor symptoms [2]. Constipation is the most prominent GI dysfunction in PD, and has currently been considered as a premotor symptom of the disease [3]. Furthermore, it has been claimed that LB pathologic manifestation in enteric nervous system (ENS) is much earlier than that in midbrain, whose symptoms are distinct [4]. Thus, GI damage may be a very important factor to PD neurodegeneration.
Even though exact mechanisms of PD remain elusive, converging researches showed that inflammation is of great significance to PD pathogenesis [5]. Neuro-inflammation characterized by expression of inflammatory cytokines is common for ageing brain and most neurodegenerative diseases [6], which is accompanied by activated glial cells. Toll-like receptor (TLR)-mediated pathway was known as a major pathway regulating inflammatory, and it was reported that TLRs could distinguish exogenously conserved structures termed pathogen-associated molecular patterns [7] and endogenous damage-associated molecular patterns (DAMPs) [8]. Interestingly, misfolded or aggregated α-syn, as one of DAMPs, can be recognized by TLRs. Recent literature proposed that the α-syn is capable of regulating inflammatory molecules by stimulating TLR2 in the microglia [9], and its phosphorylated form evokes systemic inflammatory cascade exacerbating subsequent DA degeneration in PD course [10]. Besides, our prior papers revealed p-α-syn aggregates and the excessive release of inflammatory cytokines, including tumor necrosis factor α, interleukin 1β (IL-1β) and IL-6 in Schwann cells (SCs) of sural nerves for PD patients [11, 12]. In another research of PD animal models, we demonstrated that pathological p-α-syn might combine TLR2 affecting activation of SCs, inflammatory responses, as well as motor and sensory function via TLR2 signaling [13]. Similar to the microglia cells, SCs can generate numerous pattern recognition receptors which help recognize the exogenous and endogenous hazard signals. In addition, they initiate and regulate local immune responses through antigen presentation, cytokine, and chemokine secretion [14].
The p-α-syn deposition, autonomic nerve loss, and GI dysfunction were shown in an ENS research from Greene et al. [15]. Nevertheless, little is known about pathological pathways that induce ENS alterations of PD. Considering the important role of p-α-syn and TLR2 in central nervous system (CNS) of PD and our previous findings of PD peripheral nerves, we hypothesized that enteric SCs are activated by abnormal p-α-syn accumulation, and TLR2-mediated signaling pathway in enteric SCs induces inflammatory cascades by interacting with p-α-syn, finally resulting in GI dysfunction.
Materials and Methods
Animals and Treatment
C57BL/6 J mice (male, 18–22 g, 8 weeks) were acquired from the Laboratory Animal Center of Nanjing Medical University, Nanjing, China. Mice were bred and maintained to guarantee a light/dark cycle (12:12 h) with a constant temperature of 22 ± 2 °C. Food and water were provided ad libitum.
Mice were randomized into two groups including control and model groups. According to the existing publication [16], 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine (MPTP, 20 mg/kg, 0.1 ml/10 g; Table S1) was subcutaneously administered for model mice every 3.5 days in a total of ten doses. Mice in control group were treated with normal saline via subcutaneous injection at the same dose and frequency. Then, intraperitoneal administration of probenecid (250 mg/kg, 10 μl/10 g; Table S1) was given 1 h after MPTP or saline injection.
To evaluate the involvement of TLR2 in PD intestinal impairments, we utilized CU-CPT22 for further experimentations. Mice in four groups (vehicle, MPTP, MPTP + CU-CPT22, and CU-CPT22) were included. Based on the previous literature [17], CU-CPT22 (3 mg/kg, 40 μl/10 g; Table S1) was injected intraperitoneally 30 min before MPTP administration in MPTP + CU-CPT22 group or separately in CU-CPT22 group.
All experimental protocols were in strict accordance with guidelines of the Institutional Animal Care, and approved by the Committee of Nanjing Medical University.
Behavior Analysis
Rotarod Test
Rotarod test was employed to explore the motor balance and coordination of mice. Animals were first acclimatized to the equipment for three rounds prior to the experiment. The test was performed at accelerating speeds from 5 to 30 r/min in a total of 300 s, and the latency time on the rod was measured.
Pole Test
For pole test, each mouse was placed separately on a rough-surfaced wooden pole (50-cm height) with its head upward. Briefly, mice were accustomed on the pole and descended for three times. During the test, the time of turning head downward and the total time to climb down the pole were both recorded.
GI Function Assessment
GI function was measured 7 days after the last dose. Animals were fasted overnight and given access to food for 2 h. Then, each mouse was placed separately without water or food for 1 h. After being counted and weighed, stools were dried overnight at 65 °C to determine the dry weight. Water content was calculated with the equation: stool water content (%) = [(wet weight – dry weight)/wet weight] × 100% [16, 17].
Histopathology, Immunohistochemistry, and Immunofluorescence Staining
Mice were intraperitoneally euthanized and perfused. Brain and colonic tissues were removed and kept in 4% paraformaldehyde for 24 h. Then, they were dehydrated in 20% and 30% sucrose-phosphate buffer saline, and fixed with optimal cutting temperature compound. Hematoxylin–eosin (H&E) staining was first used to observe the morphological structures of colon. For immunohistochemistry analysis, slices were treated with 3% hydrogen peroxide and incubated with anti-tyrosine hydroxylase (TH, for slices of midbrain and striatum; Table S1) or anti-neurofilament (NF, for colonic slices; Table S1), followed by the corresponding mouse secondary antibody and the Diaminobenzidine staining. Number of TH+ neurons in SNc was evaluated using the Olympus BX51 Microscope and the MicroBrightField Stereo Investigator Stereology System. For immunofluorescence staining, the enteric segments were washed 3 times using PBS for 5 min, and blocked in PBST (PBS with 0.3% Triton X-100 contained) 5% bovine serum albumin contained at room temperature for 1 h, which was followed by PBS washes. The slices were incubated the following primary antibodies (Table S1): anti-p-α-syn, anti-TLR2, anti-IL-1β, anti-S100, anti-glial fibrillary acidic protein (GFAP), and anti-α-smooth muscle actin (α-SMA), and then incubated with the corresponding secondary antibodies. Hoechst was used as the indicator of nucleus. The ZEISS AXIO Fluorescence Microscope and the MicroBrightField Stereo Investigator System were applied for observation.
Western Blotting Analysis
Colon tissues were quickly isolated and placed into a − 80 °C freezer. After sufficient lysis, homogenates using radio immunoprecipitation assay lysate (protease and phosphatase inhibitors involved) were centrifuged at 4 °C for 15 min and the supernatants were collected. Protein concentration was quantified via Bradford assay kit (Bio-Rad, Hercules, CA, USA). Samples were electrophoresed and transferred to the polyvinylidene fluoride membranes (Millipore, ISEQ00010). Membranes were then blocked with 5% non-fat milk for 2 h and incubated with the following primary antibodies at 4 °C overnight (Table S1): anti-TH, anti-p-α-syn, anti-S100, anti-TLR1, anti-TLR2, anti-nod-like receptor pyrin domain containing 3 (NLRP3), anti-nuclear factor kappa B (NF-κB), anti-Caspase 1, anti-IL-1β, and anti-β-actin. The corresponding secondary antibodies were incubated at room temperature for 1 h. Signals were detected by the SuperSignal™ West Femto Maximum Sensitivity Substrate Kit (34096, Thermo Fisher) and the Tanon 5200 Automatic Chemiluminescence Imaging Analysis System. The contents of protein were normalized to the β-actin levels.
