Abstract
Stress is related to major depressive disorder (MDD). This study investigated the action that early stress, represented by maternal deprivation (MD), has on the behavior and oxidative stress of Wistar female and male rats. Also, it was evaluated whether changes induced by MD could be reversed by environmental enrichment (EE). Male and female rats were divided into a non-MD and MD group. The MD group was subdivided into 3 groups: (1) assessed on the 31st day after exposure to EE for 10 days, (2) assessed on the 41st day after exposure to EE for 20 days, and (3) assessed on the 61st day after exposure to EE for 40 days. Behavioral tests were performed (memory habituation and elevated plus maze). Oxidative stress parameters were evaluated peripherally. MD was able to promote anxiety-like behavior at postnatal day (PND) 41 and impair memory at PND 31 and PND 61 in male and PND 41 and PND 61 in female rats. MD was associated with increased oxidative stress parameters (reactive species to thiobarbituric acid levels (TBARS), carbonylated proteins, nitrite/nitrate concentration), and altered antioxidant defenses (superoxide dismutase (SOD) and catalase (CAT), and sulfhydryl content) in different stages of development. The EE was able to reverse almost all behavioral and biochemical changes induced by MD; however, EE effects were sex and developmental period dependent. These findings reinforce the understanding of the gender variable as a biological factor in MDD related to MD and EE could be considered a treatment option for MDD treatment and its comorbidities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The major depressive disorder (MDD) is a common mental illness that causes significant disability and declining quality of life [1, 2]. Individuals with this mood disorder have impairment in social, work, family, and cognitive performance [3, 4]. The onset and outcomes of MDD are very varied, with different causes and outcomes across the life course [5]. Also, it is worth mentioning that meta-analysis evidenced that MDD is associated with cognitive impairment [6, 7] and anxiety [8].
MDD affects all ages; however, mid-adolescence to mid-40 s is the most probable period for the onset of the first depressive episode, with an average age of onset in the mid-20 s [1, 9]. Moreover, MDD is more prevalent in women, with the gender difference peaked in adolescence [10].
An overlap of multiple factors seems to determine the vulnerability to MDD. Populations affected by the disorder presents genetic, environmental, hormonal, and/or immunological alterations that lead to structural and biochemical changes in the central nervous system (CNS) that may be associated with the evolution of depressive symptoms [11]. Some factors that may be involved in the pathophysiology of MDD are as follows: decreased monoaminergic neurotransmitters, increased glutamate levels, decreased brain-derived neurotrophic factor, deregulation in the hypothalamic–pituitary–adrenal (HPA) axis, neuroinflammation, and oxidative stress [1, 12,13,14].
Notably, meta-analyses have already shown that patients with MDD have increased oxidative stress markers [15, 16], suggesting that MDD is accompanied by oxidative stress dysregulation. An increase in oxidative stress induces damage to the brain and has a negative impact on CNS functions [17].
It is well established that stressful experiences, especially childhood stress, can be determinant for vulnerability to MDD or depressive symptoms [18, 19]. Childhood trauma increases an individual’s vulnerability to the development of MDD and anxiety disorder, and it is also associated with a more serious and chronic course of these disorders [20]. Besides, the severity of childhood trauma experience contributes to increased HPA axis activity and lack of response to antidepressant treatment [21]. A meta-analysis also evidenced that childhood maltreatment was associated with an elevated risk of developing recurrent and persistent depressive episodes and was associated with lack of response or remission during treatment for MDD [22]. In line with this, studies in rodents use models such as maternal separation or maternal deprivation (MD) to investigate behavioral and biochemical mechanisms involved with early life stress consequences [23,24,25,26].
On the other hand, a positive environment is known to be critical for brain development, with beneficial consequences throughout the entire life span [27]. In the laboratory animals, positive stimuli like environmental enrichment (EE), with physical and/or social stimulation, have revealed significant effects on brain neurochemistry (for example, influence in neurotransmitter system, such as serotonin and acetylcholine, increased neurotrophic factors, stimulates adult hippocampal neurogenesis), resulting in behavior changes, including changes in MDD, anxiety, cognition, and stress resiliency [28,29,30,31,32].
However, few studies assess the effect of MD and EE at different stages of the development and if gender influences the responses of these protocols. Thus, this study aimed to investigate the effects that early life stress, represented by MD, has on behavior and oxidative stress at different stages of the development of male and female rats. Besides, it aimed to assess whether there are differences concerning sex and developmental period, and whether EE can reverse these changes caused by early life stress.
Methods
Animals
Female Wistar rats with 3 months of age and weighing 250–280 g were obtained from the breeding colony of Universidade do Extremo Sul Catarinense (UNESC, Criciúma, SC, Brazil) and were housed for 1 week in the presence of males for mating purposes. At the end of 7 days, the pregnant rats were housed individually with ad libitum access to food and water. The pregnant rats were housed individually for the birth of the pups and their identification. All mothers and pups were kept on a 12-h light/dark cycle (06:00 a.m. to 06:00 p.m.) at a temperature of 23 ± 1 °C. One day after birthing occurred, the MD protocol was applied to in a percentage of male and female pups from days 1 to 10 after birth (MD group); other males and female were used as controls (non-MD group). All experimental procedures that involved animals were performed in accordance with the NIH Guide for the Care and Usage of Laboratory Animals, within the Brazilian Society for Neuroscience and Behavior recommendations for animal care. The experimental protocol was approved by the ethics committee from UNESC under protocol number: 070/2018–1.
Maternal Deprivation (MD)
The MD protocol consisted of removing the mother from the residence box and taking her to another room. The pups were maintained in their home cage (grouped in the nest in the presence of maternal odor). The pups were deprived of the mother for 3 h per day during the first 10 days (Fig. 1). We prefer this protocol because it does not require the manipulation of the pups [33,34,35,36]. At the end of each daily MD session, the mothers were returned to their home boxes; this procedure was carried out during the light part of the cycle, between 8:00 a.m. and 12:00 p.m. The control rats (non-MD group) remained in their resident boxes together with their mothers throughout the experiment. After MD protocol, pups remained with their mothers for 21 days, when the pups were weaned. Then, the animals were again divided into new experimental groups: (1) non-MD (control); (2) MD + without EE, and (3) MD + EE. Individual groups of rats (male and female) were evaluated at different periods of development after postnatal days (PND) 31, 41, and 61 (n = 12 animals/group for each stage of development: n = 12 for males and n = 12 for female). In the different stages of development and in the different experimental groups, behavior tests were performed (n = 12 for males and n = 12 for female for all tests: memory habituation and plus maze). The oxidative stress parameters (n = 05 for males and n = 05 for female) were performed as described in the “Methods” section.
Maternal deprivation (MD) was performed during the first 10 days of life (3 h a day). Different groups of male and female rats were evaluated on PND 31, 41, and 61. Environmental enrichment (EE) was carried out for 3 h/day. The group evaluated on PND 31 was submitted to EE for 10 days, the group evaluated on PND 41 was submitted to EE for 20 days, and the group evaluated on PND 61 was submitted to EE for 40 days. In each of the developmental stages, behavior tests were performed (memory habituation, forced swimming test, and the elevated plus maze). After, the animals were euthanized for serum collection oxidative stress parameters
Environmental Enrichment (EE) and Experimental Groups
EE began after weaning [37,38,39]. For the EE procedure, MD + EE group was exposed to the EE for 3 h daily, for different periods. One group of animals was exposed to EE for 40 days, another group for 20 days, and a third for 10 days [40]. Each of these groups had experimental groups 1 (non-MD) and 2 (MD) for each stage of development. More details can be seen in Fig. 1. EE consisted of a large cage (40 × 60 × 90 cm) with three floors, ramps, running wheels, and several objects of different shapes and textures. Small changes were made once a week by adding new objects and withdrawing others [37, 41]. The running wheels and stairs enhanced voluntary exercise, a seesaw provided somatosensory stimulation, and large tubes, a set of tunnels, LEGOH blocks, wood pieces, and hanging items provided cognitive stimulation [42].