Statistical Analysis
Data were analyzed by GraphPad Prism 8.0. Normal distribution of the data was assessed by the Kolmogorov–Smirnov test before statistical analysis. All data here were determined to be normally distributed. Comparisons between two groups were assessed via independent sample Student’s t-test. For multiple groups, one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was adopted. Pearson’s correlations were performed to explore association between TLR2 expression and GI function. Results were displayed as the mean ± standard deviation (SD). The “n’’ represents the number of animals or samples in each group. Statistical significance was accepted when p < 0.05.
Results
DA Degeneration of SNc-Striatal Regions and Neuronal Cell Death in the Colon Muscle Layers of MPTP-Treated Animals
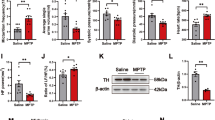
Immunohistochemistry staining of TH was applied to measure the number of DA neurons in PD-relevant brain areas. As is shown in representative photomicrographs, there was a significant reduction (****p < 0.0001) of TH-immunoreactive neurons in SNc for MPTP group compared with saline group (Fig. 1A), which reveals that MPTP administration in our study reproduced the neuropathological features of human Parkinsonism. The striatal TH expression was also reduced in MPTP mice compared to control mice (Fig. 1B). In order to investigate the effect of MPTP on GI neurons and axons, NF staining of the two groups was conducted via immunohistochemistry method. The colon sections from MPTP-treated mice exhibited significant neuronal apoptosis and axonal degeneration in muscle layers when compared with saline mice (***p < 0.001, Fig. 1C). From western blotting analysis, it was suggested that significant loss of colonic DA neurons existed in MPTP-injected group compared to the control group (***p < 0.001, Fig. 1D). Additionally, the H&E staining of colon was also conducted and we discovered that the number of goblet cells was significantly increased in the colonic mucosa of mice injected with MPTP (Fig. S1), possibly due to impaired intestinal mucosal barrier caused by pathological protein deposition and inflammatory activation after MPTP treatment. Fonseca et al. [18] detected that the number of duodenum goblet cells of gerbils infected with bacteria was remarkably higher than that of uninfected gerbils, which is in consistent with our outcomes.
DA loss of SNc-striatal regions and neuronal death of the colonic muscularis in MPTP mice. A Photomicrographs of TH staining (black arrow) in SNc (scale bar: 100 µm) and quantitative analysis of DA number for the two groups. Compared with saline group, there was a reduction of TH+ cells in SNc after MPTP exposure. B Photomicrographs of TH staining (black arrow) in striatum for the two groups. The expression of striatal TH was markedly decreased in MPTP mice compared with the control mice. Scale bar: 200 µm for the left columns and 40 µm for the right columns. C Representative photomicrographs of immunohistochemical staining for NF (black arrow) in colonic slices of saline and MPTP groups and quantitative analysis of NF staining for the two groups. In control group (left column), the nerve plexus of colonic segments was intact; however, degeneration and loss of neuronal fibers were markedly noticed in MPTP group (right column). Scale bars: 100 µm for the upper columns and 40 µm for the lower columns. D Western blotting analysis of TH in colon samples for saline and MPTP groups. When compared to the control mice, colonic TH was notably declined in MPTP-lesioned mice. Data were expressed as mean ± SD and analyzed by unpaired Student’s t-test (n = 6). For western blotting analysis, data were normalized to the β-actin expression. ***p < 0.001, ****p < 0.0001 compared to saline group. DA: Dopaminergic; SNc: substantia nigra pars compacta; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TH: tyrosine hydroxylase; NF: neurofilament; SD: standard deviation
Worse Motor Performance and GI Autonomic Function for Mice Following MPTP Treatment
The voluntary movement performances were monitored to evaluate motor impairment of chronic MPTP mice. MPTP group exhibited remarkably shorter latency periods (**p < 0.01) on rotarod, with both the time to turn (**p < 0.01) and the total time (**p < 0.01) significantly extending during pole test than in saline mice (Fig. S2A). To ascertain whether GI motility altered under the present MPTP regimen, we performed 1-h stool collection test among the two groups (Fig. S2B). With respect to control group, a significant decline in the number of defecation particles was seen after MPTP injection (**p < 0.01), implicating difficulty of GI peristalsis in MPTP group. Fecal water content, an indicator of constipation degree, was also reduced for mice after 6 weeks of MPTP exposure (***p < 0.001).
Increased Expression of p-α–syn, S100 and Inflammatory Molecules in the Colon Tissues of MPTP Mice
Western blotting analysis showed that long-term MPTP challenge effectively induced colonic p-α-syn aggregates (***p < 0.001; Fig. 2A), while administration of saline hardly promoted the expression. Activation of S100 (***p < 0.001) was also observed in MPTP-induced group, which reflects the recruitment of enteric SCs caused by MPTP (Fig. 2A). TLRs contain 10 receptors in humans and 12 for mice. Previous researches demonstrated that TLRs showing abundant expression in microglia were TLR1-4, among which TLR2 was the highest [19, 20]. In this study, we analyzed TLRs expression and noted that TLR2 was markedly elevated in MPTP group (***p < 0.001; Fig. 2B), while no remarked differences of other receptors were detected. Analysis of downstream TLR2 signaling suggested significant increases of NF-κB (**p < 0.01), NLRP3 (***p < 0.001), Caspase 1 (*p < 0.05), and IL-1β (**p < 0.01) in MPTP group compared with saline group (Fig. 2B–E). Thus, MPTP-treated mice model ideally exhibited PD peripheral neuropathy, and we speculate that TLR2-mediated signaling pathway possibly participated in the pathological processes of p-α-syn deposition and SCs reaction in colon tissues.