Behavioral Tests
The behavioral tests were performed at PND 21, 31, and 41. All behavior tests were performed during light part of the cycle.
Habituation to the Open Field Test
The habituation to the open field test evaluates motor performance in the training section and non-associative memory in the retention test session. This apparatus consisted of a brown plywood arena 45 × 60 cm surrounded by wooden walls 50 cm high and containing a frontal glass wall. The floor of the open field is divided by black lines into nine rectangles (15 × 20 cm each). The animals were gently placed in the left rear quadrant and left to explore the arena for 5 min (training session). Twenty-four hours later, they were submitted to a similar open field session (test session). Any crossing (frequency with which the mice crossed one of the grid lines with all four paws) and rearing (frequency with which the animal stood on their hind legs in the maze) performed in both sessions were counted [43]. The decrease in the number of crossings and rearings between the two sessions in all experimental groups was taken as a measure of the retention of habituation [44].
Elevated Plus Maze
The elevated plus maze apparatus was made of wood and consisted of two opposed open arms (50 × 10 × 2 cm) and two opposed closed arms (50 × 10 × 40 cm), all facing a central platform (10 × 10 cm), elevated 45 cm from the floor. Rats from all groups were placed in the center of an elevated plus maze facing one of the closed arms. During a 5-min test performed in a dark room illuminated with red light, the number of entries into each arm and the time spent there were registered [45].
Oxidative Stress Parameters
After the behavioral tests were complete, the animals were killed by decapitation and the blood was collected and placed in microcentrifuge tubes. Serum aliquots were obtained from the collected blood by centrifugation at 10,000 RPM for 10 min.
Protein Carbonyls
The oxidative damage to proteins in the serum was assessed by the determination of carbonyl groups based on the reaction with dinitrophenylhydrazineas previously described by Levine et al. [46]. Briefly, proteins were precipitated by the addition of 20% trichloroacetic acid and redissolved in dinitrophenylhydrazine, and the absorbance was read at 370 nm. Results were expressed as protein carbonyls per milligram of protein.
Thiobarbituric Acid Reactive Substances (TBARS)
Lipid peroxidation was measured via the formation of TBARS [47]. Serum was washed with buffered saline (PBS), harvested, and lysed. Thiobarbituric reactive species, obtained by acid hydrolysis of 1,1,3,3-tetra-ethoxy-propane (TEP), were used as the standard for the quantification of TBARS. Thiobarbituric acid (TBA) 0.67% was added to each tube, and the tubes were vortexed. The reaction mixture was incubated at 90 °C for 20 min, and the reaction was stopped by placing the samples on ice. The optical density of each solution was measured in a spectrophotometer at 535 nm. Data were expressed as nanomoles of malondialdehyde (MDA) equivalents per milligram of protein.
Measurement of Nitrite/Nitrate Concentration
Total nitrite concentrations were measured in the serum using the Griess reaction, by adding 100 μL of Griess reagent 0.1% (w/v) naphthyl ethylenediamine dihydrochloride in H2O and 1% (w/v) sulphanilamide in 5% (v/v) concentrated H3PO4, vol. [1:1] to the 100-μL sample. Absorbance was recorded in a spectrophotometer at 550 nm [48]. Data were expressed as nanomoles of nitrite/nitrate concentration per milligram of protein.
Sulfhydryl Content
Oxidative damage was analyzed by the amount of thiol groups in the serum homogenate, using the 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) method. Briefly, 30 μL of a sample was mixed with 1 mL of PBS/1 mM ethylenediamine tetraacetic acid (EDTA) (pH 7.5). The reaction was initiated by the addition of 30 μL of 10 mM DTNB stock solution in PBS. After 30 min of incubation at room temperature and absorbance at 412 nm, the amounts of TNB (5-thio-2-nitrobenzoic acid) formed [equivalent to the amount of sulfhydryl (SH) groups] were calculated and expressed as nanometer per milligram of protein.
Antioxidant Enzyme Activity
For the determination of the catalase activity, serum was sonicated in a 50-mM phosphate buffer, and the resulting suspension was centrifuged at 3,000 g for 10 min. The supernatant was used for the enzymatic assay. Catalase activity was measured by the rate of decrease in hydrogen peroxide (H2O2) absorbency at 240 nm [49] and results were expressed as catalase activity (U/mg of protein). Superoxide dismutase (SOD) activity was assayed by measuring the inhibition of adrenaline auto-oxidation, as previously described by Bannister and Calabrese [50]. Results were expressed as SOD activity (U/mg of protein).
Statistical Analysis
The data are presented as mean ± standard error of mean (S.E.M). In the habituation to the open field, the differences between the training and test sessions were analyzed by the Student t test for paired samples. For the elevated plus maze, and oxidative stress parameters, the differences between the experimental groups were analyzed by one-way ANOVA followed by post hoc Tukey. Differences between sex and groups interaction were determined by two-way ANOVA. Statistical significance was considered for P values less than 0.05.
Results
Habituation Memory in the Open Field Test at 31 PND for the Non-MD Group, MD Group, and MD Group Exposed to 10 Days of EE
The results of habituation memory evaluated in the open field test at 31 PND in the MD animals and exposed to EE for 10 days are in Fig. 2. In male rats, the number of crossings was reduced in the test session when compared to the training session in the non-MD group (t = 7.462; p < 0.0001), MD group (t = 3.653; p = 0.003), and MD + EE for 10 days (t = 4.157; p = 0.001). The number of rearings was different from the male non-MD group (t = 6.555; p < 0.0001), but not in the MD group (t = 1.756; p = 0.105). However, in MD exposed to EE for 10 days, a reduction in the test session was demonstrated, compared to the training session (t = 1.756; p = 0.105). In female rats, a reduction in the number of crossings during test session were found in the non-MD group (t = 4.161; p = 0.003), MD group (t = 5.588; p < 0.0001), and EE exposed for 10 days group (t = 2.467; p = 0.027). In addition, a reduction in number of rearings was found in the non-MD group (t = 2.440; p = 0.041) and MD group (t = 7.143; p < 0.0001), but not in the group exposed to EE for 10 days (t = − 1.584; p = 0.137). Two-way ANOVA did not reveal differences for sex and groups interaction in the crossings’ (F 2–67 = 0.366; p = 0.694) and rearings’ (F 2–67 = 1.165; p = 0.317) numbers at 31 PND.