Increase of colonic p-α-syn, SCs recruitment and TLR2-mediated signaling, as well as the correlation between TLR2 and GI function in mice after treatment with MPTP. A–E Western blotting analysis of p-α-syn (A), S100 (A) TLR2 (B), NF-κB (B), NLRP3 (C), Caspase 1 (D), and IL-1β (E) in colon samples for saline and MPTP groups (n = 6). There was significant increased expression of colonic p-α-syn, SCs and TLR2-mediated inflammatory pathway in MPTP group compared to saline group. F–G Pearson’s correlations between the protein level of TLR2 in colon and gastrointestinal function for MPTP group (n = 11). The expression of TLR2, increased with MPTP treatment, was inversely associated with fecal particle number as well as water content. H–I Quantitative analysis of S100 (H) and GFAP (I) immunofluorescence staining of myenteron in saline and MPTP mice (n = 6). In comparison with saline group, activation of S100 and GFAP was prompted in colonic muscularis of mice for MPTP group. Data were expressed as mean ± SD. Data were normalized to the β-actin expression for western blotting analysis and analyzed by unpaired Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001 compared to saline group. p-α-syn: phosphorylated α-synuclein; SCs: Schwann cells; TLR2: toll-like-receptor 2; GI: gastrointestinal; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NF-κB: nuclear factor kappa B; NLRP3: nod-like receptor pyrin domain containing 3; IL-1β: interleukin-1β; GFAP: glial fibrillary acidic protein; SD: standard deviation
Upregulated Enteric TLR2 Level in MPTP Group Correlated with GI Autonomic Nerve Dysfunction
To explore the underling association between TLR2 expression and GI autonomic nerve function in MPTP group, Pearson’s correlations were applied. The results showed that there was a robust relationship between increased TLR2 levels in colonic tissues and damaged GI autonomic function, where fecal particle number (**p < 0.01; Fig. 2F) and stool water content (*p < 0.05; Fig. 2G) were included, indicating that TLR2 signaling was associated with GI impairments for MPTP-exposed mice.
Co-localization of p-α-syn, Inflammatory Molecules, and Enteric SCs in Muscular Layer of Colon from MPTP-Treated Mice
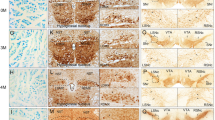
Immunofluorescence staining was employed to assess peripheral PD pathology, TLR2 signaling and responses of enteric SCs in colon slices. To confirm the location of SCs activation in GI tract, S100 and GFAP were immunofluorescent stained for colonic sections in saline and MPTP groups, respectively. Quantitative analysis demonstrated that significant elevations were observed in intestinal muscularis for MPTP group (both ***p < 0.001; Fig. 2H–I) compared to saline mice. Combined with the severe neuronal injury of GI plexus after MPTP injection in Fig. 1C, we consider the SCs activation here to be a denervated response. As displayed in Figs. 3A, 4A, absence of p-α-syn in saline group and abundant expression in MPTP mice were noted. The p-α-syn for MPTP group was predominantly distributed in the myenteric plexus of colon. Additionally, p-α-syn was primarily co-localized in S100+ and GFAP+ cells of muscular layer, both of which were in lumpy shapes, with some punctate staining also observed (Figs. 3A, 4A). The outcomes above declared that p-α-syn was mostly expressed in SCs of pathological states. Specifically, MPTP mice had a greater number of TLR2+ cells co-localized with S100 and GFAP in muscular layer of colon compared with control mice (Figs. 3B, 4B). What is more, the co-localization of TLR2 with p-α-syn was noticed in the colon muscularis for MPTP group (Fig. 3C). In addition, the intensity of IL-1β staining was significantly higher in MPTP group than in saline group, with IL-1β principally co-localized with S100+ and GFAP+ cells in GI muscularis and less in epithelial layer (Figs. 3D and 4C). To determine the level of the above pathological factors in other colonic cells, such as mesenchymal cells, we performed immunofluorescence staining using α-SMA (a marker of mesenchymal cells) which was widely applied in intestinal inflammatory diseases [21, 22], with no co-localizations detected in double staining of α-SMA with p-α-syn, TLR2, and IL-1β (Fig. S3). Figure S4 displays the co-localization of S100 and GFAP in MPTP group. Therefore, p-α-syn accumulation and inflammatory reaction mainly occurred in myenteric plexus of colonic SCs after MPTP exposure, while p-α-syn and TLR2 had some degree of interaction for MPTP mice.
Immunofluorescence of p-α-syn, inflammatory responses and S100 in colon slices for MPTP-challenged and saline groups. A Representative photomicrographs of immunofluorescence staining for p-α-syn (Green) and S100 (Red) in colon sections from saline and MPTP groups. The p-α-syn in MPTP group was principally co-localized with S100+ cells in myenteric plexus of colon, mainly in a lumpy shape and partly in a punctate shape (white arrow). B Representative photomicrographs of immunofluorescence staining for TLR2 (Green) and S100 (Red) in colon slices from saline and MPTP mice. Co-localization of TLR2 with S100 was observed for MPTP-treated mice, and the location was the muscularis of colon sections (white arrow). C Representative photomicrographs of immunofluorescence staining for TLR2 (Green) and p-α-syn (Red) in colon sections from MPTP group. TLR2 was co-localized with p-α-syn in the muscular layer of colon after MPTP challenge (white arrow). D Representative photomicrographs of immunofluorescence staining for IL-1β (Green) and S100 (Red) in colon slices from saline and MPTP groups. A significant elevation of IL-1β was mainly noticed in intestinal muscularis for MPTP group, and the inflammation marker was mostly co-localized with S100+ cells (white arrow). Scale bars: 100 µm for left three columns and 40 µm for the right column. p-α-syn: phosphorylated α-synuclein; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TLR2: toll-like-receptor 2; IL-1β: interleukin 1β
Immunofluorescence of p-α-syn, inflammatory responses and GFAP in colon slices for MPTP-treated and saline groups. A Representative photomicrographs of staining for p-α-syn (Green) and GFAP (Red) in colon slices from saline and MPTP groups. MPTP mice showed co-localization of p-α-syn with GFAP+ cells in colonic myenteric plexus, mainly with lumpy or punctate shape (white arrow). B Representative photomicrographs of immunofluorescence staining for TLR2 (Green) and GFAP (Red) in colon from saline and MPTP mice. TLR2 was co-located with GFAP+ cells in intestinal muscularis for MPTP mice (white arrow). C Representative photomicrographs of staining for IL-1β (Green) and GFAP (Red) in colon sections from saline and MPTP groups. IL-1β staining was mostly co-localized with GFAP+ cells in colonic myenteric layer for MPTP mice (white arrow). Scale bars: 100 µm for left three columns and 40 µm for the right column. p-α-syn: phosphorylated α-synuclein; GFAP: glial fibrillary acidic protein; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TLR2: toll-like-receptor 2; IL-1β: interleukin 1β
GI Autonomic Nerve Functional Recovery in Response to the TLR2 Inhibitor
One-hour stool frequency and stool weight were performed again for animals after CU-CPT22 injection in some mice groups (MPTP + CU-CPT22 group and CU-CPT22 group). Fecal particle number (**p < 0.01 vs saline group) and water content (***p < 0.001 vs saline group) were significantly reduced in MPTP-injected mice (Fig. 5), and water content displayed a considerable increase (##p < 0.01 vs MPTP group; Fig. 5B) when MPTP mice were simultaneously treated with CU-CPT22. In contrast, fecal particle number was less affected in MPTP + CU-CPT22 group (Fig. 5A). Rapid intestinal transit limits GI water absorption leading to liquid stools, whereas slow intestinal transit results in extensive water absorption leading to harder stools [23]. This suggests that the inhibition of TLR2-mediated pathway could improve GI absorption and movement following MPTP exposure, which might be beneficial to alleviating symptoms of constipation.
Attenuated GI autonomic nerve dysfunction induced by MPTP after the challenge of a TLR2 inhibitor. To investigate the participation of TLR2 in gastrointestinal autonomic function, CU-CPT22 inhibiting TLR2 signaling was utilized. A–B Quantitative analysis of fecal particle number (A) and water content (B) among four groups. The fecal particle number was partly affected by MPTP lesioning plus CU-CPT22 treatment, whereas water content was significantly elevated following CU-CPT22 treatment relative to MPTP-lesioned mice. Data were displayed as mean ± SD and analyzed via one-way ANOVA followed by Tukey’s post hoc Test (n = 8). **p < 0.01, ***p < 0.001 compared to saline group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared to MPTP group. GI: gastrointestinal; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TLR2: toll-like-receptor 2; SD: standard deviation; ANOVA: analysis of variance
Improvement of p-α-syn Accumulation, TLR2-Mediated Inflammatory Pathway, and Activated Enteric SCs Induced by MPTP in Colonic Tissues of Mice After TLR2 Suppression
To establish the possible effect of TLR2 on GI dysfunction after MPTP administration, we examined protein expression of gut tissues as determined by western blotting with CU-CPT22 intervention. Group of CU-CPT22 plus MPTP exposure was partially protected from MPTP-induced manifestations of p-α-syn deposition (#p < 0.05 vs MPTP group; Fig. 6A) and SCs reaction (##p < 0.01 vs MPTP group; Fig. 6A), and it turned out that the expression of TLR2 was reduced in MPTP + CU-CPT22 mice compared to the MPTP group (#p < 0.05; Fig. 6B). Moreover, there was a decline of NLRP3 activation (##p < 0.01 vs MPTP group), accompanied by decreased levels of NF-κB (##p < 0.01 vs MPTP group) and IL-1β (#p < 0.05 vs MPTP group) in MPTP + CU-CPT22 group (Fig. 6B–D). Since CU-CPT22 can also have an antagonistic effect on TLR1, we evaluated colonic TLR1 after CU-CPT22 administration and found that there was no significant differences of the levels among four groups (Fig. S5). Collectively, negative manipulation of TLR2 signaling might abrogate the deposition of pathological protein, ENS responses, and inflammatory activation resulted from MPTP injection.
Recovery of p-α-syn accumulation, activation of enteric SCs, and TLR2 signaling in colon samples mediated by MPTP following TLR2 suppression. A–D Western blotting analysis for p-α-syn (A), S100 (A), TLR2 (B), NF-κB (B), NLRP3 (C), and IL-1β (D) of colon tissues among four mice groups. Data were presented as mean ± SD (n = 6). Data were normalized to the β-actin expression and analyzed via one-way ANOVA followed by Tukey’s post hoc Test. **p < 0.01, ***p < 0.001 compared to saline group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 compared to MPTP group. p-α-syn: phosphorylated α-synuclein; SCs: Schwann cells; TLR2: toll-like-receptor 2; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MPTP + CU: MPTP + CU-CPT22; NF-κB: nuclear factor kappa B; NLRP3: nod-like receptor pyrin domain containing 3; IL-1β: interleukin-1β; SD: standard deviation; ANOVA: analysis of variance
Discussion
As a complicated neurodegenerative disease, PD is featured by p-α-syn aggregates and gradually described with its etiology not totally understood [10]. Abnormal deposition of p-α-syn was reported in CNS and peripheral tissues [24]. Several articles have regarded colonic α-syn to be a possible biomarker in PD of early stages, long before the occurrence of LB pathology in CNS [25]. Researches claimed that the pathogenesis and progression of PD, including α-syn aggregation, are related to neuro-inflammation, which are accompanied by elevated glial reaction [26,27,28]. As our previous work reported, intestinal SCs acted like glia involved in inflammatory process [11]. Thus, it is urgent to concentrate on ENS pathology alterations and the relevant role of SCs to determine its mechanism during PD development. In the current study, we used MPTP-induced PD mouse model to investigate whether TLR2-mediated pathway is associated with PD GI autonomic nerve dysfunction (Fig. 7).
MPTP administration induces p-α-syn accumulation in intestinal tract and enteric autonomic nerve dysfunction. The above phenomena might be mediated by the TLR2-mediated pathway. MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TLR2: toll-like-receptor 2; p-α-syn: phosphorylated α-synuclein; NF-κB: nuclear factor kappa B; NLRP3: nod-like receptor pyrin domain containing 3; IL-1β: interleukin-1β
Two groups were first randomized by systemic administration of saline or MPTP to resemble healthy control and PD model. DA loss in SNc-striatal regions (Fig. 1A–B) and poor motor performances (Fig. S2A) in MPTP group suggested the success in our modeling, which was confirmed to other publications [29]. Figure 1C–D shows that MPTP administration resulted in severe colonic nerve damages and even DA degeneration [30]. Currently, it is not clear whether the enteric neuronal loss was incurred by PD pathology. The suppressed gastric emptying and constipation in rats with 6-Hydroxydopamine lesion were reported to be related to the DA increase and the decrease of neuronal nitric oxide synthase in GI tract [31]. Nevertheless, Singaram et al. have claimed the DA deletion in most PD patients of the colonic myenteric and submucosal plexus, with other types of neurons unaffected [32]. Besides, significant drops in stool particle number and water content were discovered in MPTP-induced mice from GI function assessment (Fig. S2B). Consistently, similar abnormalities of GI tract were noted in MPTP animal models [33,34,35]. The deterioration of DA neurons in GI cannot fully explain the delayed gastric emptying in the present study because of the inhibiting effects on intestinal motility. Moreover, apart from DA neurons, some non-DA neurons including cholinergic and nitric oxidergic systems might be connected with PD GI dysfunctions. GI dysfunction in MPTP mice here can be understood as an imbalance of inhibitory/excitatory neuromuscular activity in the digestive system; this may be due to an imbalance between inhibitory and excitatory neurotransmitters. Further investigations are warranted.
Western blotting analysis verified the elevated expression of colonic p-α-syn, SCs activation, as well as TLR2-mediated inflammatory responses following chronic MPTP exposure (Fig. 2A–E). The elevated colonic p-a-syn expression in MPTP mice (Fig. 2A) is similar to our previous outcomes of p-α-syn deposition in peripheral nerves from PD patients and animal models [12, 13]. Prior studies have noticed total α-syn expression in both PD and healthy colon tissues [36]. The p-a-syn has been regarded as a pathological form of α-syn, while total α-syn is believed to be non-differentially expressed in both physiological and pathological states by publications of our team and others [12, 13, 37]. The elevation of colonic S100 in MPTP mice (Fig. 2A) can be inferred as compensatory proliferation and reparative activation of SCs owing to enteric plexus injury (Fig. 1C) following MPTP modeling [38]. Indeed, PD neuro-inflammation is related to astrocytic and microglial activation, which can be the pervasive reason of neuronal damage [28]. These facts were consistent with our recent research regarding the link between activated SCs and increased inflammatory cytokines in sural nerves for PD patients [11]. Expressed in various cell populations, TLRs can not only combat microbial products but also regulate the repair, development and regeneration of tissues [39]. Previous reports emphasized that TLRs are functional in SCs, with bacterial lipoprotein yielding the strongest response [40]. According to the prior literature, TLR2 polymorphism is concerned with increased chances for PD onset [41]. NF-κB is crucial for neuro-inflammation, which accepts the signal transduction in the cytoplasm and transfer to nucleus [42,43,44]. A research proposed that NF-κB is immune-positive in LB, and functions in p-α-syn-mediated DA death [45]. NLRP3 acts as an inflammasome [46] and plays important roles in MPTP-generated neuro-inflammation [47, 48]. It mediates pro-Caspase 1 cleaving into Caspase 1 and subsequently increases the level of IL-1β [49]. Furthermore, a remarked association between higher TLR2 levels and damaged GI function (Fig. 2F–G) in MPTP group reflects the contribution of TLR2 on PD GI autonomic impairments. However, we cannot exclude the involvement of CNS in controlling GI movement, and further exploration is necessary.