EE effects for 10 days in rats subjected to MD in the open field habituation test. The experimental groups: non-MD, MD, and MD + EE 10 days of female rats in the number of crossings (A) and rearing (B) and male rats in the number of crossings (C) and rearings (D) were recorded in the training and test sessions. Values are expressed as mean \(\pm\) SEM. *p < 0.05 compared to the training session
Habituation Memory in the Open Field Test at 41 PND Non-MD Group, MD Group, and MD Group Exposed to 20 Days of EE
The results showed that the male rats at 41 PND subjected to habituation memory test present a reduction in the number of crossing in the non-MD group (t = 5.52; p < 0.0001), MD group (t = 8.125; p < 0.0001) and MD + EE group (t = 4.013; p = 0.002). The same was found in the number of rearings for the non-MD group (t = 3.883; p = 0.003), MD group (t = 6.387; p < 0.0001), and MD + EE group (t = 3.027; p = 0.013) during test session. These data indicate environmental habituation in those groups. Similarly, female rats showed a reduction in the number of crossings in the test session of non-MD group (t = 4.995; p < 0.0001), MD group (t = 6.605; p < 0.0001), and MD + EE group (t = 4.480; p = 0.002). Thus, these data demonstrated the learning acquisition proposed by the environment recognition test. On the other hand, when the number of rearing was evaluated in female rats subjected to MD, no significant difference was found between training and test sessions (t = 0.175; p = 0.863). But there is a decrease in the number of rearings in the non-MD group (t = 4.522; p = 0.001) and MD exposed to EE for 20 days (t = 3.747; p = 0.005), indicating the memory preservation of the experience from the environment and effects induced by MD was reversed in these parameters. Two-way ANOVA did not reveal differences for sex and groups interaction in the crossings’ (F 2–69 = 1.698; p = 0.190) and rearings’ (F 2–72 = 0.829; p = 0.440) numbers at 41 PND (Fig. 3).
EE effects for 20 days in rats subjected to MD in open field habituation test. Experimental groups: non-MD, MD, and MD + EE 20 days of female rats in the number of crossings (A) and rearings (B) and male rats in the number of crossings (C) and rearings (D) were recorded in training and test sessions. Values are expressed as mean \(\pm\) SEM. *p < 0.05 compared to the training session
Habituation Memory in the Open Field Test at 61 PND Non-MD Group, MD Group, and MD Group Exposed to 40 Days of EE
In the non-MD group, a reduction in the number of crossing in male (t = 4.405; p = 0.001) and female (t = 3.957; p = 0.002) rats was observed. The number of rearings was decreased in male (t = 2.777; p = 0.020) and female (t = 3.746; p = 0.003) from session test. However, no significant difference was found between training and test sessions in rats subjected to MD, both in the number of crossing of males (t = 0.754; p = 0.464) and female (t = 0.706; p = 0.492) and in the number of rearings of females (t = 1.454; p = 0.168). Therefore, this data demonstrated a cognitive impairment of the MD group. After the EE exposition by 40 days, the number of crossings in male (t = 4.296; p = 0.001) and female (t = 4.859; p < 0.0001), as well as the number of rearing in male (t = 4.296; p = 0.001) and female (t = 4.859; p < 0.0001), was reduced. These results suggest that an EE for 40 days can reverse cognitive impairment. Two-way ANOVA did not reveal differences for sex and groups interaction in the crossings’ (F 2–75 = 0.745; p = 0.478) and rearings’ (F 2–78 = 0.829; p = 0.440) numbers at 61 PND (Fig. 4).
EE effects for 40 days in rats subjected to MD in open field habituation test. Experimental groups: non-MD, MD, and MD + EE 40 days of female rats in the number of crossings (A) and rearings (B) and male rats in the number of crossings (C) and rearings (D) were recorded in training and test sessions. Values are expressed as mean \(\pm\) SEM. *p < 0.05 compared to the training session
Anxiety-Related Behavior in the Elevated Plus Maze Test at PND 31 Non-MD Group, MD Group, and MD Group Exposed to EE for 10 Days
No alterations were found in open arms time of female rats exposed to EE at PND 31 (F = 0.405; p = 0.671), even as in the number of open arm entries (F = 0.49; p = 0.616). In male rats, there was an increase in open arms time in the MD + EE group for 10 days (F = 4.126; p = 0.028). However, the number of open arm entries made by male rats did not exhibit significant alterations (F = 1.392; p = 0.267). Two-way ANOVA did not reveal differences for sex and groups interaction on the open arm time (F = 2.291; p = 0.112) and on the open arm entries (F = 2.048; p = 0.140) at 31 PND (Fig. 5). The time spent in the closed arms was decreased in the male of rats exposed to EE for 10 days compared to non-MD group (F = 3.891; p = 0.034). In female, there was no difference between groups in the time spent in the closed arms (F = 2.628; p = 0.090) (Table 1, supplementary data).
EE effects for 10 days in rats subjected to MD in anxiety-related behavior. The open arms time of female (A) and male (C) rats and the number of open arms entries of female (B) and male (D) rats were measured in all experimental groups. Values are expressed as mean \(\pm\) SEM. *p < 0.05 compared to the non-MD group
Anxiety-Related Behavior in the Elevated Plus Maze Test at PND 41 Non-MD Group, MD Group, and MD Group exposed to EE for 20 days
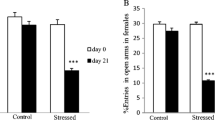
Female MD rats had shorter open arms time (F = 6.676; p = 0.005) and had a lower percentage of open arms entries (F = 9.435; p = 0.001) when compared to the non-MD group, indicating an anxiety-like behavior in this test. However, when exposed to EE, the MD female rats group displayed an increase in open arms time and the number of open arms entries, demonstrating a reduction in anxiety-like behavior. In male rats, a reduction in open arms time in the MD group was observed (F = 4.201; p = 0.033) compared to non-MD. No significant alterations in the number of open arms entries in all experimental groups evaluated were found at 41 PND (F = 2.440; p = 0.117). Two-way ANOVA revealed differences for sex and groups interaction on open arms time at 41 PND (F 2–40 = 3.358; p = 0.044). Effects were observed for sex in the MD groups. However, two-way ANOVA did not reveal differences for sex and groups interaction on open arms entries (F 2–40 = 0.877; p = 0.423) (Fig. 6). The time spent in the closed arms was increased in MD female and decreased in the female rats exposed to EE for 20 days compared to non-MD group (F = 7.777; p = 0.003). In male, there was no difference between groups in the time spent in the closed arms (F = 2.768; p = 0.091) (Table 2, supplementary data).
EE effects for 20 days in rats subjected to MD in the elevated plus maze test. The open arms time of female (A) and male (C) rats and the number of open arms entries of female (B) and male (D) rats were measured in all experimental groups. Values are expressed as mean \(\pm\) SEM. *p < 0.05 compared to the non-MD group; #p < 0.05 compared to the MD group; $p < 0.05 vs. sex and groups interaction according to two-way ANOVA
Anxiety-Related Behavior in the Elevated Plus Maze Test at PND 61 Non-MD Group, MD Group, and MD Group Exposed to EE for 40 Days
Female rats showed an increased time (F = 4.806; p = 0.018) and number of entries (F = 4.532; p = 0.022) in the open arms for the group that was exposed to EE only, when compared to non-MD. These data indicate that EE exposition induced an anxiolytic-like behavior in the MD female rats. Male rats did not exhibit significant alterations in both time (F = 1.134; p = 0.339) and number of entries in the open arms in none of experimental groups (F = 0.281; p = 0.758). Two-way ANOVA did not reveal differences for sex and groups interaction at 61 PND on open arm time (F 2–46 = 0.977; p = 0.384) and open arms entries (F 2–47 = 2.644; p = 0.081) (Fig. 7). The time spent in the closed arms was decreased in the female (F = 4.285; p = 0.026) and male (F = 3.820; p = 0.037) rats exposed to EE for 40 days compared to the MD group (Table 23, supplementary data).