For immunofluorescence staining, we defined S100 and GFAP as enteric SCs which are different from satellite glia (Fig. S3A) in the peripheral regions [11, 13, 50], and indicated that SCs expression was significantly increased in GI muscularis for MPTP mice (Fig. 2H–I). MPTP mice exhibited the co-localizations of elevated p-α-syn positive structures with S100+ and GFAP+ cells (Figs. 3A and 4A) in colonic myenteric plexuses rather than α-SMA+ cells (Fig. S3A), which is consistent with our prior studies [12, 13, 51]. Under physiological conditions, SCs play a role of neuroprotection and nerve repair in neural tissues [52]. Nevertheless, they can be activated and release inflammatory factors under pathological conditions such as intestinal disorders [53, 54]. Inflammatory factors were detected to be co-labeled with S100+ and GFAP+ cells (Figs. 3B, D and 4B–C) in enteric muscular layer of MPTP-induced mice, which implicates that the increased neuro-inflammation is activated in SCs of colon muscularis after MPTP treatment. In the present study, we generalize for the first time that p-α-syn was deposited in SCs that innervate GI motility of MPTP mice rather than saline group. It is suggested that p-α-syn accumulation may be the main causes of intestinal denervation and GI symptoms.
SCs can express a wide range of TLRs and that all TLR ligands tested may stimulate NF-κB activation [40]. Interestingly, recent evidence revealed an upregulation of microglial TLR2 in SNc of PD [55]. The co-localization of TLR2 with the p-α-syn in MPTP group (Fig. 3C) may be thought as an indicator of vibrant interaction between TLR2 and p-α-syn following MPTP administration. TLR2 is highly expressed in the influenced brain areas of PD and related to the level of aberrant α‑syn [56]. In turn, α-syn of oligomeric and fibrous forms was verified to activate microglia by binding to TLR2 mostly in a conformation-dependent manner, manifesting the potential for a detrimental feedback loop [57,58,59]. Similarly, pathological p-α-syn combined TLR2 to trigger activation of SCs and the downstream inflammatory responses. Furthermore, the enteric plexus innervating colonic tissues was first injured after MPTP administration, followed by a compensatory response and activation of SCs. On the one hand, they repair damaged GI neuronal plexus in the body; on the other hand, TLR2-mediated inflammatory signaling pathway was activated, leading to the aggravated injury of nerve plexus. This is actually a double-edged sword. Moreover, it would be meaningful and interesting to investigate the co-localization of TLR2, p-α-syn and S100B/GFAP in colon tissue specimens from control and PD patients. Till now, no literatures have been found relevant to this issue and we will collect relevant samples conducting experiments in the future work. On the basis of our results, we conclude that TLR2-mediated inflammation may be a contributing factor to p-α-syn pathology in SCs of colon muscle layer for MPTP-lesioned mice, causing GI autonomic nerve dysfunction thereby influencing number of stool particles and water content.
To explore the roles of TLR2 in PD intestinal autonomic disorders, mice were treated with CU-CPT22 in the following study [60]. Recovery of stool water content was noted in MPTP combined with CU-CPT22 group when compared to MPTP group; in contrast, particle number was less affected which may be due to the small sample size (Fig. 5). This result indicates that TLR2 intervention participated in improvement of constipation and especially in the enhancement of intestinal absorption function. Additionally, reduced p-α-syn level stimulated by MPTP in mice under the condition of drug inhibition (Fig. 6A), demonstrating protection against a plethora of PD-like consequences following MPTP challenge. Indeed, enteric SCs recruiting (Fig. 6A) was decreased after TLR2 inhibition, suggesting suppression of the compensatory hyperplasia and activation of reactive glial cells. The expression of TLR2 and its downstream inflammatory cytokines in MPTP + CU-CPT22 group was lower than purely MPTP-exposed group (Fig. 6B–D). Overall, there were less GI autonomic nerve dysfunction, peripheral degeneration, and inflammatory reaction in MPTP plus CU-CPT22 group when compared with MPTP animals, which establishes that TLR2 plays a critical role in GI autonomic impairments induced by MPTP. Specifically, restoration of GI autonomic function in MPTP + CU-CPT22 mice might be attributed to ameliorated inflammation and decreased SCs reactivity. It was covered that CU-CPT22 performs systemic precondition on MPTP group, which can protect motor nerve fibers and improve motor capacity in a prior animal study [61]. CU-CPT22 can regulate the activated pro-inflammatory phenotypes to help relief symptoms in synucleinopathies and other diseases [9, 62, 63], further confirming our research. An investigation of TLR2 knockout mice depicted that TLR2 pathway exerted an important influence on ENS of inflammatory bowel diseases [64]. Knockout or knockdown of TLR2 in PD animal models could attenuate neurodegeneration indicated by p-α-syn deposition, which is in accordance with our study here [65, 66]. Thus, we hypothesize that the suppression of TLR2-mediated signaling pathway may be one of effective ways to treat PD GI complication by inhibiting parkinsonian pathology and neuro-inflammation.
In brief, our study found that SCs injury can lead to intestinal nerve plexus injury and thus GI dysfunction. Meanwhile, the destroyed function of intestinal mucus secretion and the increased sphincter muscle tension resulted in the inhibited defecation reflex, which may be involved in the occurrence of intestinal symptoms in PD patients. In addition, the injured enteric nerve plexus and inflammatory responses caused the damage of intestinal endothelial cell and mucosal barrier. Then, more harmful elements poured into the GI tract, thereby affecting the intestinal environment. The increase of colonic p-α-syn deposition exacerbated the injury of enteric nerve plexus. In turn, intestinal inflammatory responses can contribute to the imbalance of intestinal flora, which remains to be followed up.
Our findings from this animal model provide information about how effectively TLR2 signaling affects GI autonomic nerve dysfunction in humans. Novel therapeutic targets aimed at modifying neuro-inflammation may intervene in the first stage of PD neurodegeneration. Nonetheless, whether TLR2 pathway-specific compounds are warranted, and how such compounds work mechanistically including their participation in immune responses [67] need to be determined by application of advanced technologies such as transgenic animals. Moreover, our evidence suggests that association of TLR2 with p-α-syn is bona fide, and further investigation on TLR2-mediated pathway is indispensable. Currently, treatments designed to mere relief of non-motor symptoms remain largely inadequate. Modifying therapies for underlying pathogenesis of the disease provide the possibility to slow down or stop the neurodegenerative courses.
However, some limitations of this work need to be considered. Firstly, it is probable that apart from inflammatory pathway mediated by TLR2, other signaling pathways such as the microbiota-gut-brain axis [68], GBA mutation [69], and δ-secretase [70] may contribute to GI autonomic nerve dysfunction of PD. They may be involved in neuro-modeling of the gut of PD patients/pre-clinical models. Future efforts to explore the effects of other pathways on PD pathology will provide more conclusive evidence. Another possible limitation is that occurrence and development of human diseases are extremely complicated, and it is difficult to truly simulate PD onset using existing animal models. Finally, only part of SCs were noted to express p-α-syn in this study. The possible reason for this result is that the chronic PD model here was taken at 5 weeks after MPTP injection and the disease course was relatively short. At this time, p-α-syn was detected in some of SCs. We hypothesized that more SCs might express p-α-syn with the prolongation of the courses. Besides, SCs may have the ability of phagocytic clearance, which can clear the abnormal deposition of p-α-syn in the cytoplasm. Further explorations about this are required in the future.
Conclusions
In this study, for the first time, we innovatively discovered the correlation between both abnormal p-α-syn deposition and TLR2-mediated signal transduction in intestinal SCs and ENS autonomic nerve dysfunction via MPTP modeling. Anti-inflammatory agents that target TLR2 signaling pathway might be a promising treatment option to avoid p-α-syn deposition and mitigate GI autonomic nerve dysfunction.
Data Availability
Data are available upon reasonable request.
References
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912. https://doi.org/10.1016/s0140-6736(14)61393-3
Braak H, Vos RAID, Bohl J, Tredici KD (2006) Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396:67–72. https://doi.org/10.1016/j.neulet.2005.11.012
Fasano A, Visanji NP, Liu LWC, Lang AE, Pfeiffer RF (2015) Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 14:625–639. https://doi.org/10.1016/S1474-4422(15)00007-1
Braak H, Tredici KD, Rüb U, Vos RAID, Steur ENHJ, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211. https://doi.org/10.1016/s0197-4580(02)00065-9
Xanthos DN, Sandkuhler J (2014) Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci 15:43–53. https://doi.org/10.1038/nrn3617
Wang Q, Liu Y, Zhou J (2015) Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener 4:19. https://doi.org/10.1186/s40035-015-0042-0
Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650. https://doi.org/10.1016/j.immuni.2011.05.006
Piccinini AM, Midwood KS (2010) DAMPening inflammation by modulating TLR signalling. Mediators Inflamm 2010:672395. https://doi.org/10.1155/2010/672395
Daniele SG, Beraud D, Davenport C, Cheng K, Yin H, Maguire-Zeiss KA (2015) Activation of MyD88-dependent TLR1/2 signaling by misfolded alpha-synuclein, a protein linked to neurodegenerative disorders. Sci Signal 8:ra45. https://doi.org/10.1126/scisignal.2005965
Rocha EM, De Miranda B, Sanders LH (2018) Alpha-synuclein: pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol Dis 109:249–257. https://doi.org/10.1016/j.nbd.2017.04.004
Zhang H, Wu J, Shen F-F, Yuan YS, Li X, Ji P et al (2020) Activated Schwann cells and increased inflammatory cytokines IL-1beta, IL-6, and TNF-alpha in patients’ sural nerve are lack of tight relationship with specific sensory disturbances in Parkinson’s disease. CNS Neurosci Ther 26:518–526. https://doi.org/10.1111/cns.13282
Zhang H, Zhu L, Sun L, Zhi Y, Ding J, Yuan YS et al (2019) Phosphorylated alpha-synuclein deposits in sural nerve deriving from Schwann cells: a biomarker for Parkinson’s disease. Parkinsonism Relat Disord 60:57–63. https://doi.org/10.1016/j.parkreldis.2018.10.003
Sun L, Jiang WW, Wang Y, Yuan YS, Rong Z, Wu J et al (2021) Phosphorylated α-synuclein aggregated in Schwann cells exacerbates peripheral neuroinflammation and nerve dysfunction in Parkinson’s disease through TLR2/NF-κB pathway. Cell Death Discov 7:289. https://doi.org/10.1038/s41420-021-00676-w
Ydens E, Lornet G, Smits V, Goethals S, Timmerman V, Janssens S (2013) The neuroinflammatory role of Schwann cells in disease. Neurobiol Dis 55:95–103. https://doi.org/10.1016/j.nbd.2013.03.005
Greene JG (2011) Animal models of gastrointestinal problems in Parkinson’s disease. J Parkinson’s Dis 1:137–149. https://doi.org/10.3233/JPD-2011-11033
Hu ZL, Sun T, Lu M, Ding JH, Du RH, Hu G (2019) Kir6.1/K-ATP channel on astrocytes protects against dopaminergic neurodegeneration in the MPTP mouse model of Parkinson’s disease via promoting mitophagy. Brain Behav Immun 81:509–522. https://doi.org/10.1016/j.bbi.2019.07.009
Lin F, Shan W, Zheng Y, Pan L, Zuo Z (2021) Toll-like receptor 2 activation and up-regulation by high mobility group box-1 contribute to post-operative neuroinflammation and cognitive dysfunction in mice. J Neurochem 158:328–341. https://doi.org/10.1111/jnc.15368
Fonseca JF, Alvim LB, Nunes ÁC, Oliveira FMS, Amaral RS, Caliari MV et al (2019) Probiotic effect of Bifidobacterium longum 5(1A) and Weissella paramesenteroides WpK4 on gerbils infected with Giardia lamblia. J Appl Microbiol 127:1184–1191. https://doi.org/10.1111/jam.14338
Kouli A, Horne CB, Williams-Gray CH (2019) Toll-like receptors and their therapeutic potential in Parkinson’s disease and alpha-synucleinopathies. Brain Behav Immun 81:41–51. https://doi.org/10.1016/j.bbi.2019.06.042