EE effects for 40 days in rats subjected to MD in the elevated plus maze test. The open arms time of female (A) and male (C) rats and the number of open arms entries of female (B) and male (D) rats were measured in all experimental groups. Values are expressed as mean \(\pm\) SEM. *p < 0.05 compared to the non-MD group
Oxidative Stress Parameters at PND 31 for Non-MD Group, MD Group, and MD Group Exposed to EE for 10 Days
Serum levels of carbonyl protein were not altered in male rats (F = 2.482; p = 0.116) and female rats (F = 1.183; p = 0.328), both in the MD group and in the MD + EE group. No changes in TBARS levels were observed in female rats (F = 1.444; p = 0.254) in all experimental groups. Two-way ANOVA did not reveal differences for sex and groups interaction on the carbonyl levels (F 2–34 = 0.171; p = 0.843). However, in male rats, TBARS levels were shown to be increased in the MD group, compared to non-MD, while in the group that was exposed to EE by 10 days, there was a decrease in damage to lipids (F = 8.465; p = 0.003). Two-way ANOVA did not reveal differences for sex and groups interaction on the TBARS levels (F 2–34 = 2.743; p = 0.078). In the nitrite/nitrate concentrations, no significant changes were found in male (F = 2.209; p = 0.137) and female (F = 0.340; p = 0.717) rats, in any of the groups. Two-way ANOVA did not reveal differences for sex and groups interaction on the nitrite/nitrate concentrations (F 2–34 = 0.018; p = 0.981). The sulfhydryl content was reduced in female (F = 6.094; p = 0.011) and male (F = 18.527; p < 0.0001) rats in the MD groups compared to non-MD. EE exposition by 10 days increased the sulfhydryl content only in female, compared to the MD group. Two-way ANOVA revealed differences for sex and groups interaction on the sulfhydryl content (F 2–31 = 3.984; p = 0.028). Effects were observed for sex in the EE groups (Fig. 8).
EE effects for 10 days in the serum from female and male rats at PND 31 subjected to MD in parameters of oxidative damage: carbonyl protein (A), TBARS levels (B), nitrite/nitrate concentration (C), and sulfhydryl content (D). Values are expressed as mean \(\pm\) SEM. *p < 0.05 compared to the non-MD group; #p < 0.05 compared to the MD group; $p < 0.05 vs. sex and groups interaction according to two-way ANOVA
The activity of the SOD enzyme was not significantly altered in the serum of female (F = 0.866; p = 0.437) and male (F = 0.554; p = 0.586) rats, in none of the experimental groups. Two-way ANOVA did not reveal differences for sex and groups interaction on the SOD activity (F 2–34 = 2.515; p = 0.095) (Fig. 9). CAT activity was reduced in both male (F = 14.453; p < 0.0001) and female (F = 19.473; p < 0.0001) rats in the MD group. The MD + EE for 10 days group showed no change in CAT levels. Two-way ANOVA did not reveal differences for sex and groups interaction on the CAT activity (F 2–34 = 0.042; p = 0.958) (Fig. 9).
Oxidative Stress Parameters at PND 41 for Non-MD Group, MD Group, and MD Group Exposed to EE for 20 Days
There were no changes in the levels of carbonyl protein (F = 2.974; p = 0.070) and in the concentration of nitrite/nitrate (F = 2.051; p = 0.151) in female rats from all experimental groups. However, an increase in carbonyl protein levels (F = 8.926; p = 0.02) and nitrite/nitrate concentrations (F = 4.950; p = 0.020) was observed in male rats in the MD group. However, after exposure to EE by 20 days, male rats had a reduction in these parameters. Two-way ANOVA revealed differences for sex and groups interaction on the carbonyl levels (F 2–41 = 3.774; p = 0.031). Effects were observed for sex in the MD groups. However, two-way ANOVA did not reveal differences for sex and groups interaction on the nitrite/nitrate concentration (F 2–41 = 1.178; p = 0.317). The levels of TBARS in the serum of the female rats were not altered in any of the experimental groups studied (F = 1.646; p = 0.214). In male MD rats, there was an increase in TBARS levels (F = 8.620; p = 0.003), compared to non-MD. However, after EE exposition, a reduction in these levels was observed. Two-way ANOVA revealed differences for sex and groups interaction on the TBARS levels (F 2–39 = 4.553; p = 0.016). Effects were observed for sex in the MD groups. The sulfhydryl concentration was reduced in male rats subjected to MD protocol (F = 26.982; p < 0.0001); however, when male and female (F = 3.590; p = 0.043) rats were exposed to EE for 20 days, the sulfhydryl concentration increased. Two-way ANOVA revealed differences for sex and groups interaction on the sulfhydryl concentration (F 2–38 = 5.266; p = 0.009). Effects were observed for sex in the non-MD group (Fig. 10).
EE effects for 20 days on the serum of female and male rats at PND 41 subjected to MD in parameters of oxidative damage: carbonyl protein (A), TBARS levels (B), nitrite/nitrate concentration (C), and sulfhydryl content (D). Values are expressed as mean \(\pm\) SEM. *p < 0.05 compared to the non-MD group; #p < 0.05 compared to the MD group; $p < 0.05 vs. sex and groups interaction according to two-way ANOVA
The activity of the SOD enzyme was shown to be increased in the serum of female (F = 8.131; p = 0.002) rats subjected to MD and reduced in male rats from the MD + EE for 20 days group (F = 8.702; p = 0.002). Two-way ANOVA revealed differences for sex and groups interaction on the SOD activity (F 2–41 = 9.676; p < 0.001). Effects were observed for sex in the non-MD group (Fig. 11). The CAT enzyme activity decreased both in female (F = 18.367; p < 0.0001) and in male (F = 11.982; p = 0.0001) rats subjected to MD. However, when males and females were exposed to EE for 20 days, catalase activity increased. Two-way ANOVA did not reveal differences for sex and groups interaction on the CAT activity (F 2–41 = 1.944; p = 0.155) (Fig. 11).
EE effects for 20 days in the serum of female and male rats at PND 41 subjected to MD in antioxidant enzymes: SOD (A) and catalase (B). Values are expressed as mean \(\pm\) SEM. *p < 0.05 compared to the non-MD group; #p < 0.05 compared to the MD group; $p < 0.05 vs. sex and groups interaction according to two-way ANOVA
Oxidative Stress Parameters at PND 61 for Non-MD Group, MD Group, and MD Group Exposed to EE for 40 Days
In female rats, no significant changes were found in the levels of carbonyl protein in any of the groups (F = 1.115; p = 0.346). However, in males subjected to MD, the levels of carbonyl protein were increased, while in male rats from the MD + EE for 40 days group, a reduction in protein damage was showed (F = 5.977; p = 0.012). Two-way ANOVA did not reveal differences for sex and groups interaction on the carbonyl levels (F 2–37 = 0.777; p = 0.466). TBARS levels were increased in the serum of female (F = 7.639; p = 0.003) and male (F = 8.679; p = 0.003) rats of the MD group, but only males exposed to EE had TBARS levels reduced. Two-way ANOVA did not reveal differences for sex and groups interaction on the TBARS levels (F 2–37 = 3.203; p = 0.052). In the nitrite/nitrate concentration, no significant changes were found in male (F = 1.858; p = 0.190) or female (F = 1.964; p = 0.164) rats from all experimental groups. Two-way ANOVA did not reveal differences for sex and groups interaction on the nitrite/nitrate concentration (F 2–37 = 0.202; p = 0.817). Regarding the sulfhydryl concentration, a reduction was found in females (F = 5.417; p = 0.013) and males (F = 17.392; p < 0.0001) subjected to MD. After EE exposition, both sexes showed an increase in the levels of sulfhydryl, compared to the MD group. Two-way ANOVA did not reveal differences for sex and groups interaction on the sulfhydryl concentration (F 2–35 = 1.710; p = 0.195) (Fig. 12).
EE effects for 40 days on the serum of female and male rats at PND 61 subjected to MD in parameters of oxidative damage: carbonyl protein (A), TBARS levels (B), nitrite/nitrate concentration (C), and sulfhydryl content (D). Values are expressed as mean \(\pm\) SEM. *p < 0.05 compared to the non-MD group; #p < 0.05 compared to the MD group
The activity of the SOD enzyme was increased in the serum of female rats subjected to MD and reduced in the female rats exposed to EE (F = 7.595; p = 0.003). In males, SOD activity did not change significantly in any experimental group studied (F = 0.830; p = 0.455). Two-way ANOVA revealed differences for sex and groups interaction on the SOD activity (F 2–37 = 3.336; p = 0.046). Effects were observed for sex in the EE groups. In females, CAT activity did not change significantly in any of the groups (F = 2.159; p = 0.139). In males (F = 11.822; p = 0.001) CAT decreased in the MD group; however, after EE exposition by 40 days, there was an increase in the activity of this enzyme in the serum. Two-way ANOVA did not reveal differences for sex and groups interaction on the CAT activity (F 2–37 = 0.166; p = 0.847) (Fig. 13).
EE effects for 40 days on the serum of female and male rats at PND 61 subjected to MD in antioxidant enzymes: SOD (A) and catalase (B). Values are expressed as mean \(\pm\) SEM. *p < 0.05 compared to the non-MD group; #p < 0.05 compared to the MD group; $p < 0.05 vs. sex and groups interaction according to two-way ANOVA
Discussion
In the present study, we showed that rats exposed to MD have behavior changes that were influenced according to sex and stage of life. Males and females at PND 41 exposed to MD developed an anxiety-like behavior. However, the same was not observed at PND 31 or PND 61. In another protocol of MD, both male and female rats showed an anxiety-like behavior at PND 52–60 when the MD protocol was applied at PND 3, but not when the protocol was applied PND 11 [51], with similar results in both sexes, but with differences according to the day that MD protocol was applied. Another study evidenced that MD (3 h daily from PND 1 through 14) decrease open arm time in adulthood (PND 61–63) in both males and females rats (without sex separation) [26]. Similarly, another MD protocol (3 h daily from PND 3 through 12) also demonstrated anxious-like behavior in adult male and female rats, although the effects are greater in males [52]. In contrast, MD (24 h at PND 9) increased the percent of open arms entries with regard to total entries in males but not females in adulthood [53]. Analyzing this data, we can assume that probably a longer MD protocol ends up influencing more anxious behavior. Also, a review of the effects of MD on behavior (depressive and anxious) suggests that some inconsistencies in the results may be justified by the cognitive impairment associated with MD protocols [54].
The cognitive impairment related to early stress was explored in this experiment through the assessment of habituation memory. In male rats, MD protocol generated cognitive impairment at PND 31 and PND 61. In females, cognitive impairment was observed at PND 41 and PND 61. These results show that cognitive impairments related to early stress can manifest later in development, especially in females. In line with this, other studies also evidenced that MD protocols can induce cognitive impairment [55, 56]. However, it should be noted that, as far as we know, this was the first study that compared the effects according to sex and the stage of life.
Regarding the effects of EE on behaviors and cognition, we observed that (a) EE decreased anxiety-like behavior at PND 31 in males and PND 41 and PND 61 in females, suggesting that the effect of EE on females is later and on males it occurs earlier, and (b) EE improved memory in females at PND 41 and PND 61 and in males at PND 31 and PND 61; however, in females at PND 31, the association of MD and EE worsened cognition. That is, exposure to EE managed to revert, practically all behavioral changes caused by early life stress. It is worth noting that differences were observed according to sex.
Literature data evidenced that EE can ameliorate behavioral depression, memory impairment, and reduced anxiety behavior in rats exposed to chronic stress [31, 57]. Another study demonstrated that a single short episode of EE in adulthood reduced anxiety-like behavior in maternally separated rats [58]. There is also evidence that EE during the peripubertal period reverses the effects of maternal separation on both HPA axis and behavioral responses to stress [59]. Noteworthy, how reviewed by Sampedro-Piquero and Begega [60], EE is a protocol to improve cognitive functions and reduce anxiety-like behaviors across the lifespan.
Interestingly, it is already shows that the effects of EE are sex-specific. Mice exposed for 4 weeks to EE during adolescence altered emotionality-related behavior in a sex-specific manner: the time spent on the open arm (elevated T-maze) was longer in enriched male but not in female mice [61]. It is worth noting that some neurochemical mechanisms involved with the effects of EE (example: glucocorticoid receptor mRNA expression, and BDNF mRNA) also suffer influence according to the sex of the animal [61, 62]. Thus, these differences could, at least in part, justify some behavioral differences observed in this study. Unexpectedly, the present study demonstrated that at PND 31, females exposed to EE worsened cognition. Although we cannot say why this has occurred, we suggest that the manipulation of the females and/or the hormonal changes characteristic of this range of development may be involved with this result.
After evaluating behavioral issues, we investigated the impact of MD on the parameters involved in oxidative stress. As already mentioned, meta-analyses have already shown that patients with MDD have increased oxidative stress markers [15, 16]. Oxidative stress occurs when there is an imbalance between reactive oxygen and nitrogen species and antioxidants. Low concentrations of reactive oxygen and nitrogen species can function as signaling molecules and participate in the regulation of cellular activities. However, in high concentrations, reactive oxygen and nitrogen species can cause mitochondrial dysfunction, cell damage, and even apoptosis [63, 64]. Thus, controlling the formation of reactive oxygen species and nitrogen species and enhancing the antioxidant system are important when thinking about the proper functioning of the brain [17]. The human body has a variety of antioxidants that serve to counterbalance the effect of oxidants. There are non-enzymatic antioxidants (example: ascorbic acid, α-tocopherol, glutathione, thiol group/sulfhydryl group), and enzymatic antioxidants, such as SOD and CAT [65]. Noteworthy, there are several markers that can be used in clinical and preclinical research to assess oxidative stress. Among them, we can mention protein carbonyl levels, TBARS, and nitrite/nitrate concentrations [66,67,68].
Our present findings demonstrated that females exposed to MD had fewer changes in oxidative stress/antioxidant markers than males. Females at 31 PND exposed to MD had decreased sulfhydryl content and CAT enzyme activity, suggesting less antioxidant capacity. Females at 41 PND exposed to MD also had less CAT activity but had an increase in the SOD enzyme activity (probably by compensatory mechanisms). The 61-day-old females exposed to MD had an increase in TBARS levels, a decrease in sulfhydryl content, and an increase in SOD activity. In male rats exposed to MD, there was an increase in TBARS levels at the 3 ages evaluated (PND 31, 41, and 61), as well as a decrease in sulfhydryl content and CAT activity. Also, males at PND 41 and 61 had an increase in protein carbonyl. Finally, 41-day-old males also had a higher nitrite/nitrate ratio. Together, our data suggest that males exposed to MD generate more oxidative stress than females, especially at PND 41.
Notably, a preclinical study demonstrated that MD induced gender-dependent neuronal and astroglial changes in the brain (hippocampus and cerebellar cortex) of rats that, in general, were more marked in males [69]. Another study demonstrated that MD resulted in sex-dependent alterations in neuroinflammation [70].