Fiebich BL, Batista CRA, Saliba SW, Yousif NM, de Oliveira ACP (2018) Role of microglia TLRs in neurodegeneration. Front Cell Neurosci 12:329. https://doi.org/10.3389/fncel.2018.00329
Martin-Rodriguez O, Gauthier T, Bonnefoy F, Couturier M, Daoui A, Chague C et al (2021) Pro-resolving factors released by macrophages after efferocytosis promote mucosal wound healing in inflammatory bowel disease. Front Immunol 12:754475. https://doi.org/10.3389/fimmu.2021.754475
Scheibe K, Kersten C, Schmied A, Vieth M, Primbs T, Carle B et al (2019) Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology 156:1082-1097.e1011. https://doi.org/10.1053/j.gastro.2018.11.029
Blake MR, Raker JM, Whelan K (2016) Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 44:693–703. https://doi.org/10.1111/apt.13746
Tsukita K, Sakamaki-Tsukita H, Tanaka K, Suenaga T, Takahashi R (2019) Value of in vivo alpha-synuclein deposits in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 34:1452–1463. https://doi.org/10.1002/mds.27794
Sharma A, Kurek J, Morgan JC, Wakade C, Rao SSC (2018) Constipation in Parkinson’s disease: a nuisance or nuanced answer to the pathophysiological puzzle? Curr Gastroenterol Rep 20:1–9. https://doi.org/10.1007/s11894-018-0609-x
Delattre AM, Carabelli B, Mori MA, Kempe PG, Rizzo de Souza LE, Zanata SM et al (2017) Maternal omega-3 supplement improves dopaminergic system in pre- and postnatal inflammation-induced neurotoxicity in Parkinson’s disease model. Mol Neurobiol 54:2090–2106. https://doi.org/10.1007/s12035-016-9803-8
Cao L, Li D, Feng P, Li L, Xue GF, Li G et al (2016) A novel dual GLP-1 and GIP incretin receptor agonist is neuroprotective in a mouse model of Parkinson’s disease by reducing chronic inflammation in the brain. NeuroReport 27:384–391. https://doi.org/10.1097/WNR.0000000000000548
Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353:777–783. https://doi.org/10.1126/science.aag2590
Mendes-Pinheiro B, Soares-Cunha C, Marote A, Loureiro-Campos E, Campos J, Barata-Antunes S et al (2021) Unilateral intrastriatal 6-hydroxydopamine lesion in mice: a closer look into non-motor phenotype and glial response. Int J Mol Sci 22. https://doi.org/10.3390/ijms222111530
Anderson G, Noorian AR, Taylor G, Anitha M, Bernhard D, Srinivasan S et al (2007) Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson’s disease. Exp Neurol 207:4–12. https://doi.org/10.1016/j.expneurol.2007.05.010
Zhu HC, Zhao J, Luo CY, Li QQ (2012) Gastrointestinal dysfunction in a Parkinson’s disease rat model and the changes of dopaminergic, nitric oxidergic, and cholinergic neurotransmitters in myenteric plexus. J Mol Neurosci 47:15–25. https://doi.org/10.1007/s12031-011-9560-0
Singaram C, Ashraf W, Gaumnitz EA, Torbey C, Sengupta A, Pfeiffer R et al (1995) Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet 346:861–864. https://doi.org/10.1016/s0140-6736(95)92707-7
Lai F, Jiang R, Xie W, Liu X, Tang Y, Xiao H et al (2018) Intestinal pathology and gut microbiota alterations in a methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. Neurochem Res 43:1986–1999. https://doi.org/10.1007/s11064-018-2620-x
Han NR, Kim YK, Ahn S, Hwang TY, Lee H, Park HJ (2020) A comprehensive phenotype of non-motor impairments and distribution of alpha-synuclein deposition in parkinsonism-induced mice by a combination injection of MPTP and probenecid. Front Aging Neurosci 12:599045. https://doi.org/10.3389/fnagi.2020.599045
Natale G, Kastsiushenka O, Fulceri F, Ruggieri S, Paparelli A, Fornai F (2010) MPTP-induced parkinsonism extends to a subclass of TH-positive neurons in the gut. Brain Res 1355:195–206. https://doi.org/10.1016/j.brainres.2010.07.076
Antunes L, Frasquilho S, Ostaszewski M, Weber J, Longhino L, Antony P et al (2016) Similar alpha-Synuclein staining in the colon mucosa in patients with Parkinson’s disease and controls. Mov Disord 31:1567–1570. https://doi.org/10.1002/mds.26702
Vaikath NN, Erskine D, Morris CM, Majbour NK, Vekrellis K, Li JY et al (2019) Heterogeneity in alpha-synuclein subtypes and their expression in cortical brain tissue lysates from Lewy body diseases and Alzheimer’s disease. Neuropathol Appl Neurobiol 45:597–608. https://doi.org/10.1111/nan.12531
Clairembault T, Leclair-Visonneau L, Neunlist M, Derkinderen P (2015) Enteric glial cells: new players in Parkinson’s disease? Mov Disord 30:494–498. https://doi.org/10.1002/mds.25979
Li Y, Wang L, Chen S (2010) Endogenous toll-like receptor ligands and their biological significance. J Cell Mol Med 14:2592–2603. https://doi.org/10.1111/j.1582-4934.2010.01127.x
Goethals S, Ydens E, Timmerman V, Janssens S (2010) Toll-like receptor expression in the peripheral nerve. Glia 58:1701–1709. https://doi.org/10.1002/glia.21041
Kim C, Spencer B, Rockenstein E, Yamakado H, Mante M, Adame A et al (2018) Immunotherapy targeting toll-like receptor 2 alleviates neurodegeneration in models of synucleinopathy by modulating alpha-synuclein transmission and neuroinflammation. Mol Neurodegener 13:43. https://doi.org/10.1186/s13024-018-0276-2
Bellucci A, Bubacco L, Longhena F, Parrella E, Faustini G, Porrini V et al (2020) Nuclear factor-kappaB dysregulation and alpha-synuclein pathology: critical interplay in the pathogenesis of Parkinson’s disease. Front Aging Neurosci 12:68. https://doi.org/10.3389/fnagi.2020.00068
Baig MS, Zaichick SV, Mao M, de Abreu AL, Bakhshi FR, Hart PC et al (2015) NOS1-derived nitric oxide promotes NF-kappaB transcriptional activity through inhibition of suppressor of cytokine signaling-1. J Exp Med 212:1725–1738. https://doi.org/10.1084/jem.20140654
Dresselhaus EC, Meffert MK (2019) Cellular specificity of NF-kappaB function in the nervous system. Front Immunol 10:1043. https://doi.org/10.3389/fimmu.2019.01043
Boyko AA, Troyanova NI, Kovalenko EI, Sapozhnikov AM (2017) Similarity and differences in inflammation-related characteristics of the peripheral immune system of patients with Parkinson’s and Alzheimer’s diseases. Int J Mol Sci 18:2633. https://doi.org/10.3390/ijms18122633
Franchi L, Munoz-Planillo R, Núñez G (2012) Sensing and reacting to microbes through the inflammasomes. Nat Immunol 13:325–332. https://doi.org/10.1038/ni.2231
Wen L, Zhang QS, Heng Y, Chen Y, Wang S, Yuan YH et al (2018) NLRP3 inflammasome activation in the thymus of MPTP-induced Parkinsonian mouse model. Toxicol Lett 288:1–8. https://doi.org/10.1016/j.toxlet.2018.02.003