A previous study by our laboratory, carried out only with males, showed changes in the activity of CAT and SOD enzymes in the brain of these animals, which varied according to age and the evaluated brain region (PFC or hippocampus). But in general, a decrease in the activity of antioxidant enzymes at the level of the CNS was observed [36], which is in line with the present study, which showed a decrease in CAT at the peripheral level. In the same study, an increase in the carbonyl protein in males at PND 30, 40, and 60 in the hippocampus and PFC was also observed [36]. In agreement with the present study, other preclinical studies demonstrated that male rats maternally deprived had higher levels of carbonyl protein in several brain regions [71, 72]. Maciel et al. [72] also evidenced that MD protocol increased the nitrite/nitrate concentration in the hippocampus and nucleus accumbens.
In our study, an increase in the SOD activity in females exposed to MD at PND 41 and 61 was observed. The increase in the antioxidant activity of SOD can be understood as an action by the body to maintain homeostasis through the damage already mentioned from early stress. Interestingly, a preclinical study evidenced that MD increased the SOD activity in the hippocampus and PFC of male rats at PND 60 [73]. Another preclinical study evidenced that reactive oxygen species levels were increased in the hippocampus of MD male rats (PND 90–95), but no changes in TBARS levels were observed in the hippocampus [74]. On the other hand, in another study, an increase in TBARS levels in the hippocampus and PFC of male rats submitted to MD was observed [75]. It is worth mentioning that preclinical studies also demonstrate that other forms of stress are also capable of increasing oxidative stress, including an increase in TBARS [76]. Clinical study also evidenced an increase in TBARS levels in individuals with MDD [77]. Besides, this same clinical study also demonstrated a decrease in sulfhydryl content in MDD individuals [77], corroborating with the findings of our study.
When we assess the effect of EE on oxidative stress/antioxidant parameters, it is observed that exposure to EE can reverse almost all changes induced by early life stress. In line with this, a preclinical study evidenced that EE was able to decrease oxidative stress markers in the brain, including TBARS levels, in male and female rats [78]. Also, EE increased CAT activity, especially in male rats, and decreased SOD activity in male rats [78]; a result that was similar to that observed in the present study. Interestingly, EE is also able to restore oxidative balance in animals chronically exposed to toluene (that induces a redox imbalance at the neuronal level) in male mice [79]. Lastly, although we cannot state the reason why the EE protocol does not reverse all changes induced by the MD, we suggest that the duration of the EE protocol (10, 20, or 40 days), as well as the period of development, may be involved in these results.
It is worth noting that, in this study, the activity of antioxidant enzymes was evaluated peripherally. As mentioned, MDD subjects have increased peripheral markers of oxidative stress [15, 16]. On the other hand, most studies with the MD and EE protocols evaluate antioxidant enzymes in the brain. Interestingly, a postmortem study showed changes in the antioxidant pathways in the brain of MDD individuals [80]. Moreover, the investigation of peripheral and central antioxidant action is also observed with other strategies with possible benefits for MDD, such as vitamin D [81]. Thus, peripherally analyzing markers related to antioxidant and oxidative stress can also help to understand the pathophysiology and treatment of MDD. Of note, we did not perform central analyses in this study because we use brain tissues to evaluated epigenetic changes in a paper that is already published [40].
This study had two main limitations. We did not monitor the maternal care difference after MD, and in the experimental design, we did not add the control group for EE (EE in non-MD). Investigating the effect of EE in non-MD animals would be the ideal experimental design. However, we consider the concept of 3Rs (replacement, reduction, and refinement—Russell, W.M.S. and Burch, R.L., 1959), in which the “reduction” suggests using fewer animals or obtaining more information from the same number of animals. As our protocol was large and many animals have been used, we prefer not to do the group EE in non-MD, since the main objective of this study was to evaluate the EE effect in rats subjected to adversity early in life.
Conclusions
In summary, our data suggest that early life stress can generate different responses between genders and according to age, both concerning to behavioral parameters, as concerning to the generation of oxidative stress. On the other hand, EE seems to be an interesting strategy to prevent most of these results. These findings reinforce the understanding of the gender variable as a biological factor in MDD related to early life stress and in the response to EE.
Data availability
Data will be made available on reasonable request.
This study is according to ethical concerns and it was approved by the ethics committee from UNESC under protocol number: 070/2018–1.
References
Malhi GS, Mann JJ (2018) Depression Lancet 392:2299–2312. https://doi.org/10.1016/S0140-6736(18)31948-2
WHO (2020) Depression. https://www.who.int/news-room/fact-sheets/detail/depression. Accessed 15 Apr 2020
American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington, VA: American Psychiatric Association
Sheehan DV, Nakagome K, Asami Y et al (2017) Restoring function in major depressive disorder: A systematic review. J Affect Disord 215:299–313
Colman I, Ataullahjan A (2010) Life course perspectives on the epidemiology of depression. Can J Psychiatry 55:622–632. https://doi.org/10.1177/070674371005501002
Burt DB, Zembar MJ, Niederehe G (1995) Depression and Memory Impairment: A Meta-Analysis of the Association, Its Pattern, and Specificity. Psychol Bull 117:285–305. https://doi.org/10.1037/0033-2909.117.2.285
Rock PL, Roiser JP, Riedel WJ, Blackwell AD (2014) Cognitive impairment in depression: A systematic review and meta-analysis. Psychol Med 44:2029–2040. https://doi.org/10.1017/S0033291713002535
Jacobson NC, Newman MG (2017) Anxiety and depression as bidirectional risk factors for one another: A meta-analysis of longitudinal studies. Psychol Bull 143:1155–1200. https://doi.org/10.1037/bul0000111
Kessler RC, Bromet EJ (2013) The epidemiology of depression across cultures. Annu Rev Public Health 34:119–138
Salk RH, Hyde JS, Abramson LY (2017) Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol Bull 143:783–822. https://doi.org/10.1037/bul0000102
Krishnan V, Nestler EJ (2008) The molecular neurobiology of depression. Nature 455:894–902. https://doi.org/10.1038/nature07455
Jeon SW, Kim YK (2017) Inflammation-induced depression: Its pathophysiology and therapeutic implications. J Neuroimmunol 313:92–98. https://doi.org/10.1016/j.jneuroim.2017.10.016
Levy MJF, Boulle F, Steinbusch HW et al (2018) Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 235:2195–2220. https://doi.org/10.1007/s00213-018-4950-4
Czarny P, Wigner P, Galecki P, Sliwinski T (2018) The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog Neuro-Psychopharmacology Biol Psychiatry 80:309–321. https://doi.org/10.1016/j.pnpbp.2017.06.036
Liu T, Zhong S, Liao X et al (2015) A meta-analysis of oxidative stress markers in depression. PLoS ONE 10:e0138904. https://doi.org/10.1371/journal.pone.0138904
Black CN, Bot M, Scheffer PG et al (2015) Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 51:164–175. https://doi.org/10.1016/j.psyneuen.2014.09.025
Salim S (2017) Oxidative stress and the central nervous system. J Pharmacol Exp Ther 360:201–205. https://doi.org/10.1124/jpet.116.237503
Shapero BG, Black SK, Liu RT et al (2014) Stressful Life Events and Depression Symptoms: The Effect of Childhood Emotional Abuse on Stress Reactivity. J Clin Psychol 70:209–223. https://doi.org/10.1002/jclp.22011
Łosiak W, Blaut A, Kłosowska J, Łosiak-Pilch J (2019) Stressful Life Events, Cognitive Biases, and Symptoms of Depression in Young Adults. Front Psychol 10:2165. https://doi.org/10.3389/fpsyg.2019.02165
Hovens JGFM, Giltay EJ, van Hemert AM, Penninx BWJH (2017) Emotional scars: impact of childhood trauma on the development of depressive and anxiety disorders later in life. Tijdschr Psychiatr 59:286–296
Nikkheslat N, McLaughlin AP, Hastings C et al (2020) Childhood trauma, HPA axis activity and antidepressant response in patients with depression. Brain Behav Immun 87:229–237. https://doi.org/10.1016/j.bbi.2019.11.024
Nanni V, Uher R, Danese A (2012) Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am J Psychiatry 169:141–151. https://doi.org/10.1176/appi.ajp.2011.11020335
Réus GZ, Stringari RB, Ribeiro KF et al (2011) Maternal deprivation induces depressive-like behaviour and alters neurotrophin levels in the rat brain. Neurochem Res 36:460–466. https://doi.org/10.1007/s11064-010-0364-3
El Khoury A, Gruber SHM, Mørk A, Mathé AA (2006) Adult life behavioral consequences of early maternal separation are alleviated by escitalopram treatment in a rat model of depression. Prog Neuro-Psychopharmacology Biol Psychiatry 30:535–540. https://doi.org/10.1016/j.pnpbp.2005.11.011
Vetulani J (2013) Early maternal separation: A rodent model of depression and a prevailing human condition. Pharmacol Reports 65:1451–1461. https://doi.org/10.1016/S1734-1140(13)71505-6
Lee JH, Kim HJ, Kim JG et al (2007) Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neurosci Res 58:32–39. https://doi.org/10.1016/j.neures.2007.01.008
Sale A, Berardi N, Maffei L (2014) Environment and brain plasticity: Towards an endogenous pharmacotherapy. Physiol Rev 94:189–234. https://doi.org/10.1152/physrev.00036.2012
van Praag H, Kempermann G, Gage FH (2000) Neural consequences of enviromental enrichment. Nat Rev Neurosci 1:191–198. https://doi.org/10.1038/35044558
Kempermann G (2019) Environmental enrichment, new neurons and the neurobiology of individuality. Nat Rev Neurosci 20:235–245
Simpson J, Kelly JP (2011) The impact of environmental enrichment in laboratory rats-Behavioural and neurochemical aspects. Behav Brain Res 222:246–264. https://doi.org/10.1016/j.bbr.2011.04.002
Shilpa BM, Bhagya V, Harish G et al (2017) Environmental enrichment ameliorates chronic immobilisation stress-induced spatial learning deficits and restores the expression of BDNF, VEGF, GFAP and glucocorticoid receptors. Prog Neuro-Psychopharmacology Biol Psychiatry 76:88–100. https://doi.org/10.1016/j.pnpbp.2017.02.025
Lehmann ML, Herkenham M (2011) Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci 31:6159–6173. https://doi.org/10.1523/JNEUROSCI.0577-11.2011
Ignácio ZM, Réus GZ, Abelaira HM et al (2017) Quetiapine treatment reverses depressive-like behavior and reduces DNA methyltransferase activity induced by maternal deprivation. Behav Brain Res 320:225–232. https://doi.org/10.1016/j.bbr.2016.11.044
Kosten TA, Lee HJ, Kim JJ (2007) Neonatal handling alters learning in adult male and female rats in a task-specific manner. Brain Res 1154:144–153. https://doi.org/10.1016/j.brainres.2007.03.081
Mello PB, Benetti F, Cammarota M, Izquierdo I (2009) Physical exercise can reverse the deficit in fear memory induced by maternal deprivation. Neurobiol Learn Mem 92:364–369. https://doi.org/10.1016/j.nlm.2009.04.004
Réus GZ, Fernandes GC, de Moura AB et al (2017) Early life experience contributes to the developmental programming of depressive-like behaviour, neuroinflammation and oxidative stress. J Psychiatr Res 95:196–207. https://doi.org/10.1016/j.jpsychires.2017.08.020
Pereira LO, Arteni NS, Petersen RC et al (2007) Effects of daily environmental enrichment on memory deficits and brain injury following neonatal hypoxia-ischemia in the rat. Neurobiol Learn Mem 87:101–108. https://doi.org/10.1016/j.nlm.2006.07.003
Rojas JJ, Deniz BF, Miguel PM et al (2013) Effects of daily environmental enrichment on behavior and dendritic spine density in hippocampus following neonatal hypoxia-ischemia in the rat. Exp Neurol 241:25–33. https://doi.org/10.1016/j.expneurol.2012.11.026
Barichello T, Fagundes GD, Generoso JS et al (2014) Environmental enrichment restores cognitive deficits induced by experimental childhood meningitis. Rev Bras Psiquiatr 36:322–329. https://doi.org/10.1590/1516-4446-2014-1443
Borba LA, Broseghini LDR, Manosso LM et al (2021) Environmental enrichment improves lifelong persistent behavioral and epigenetic changes induced by early-life stress. J Psychiatr Res 138:107–116. https://doi.org/10.1016/j.jpsychires.2021.04.008
Ohlsson AL, Johansson BB (1995) Environment influences functional outcome of cerebral infarction in rats. Stroke 26:644–649. https://doi.org/10.1161/01.STR.26.4.644
Nithianantharajah J, Hannan AJ (2006) Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 7:697–709. https://doi.org/10.1038/nrn1970
Brown RE, Corey SC, Moore AK (1999) Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6-H-2K mice. Behav Genet 29:263–271. https://doi.org/10.1023/A:1021694307672
Vianna MRM, Alonso M, Viola H et al (2000) Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the rat. Learn Mem 7:333–340. https://doi.org/10.1101/lm.34600
Gomes KM, Souza RP, Inácio CG et al (2011) Evaluation of light/dark cycle in anxiety- and depressive-like behaviors after regular treatment with methylphenidate hydrochloride in rats of different ages. Braz J Psychiatry 33:55–58. https://doi.org/10.1590/S1516-44462010005000018
Levine RL, Garland D, Oliver CN et al (1990) Determination of Carbonyl Content in Oxidatively Modified Proteins. Methods Enzymol 186:464–478. https://doi.org/10.1016/0076-6879(90)86141-H
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421. https://doi.org/10.1016/0076-6879(90)86134-H
Green LC, Wagner DA, Glogowski J et al (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138. https://doi.org/10.1016/0003-2697(82)90118-X
Aebi H (1984) [13] Catalase in Vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Bannister JV, Calabrese L (1987) Assays for superoxide dismutase. Methods Biochem Anal 32:279–312
Miragaia AS, de Oliveira Wertheimer GS, Consoli AC et al (2018) Maternal deprivation increases anxiety-and depressive-like behaviors in an age-dependent fashion and reduces neuropeptide y expression in the amygdala and hippocampus of male and female young adult rats. Front Behav Neurosci 12:159. https://doi.org/10.3389/fnbeh.2018.00159
Wigger A, Neumann ID (1999) Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav 66:293–302. https://doi.org/10.1016/S0031-9384(98)00300-X
Llorente-Berzal A, Fuentes S, Gagliano H et al (2011) Sex-dependent effects of maternal deprivation and adolescent cannabinoid treatment on adult rat behaviour. Addict Biol 16:624–637. https://doi.org/10.1111/j.1369-1600.2011.00318.x