Zhang QS, Heng Y, Chen Y, Luo P, Wen L, Zhang Z et al (2017) A novel bibenzyl compound (20C) protects mice from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid toxicity by regulating the alpha-synuclein-related inflammatory response. J Pharmacol Exp Ther 363:284–292. https://doi.org/10.1124/jpet.117.244020
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10:417–426. https://doi.org/10.1016/s1097-2765(02)00599-3
Pellegrini C, Ippolito C, Segnani C, Dolfi A, Errede M, Virgintino D et al (2020) Pathological remodelling of colonic wall following dopaminergic nigrostriatal neurodegeneration. Neurobiol Dis 139:104821. https://doi.org/10.1016/j.nbd.2020.104821
Rong Z, Shen F, Wang Y, Sun L, Wu J, Zhang H et al (2021) Phosphorylated alpha-synuclein and phosphorylated tau-protein in sural nerves may contribute to differentiate Parkinson’s disease from multiple system atrophy and progressive supranuclear paralysis. Neurosci Lett 756:135964. https://doi.org/10.1016/j.neulet.2021.135964
Jessen KR, Mirsky R, Lloyd AC (2015) Schwann cells: development and role in nerve repair. Cold Spring Harb Perspect Biol 7:a020487. https://doi.org/10.1101/cshperspect.a020487
Di Liddo R, Piccione M, Schrenk S, Dal Magro C, Cosma C, Padoan A et al (2020) S100B as a new fecal biomarker of inflammatory bowel diseases. Eur Rev Med Pharmacol Sci 24:323–332. https://doi.org/10.26355/eurrev_202001_19929
Costa DVS, Moura-Neto V, Bolick DT, Guerrant RL, Fawad JA, Shin JH et al (2021) S100B inhibition attenuates intestinal damage and diarrhea severity during clostridioides difficile infection by modulating inflammatory response. Front Cell Infect Microbiol 11:739874. https://doi.org/10.3389/fcimb.2021.739874
Doorn KJ, Moors T, Drukarch B, van de Berg W, Lucassen PJ, van Dam AM (2014) Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients. Acta Neuropathol Commun 2:90. https://doi.org/10.1186/s40478-014-0090-1
Dzamko N, Gysbers A, Perera G, Bahar A, Shankar A, Gao J et al (2017) Toll-like receptor 2 is increased in neurons in Parkinson’s disease brain and may contribute to alpha-synuclein pathology. Acta Neuropathol 133:303–319. https://doi.org/10.1007/s00401-016-1648-8
Kim C, Ho DH, Suk JE, You S, Michael S, Kang J et al (2013) Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun 4:1562. https://doi.org/10.1038/ncomms2534
Gustot A, Gallea JI, Sarroukh R, Celej MS, Ruysschaert JM, Raussens V (2015) Amyloid fibrils are the molecular trigger of inflammation in Parkinson’s disease. Biochem J 471:323–333. https://doi.org/10.1042/BJ20150617
Hoffmann O, Braun JS, Becker D, Halle A, Freyer D, Dagand E et al (2007) TLR2 mediates neuroinflammation and neuronal damage. J Immunol 178:6476–6481. https://doi.org/10.4049/jimmunol.178.10.6476
Xing W, Huang P, Lu Y, Zeng W, Zuo Z (2018) Amantadine attenuates sepsis-induced cognitive dysfunction possibly not through inhibiting toll-like receptor 2. J Mol Med (Berl) 96:391–402. https://doi.org/10.1007/s00109-018-1631-z
Ugalde-Muniz P, Fetter-Pruneda I, Navarro L, Garcia E, Chavarria A (2020) Chronic systemic inflammation exacerbates neurotoxicity in a Parkinson’s disease model. Oxid Med Cell Longev 2020:4807179. https://doi.org/10.1155/2020/4807179
Lan F, Zhang N, Holtappels G, De Ruyck N, Krysko O, Van Crombruggen K et al (2018) Staphylococcus aureus induces a mucosal type 2 immune response via epithelial cell-derived cytokines. Am J Respir Crit Care Med 198:452–463. https://doi.org/10.1164/rccm.201710-2112OC
Tian J, Song T, Wang H, Wang W, Ma X, Hu Y (2021) Toll-like receptor 2 antagonist ameliorates type 2 diabetes mellitus associated neuropathic pain by repolarizing pro-inflammatory macrophages. Neurochem Res 46:2276–2284. https://doi.org/10.1007/s11064-021-03365-3
Brun P, Giron MC, Qesari M, Porzionato A, Caputi V, Zoppellaro C et al (2013) Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology 145:1323–1333. https://doi.org/10.1053/j.gastro.2013.08.047
Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S et al (2019) Transneuronal propagation of pathologic alpha-synuclein from the gut to the brain models Parkinson’s disease. Neuron 103:627-641.e627. https://doi.org/10.1016/j.neuron.2019.05.035
Kim C, Rockenstein E, Spencer B, Kim HK, Adame A, Trejo M et al (2015) Antagonizing neuronal toll-like receptor 2 prevents synucleinopathy by activating autophagy. Cell Rep 13:771–782. https://doi.org/10.1016/j.celrep.2015.09.044
Bock S, Murgueitio MS, Wolber G, Weindl G (2016) Acute myeloid leukaemia-derived Langerhans-like cells enhance Th1 polarization upon TLR2 engagement. Pharmacol Res 105:44–53. https://doi.org/10.1016/j.phrs.2016.01.016
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M et al (2019) The microbiota-gut-brain axis. Physiol Rev 99:1877–2013. https://doi.org/10.1152/physrev.00018.2018
Patterson L, Allen J, Posey I, Shaw JJP, Costa-Pinheiro P, Walker SJ et al (2020) Glucosylceramide production maintains colon integrity in response to Bacteroides fragilis toxin-induced colon epithelial cell signaling. Faseb J 34:15922–15945. https://doi.org/10.1096/fj.202001669R
Ahn EH, Kang SS, Liu X, Chen G, Zhang Z, Chandrasekharan B et al (2020) Initiation of Parkinson’s disease from gut to brain by δ-secretase. Cell Res 30:70–87. https://doi.org/10.1038/s41422-019-0241-9
Acknowledgements
All of the authors are grateful to the members of Jiangsu Key Laboratory of Neurodegeneration, Department of Pharmacology of Nanjing Medical University for the technical assistance.
Funding
The research was supported by National Natural Science Foundation of China (Grant No. 82071431).
Author information
Authors and Affiliations
Contributions
Conceptualization: W.J., Y.C., and Y.W.; experimentation: W.J., Y.C., Y.W., J.W., Z.R., L.S., and Y.Z.; writing—original draft: W.J., Y.C., and Y.W.; writing—review and editing: K.Z. and Y.Z.; funding acquisition: K.Z. All of the authors contributed in editing the manuscript and read and approved the publication of the submitted version.
Corresponding authors
Ethics declarations
Ethics Approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Committee of Nanjing Medical University.
Consent to Participate
Not applicable.
Consent to Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, W., Cheng, Y., Wang, Y. et al. Involvement of Abnormal p-α-syn Accumulation and TLR2-Mediated Inflammation of Schwann Cells in Enteric Autonomic Nerve Dysfunction of Parkinson’s Disease: an Animal Model Study. Mol Neurobiol 60, 4738–4752 (2023). https://doi.org/10.1007/s12035-023-03345-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03345-4