Andersen SL (2015) Exposure to early adversity: Points of cross-species translation that can lead to improved understanding of depression. Dev Psychopathol 27:477–491. https://doi.org/10.1017/S0954579415000103
Ahmad F, Salahuddin M, Alsamman K et al (2018) Neonatal maternal deprivation impairs localized de novo activity-induced protein translation at the synapse in the rat hippocampus. Biosci Rep 38(3):BSR20180118. https://doi.org/10.1042/BSR20180118
Janetsian-Fritz SS, Timme NM, McCane AM et al (2018) Maternal deprivation induces alterations in cognitive and cortical function in adulthood. Transl Psychiatry 8:71. https://doi.org/10.1038/s41398-018-0119-5
Seong HH, Park JM, Kim YJ (2018) Antidepressive Effects of Environmental Enrichment in Chronic Stress-Induced Depression in Rats. Biol Res Nurs 20:40–48. https://doi.org/10.1177/1099800417730400
Koe AS, Ashokan A, Mitra R (2016) Short environmental enrichment in adulthood reverses anxiety and basolateral amygdala hypertrophy induced by maternal separation. Transl Psychiatry 6:e729. https://doi.org/10.1038/tp.2015.217
Francis DD, Diorio J, Plotsky PM, Meaney MJ (2002) Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci 22:7840–7843. https://doi.org/10.1523/jneurosci.22-18-07840.2002
Sampedro-Piquero P, Begega A (2016) Environmental Enrichment as a Positive Behavioral Intervention Across the Lifespan. Curr Neuropharmacol 15:459–470. https://doi.org/10.2174/1570159x14666160325115909
Lin EJD, Choi E, Liu X et al (2011) Environmental enrichment exerts sex-specific effects on emotionality in C57BL/6J mice. Behav Brain Res 216:349–357. https://doi.org/10.1016/j.bbr.2010.08.019
Chourbaji S, Hörtnagl H, Molteni R et al (2012) The impact of environmental enrichment on sex-specific neurochemical circuitries - Effects on brain-derived neurotrophic factor and the serotonergic system. Neuroscience 220:267–276. https://doi.org/10.1016/j.neuroscience.2012.06.016
Apel K, Hirt H (2004) Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Birben E, Sahiner UM, Sackesen C et al (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5:9–19
Guevara I, Iwanejko J, Dembińska-Kieć A et al (1998) Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin Chim Acta 274:177–188. https://doi.org/10.1016/S0009-8981(98)00060-6
Frijhoff J, Winyard PG, Zarkovic N et al (2015) Clinical Relevance of Biomarkers of Oxidative Stress. Antioxidants Redox Signal 23:1144–1170
Siwek M, Sowa-Kuaema M, Dudek D et al (2013) Oxidative stress markers in affective disorders. Pharmacol Reports 65:1558–1571. https://doi.org/10.1016/S1734-1140(13)71517-2
Llorente R, Gallardo ML, Berzal AL et al (2009) Early maternal deprivation in rats induces gender-dependent effects on developing hippocampal and cerebellar cells. Int J Dev Neurosci 27:233–241. https://doi.org/10.1016/j.ijdevneu.2009.01.002
Burke NN, Llorente R, Marco EM et al (2013) Maternal deprivation is associated with sex-dependent alterations in nociceptive behavior and neuroinflammatory mediators in the rat following peripheral nerve injury. J Pain 14:1173–1184. https://doi.org/10.1016/j.jpain.2013.05.003
Réus GZ, Carlessi AS, Titus SE et al (2015) A single dose of S-ketamine induces long-term antidepressant effects and decreases oxidative stress in adulthood rats following maternal deprivation. Dev Neurobiol 75:1268–1281. https://doi.org/10.1002/dneu.22283
Maciel AL, Abelaira HM, de Moura AB et al (2018) Acute treatment with ketamine and chronic treatment with minocycline exert antidepressant-like effects and antioxidant properties in rats subjected different stressful events. Brain Res Bull 137:204–216. https://doi.org/10.1016/j.brainresbull.2017.12.005
Marković B, Radonjić NV, Jevtić G et al (2017) Long-term effects of maternal deprivation on redox regulation in rat brain: Involvement of NADPH oxidase. Oxid Med Cell Longev 2017:7390516. https://doi.org/10.1155/2017/7390516
Menezes J, Neves BH, Souza M, Mello-Carpes PB (2017) Green tea protects against memory deficits related to maternal deprivation. Physiol Behav 182:121–127. https://doi.org/10.1016/j.physbeh.2017.10.010
Neves BH, Menezes J, Souza MA, Mello-Carpes PB (2015) Physical exercise prevents short and long-term deficits on aversive and recognition memory and attenuates brain oxidative damage induced by maternal deprivation. Physiol Behav 152:99–105. https://doi.org/10.1016/j.physbeh.2015.09.019
Lucca G, Comim CM, Valvassori SS et al (2009) Increased oxidative stress in submitochondrial particles into the brain of rats submitted to the chronic mild stress paradigm. J Psychiatr Res 43:864–869. https://doi.org/10.1016/j.jpsychires.2008.11.002
Kaufmann FN, Gazal M, Mondin TC et al (2015) Cognitive psychotherapy treatment decreases peripheral oxidative stress parameters associated with major depression disorder. Biol Psychol 110:175–181. https://doi.org/10.1016/j.biopsycho.2015.08.001
Mármol F, Rodríguez CA, Sánchez J, Chamizo VD (2015) Anti-oxidative effects produced by environmental enrichment in the hippocampus and cerebral cortex of male and female rats. Brain Res 1613:120–129. https://doi.org/10.1016/j.brainres.2015.04.007
Montes S, Yee-Rios Y, Páez-Martínez N (2019) Environmental enrichment restores oxidative balance in animals chronically exposed to toluene: Comparison with melatonin. Brain Res Bull 144:58–67. https://doi.org/10.1016/j.brainresbull.2018.11.007
Martín-Hernández D, Caso JR, Javier Meana J et al (2018) Intracellular inflammatory and antioxidant pathways in postmortem frontal cortex of subjects with major depression: Effect of antidepressants. J Neuroinflammation 15:1–12. https://doi.org/10.1186/s12974-018-1294-2
Almeida Moreira Leal LK, Lima LA, Alexandre de Aquino PE et al (2020) Vitamin D (VD3) antioxidative and anti-inflammatory activities: Peripheral and central effects. Eur J Pharmacol 879:173099. https://doi.org/10.1016/j.ejphar.2020.173099
Acknowledgements
Translational Psychiatry Program (USA) is funded by a grant from the National Institute of Health/National Institute of Mental Health (1R21MH117636-01A1, to JQ). Center of Excellence on Mood Disorders (USA) is funded by the Pat Rutherford Jr. Chair in Psychiatry, John S. Dunn Foundation and Anne and Don Fizer Foundation Endowment for Depression Research. Translational Psychiatry Laboratory (Brazil) is funded by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (GZR and JQ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (JQ and GZR), Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) (GZR and JQ), Universidade do Extremo Sul Catarinense (JQ, and GZR), and Instituto Cérebro e Mente (JQ and GZR). JQ is a 1A and GZR is 2 CNPq Researches Fellow.
Author information
Authors and Affiliations
Contributions
All authors participated in the design and interpretation of the studies, analyzed the data, and reviewed manuscript; ABM performed maternal deprivation protocol. LAB, MEMB, ABM, ACD, JPD, and JPB performed EE protocol, behavioral tests, and the sample collection. LG, LJ, and FP performed the oxidative stress analysis. GZR, TAC, and COA performed statistical analysis. LMM, MSA, and GZR wrote the manuscript. JQ reviewed the manuscript. GZR did the design of experiment and reviewed the manuscript.
Corresponding author
Ethics declarations
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Réus, G.Z., Abitante, M.S., Manosso, L.M. et al. Environmental Enrichment Rescues Oxidative Stress and Behavioral Impairments Induced by Maternal Care Deprivation: Sex- and Developmental-Dependent Differences. Mol Neurobiol 60, 6757–6773 (2023). https://doi.org/10.1007/s12035-021-02588-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02588-3