Abstract
Alzheimer’s disease (AD) is a devastating brain disorder characterized by neurofibrillary tangles and amyloid plaques. Inhibiting Tau protein and amyloid-beta (Aβ) production or removing these molecules is considered potential therapeutic strategies for AD. Genipin is an aglycone and is isolated from the extract of Gardenia jasminoides Ellis fruit. In this study, the effect and molecular mechanisms of genipin on the inhibition of Tau aggregation and Aβ generation were investigated. The results showed that genipin bound to Tau and protected against heparin-induced Tau fibril formation. Moreover, genipin suppressed Tau phosphorylation probably by downregulating the expression of CDK5 and GSK-3β, and activated mTOR-dependent autophagy via the SIRT1/LKB1/AMPK signaling pathway in Tau-overexpressing cells. In addition, genipin decreased Aβ production by inhibiting BACE1 expression through the PERK/eIF2α signaling pathway in N2a/SweAPP cells. These data indicated that genipin could effectively lead to a significant reduction of phosphorylated Tau level and Aβ generation in vitro, suggesting that genipin might be developed into an effective therapeutic complement or a potential nutraceutical for preventing AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a devastating and irreversible progressive brain illness and is the most common type of dementia diagnosed in elderly individuals. The characteristic pathologies of AD are the abnormal accumulation of Aβ and the hyperphosphorylation of Tau in the brain, which probably lead to synaptic damage and neuronal dysfunction and finally result in neuronal death and cognitive decline [1, 2]. Based on the complex pathobiology of AD, numerous possible solutions have been proposed as potential treatment strategies, including removing Aβ and Tau proteins or inhibiting their formation [3]. A large investment has been put into exploring effective therapies for AD treatment, and there are more than 100 compounds being implemented in different stages of clinical trials [4] [5]. For instance, methylene blue has been reported to reduce tau pathology and neuron death in the Tau transgenic mouse model, while the second-generation derivative of methylene blue (LMTM) was not effective in two different phase 3 trials [6, 7]. Aducanumab, a fully human IgG1 antibody, was confirmed to actively clear Aβ but deemed ineffective for AD treatment in March 2019 in two phase 3 trials. Tideglusib, a GSK-3 inhibitor, could reduce both Tau phosphorylation and amyloid deposition in transgenic mice [8], but a phase 2 trial showed no effect of this drug on decreasing the speed of cognitive or functional decline [9]. Despite the very large effort put in AD medication development, there is currently no disease-modifying treatment [4]. Therefore, it is now more important than ever to identify novel and effective therapeutics for AD.
Genipin is an aglycone derived from the iridoid glycoside, geniposide, which is isolated from the extract of G. jasminoides Ellis fruit [10]. Genipin is commonly used as a traditional Chinese medicine as an antidiabetic, anticancer, and antioxidant agent and for the treatment of inflammation-driven diseases [10]. Additionally, some findings have revealed that the G. jasminoides fruit extract (GFE) and genipin have neuroprotective effects. For example, the crude GFE can improve learning and memory abilities in mouse and rat models of dementia [11]. Geniposide can inhibit the cytotoxicity induced by Aβ1-42, regulate the metabolism of Aβ and Tau phosphorylation in vitro and in vivo, and improve memory in APP/PS1 transgenic mice [12,13,14,15,16,17]. Moreover, genipin can protect Neuro2a cells against cytotoxicity induced by a calcium ionophore in vitro [18]. It has been suggested that genipin might prevent the neurodegeneration observed in AD by attenuating endoplasmic reticulum (ER) stress. However, the molecular mechanisms through which genipin affects Aβ and Tau pathology in AD are not yet clearly understood.
In this study, the inhibitory effects of genipin on Tau phosphorylation in Tau-overexpressing cells and on Aβ production in N2a/SweAPP cells were investigated. Furthermore, the expression levels of protein kinases and autophagy-related proteins were also explored to reveal the molecular mechanisms through which genipin protects against AD.
Materials and Methods
Materials
Genipin was purchased from MedChemExpress (Monmouth Junction, NJ, USA). Tau-R3 was obtained from ChinaPeptides (Shanghai, China). Heparin sodium salt was obtained from Aladdin (Shanghai, China). Thioflavin T (ThT) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle medium (DMEM), F12-DMEM, opti-MEM, neurobasal medium, B27 supplement, streptomycin, penicillin, L-glutamine, and phosphate buffer solution (PBS) were purchased from Gibco (Grand Island, NY, USA). Fetal bovine serum (FBS) was supplied from Biological Industries (Kibbutz Beit HaEmek, Israel). The cell counting kit (CCK)-8 and bicinchoninic acid (BCA) protein assay kit were provided by Beyotime (Jiangsu, China). Protease and phosphatase inhibitors were obtained from Bimake (Shanghai, China). The following antibodies were used in this study: anti-Tau, anti-phospho-T231, anti-phospho-S396, anti-phospho-S404, anti-CDK5, anti-phospho-GSK-3β (Tyr216), anti-LC3, anti-amyloid precursor protein (APP), anti-Aβ, anti-BACE1, and anti-β-actin (Abcam, Cambridge, UK); anti-p62, anti-Beclin-1, anti-SIRT1, anti-LKB1, anti-phospho-LKB1, anti-AMPK, anti-phospho-AMPK, anti-mTOR, anti-phospho-mTOR, anti-p70S6K, anti-phospho-p70S6K, anti-PERK, anti-phospho-PERK, anti-eIF2α, and anti-phospho-eIF2α (Cell Signaling Technology, Beverly, MA, USA).
ThT Fluorescence Assay
ThT fluorescence assay was performed according to the method of our previous study [19]. Tau-R3 was prepared freshly and mixed with heparin (16-μM heparin and 20-μM ThT in 50 mM PBS). Genipin or dimethyl sulfoxide (DMSO) was then added to the mixture. After mixing, the samples were immediately incubated at 37°C and analyzed with a microplate reader (Fluoroskan Ascent FL, Thermo Scientific, USA) at different time points. The ThT fluorescence of the samples was measured with an excitation wavelength of 440 nm and an emission wavelength of 485 nm.
Transmission Electron Microscopy (TEM)
The procedure of TEM was performed based on our previous study with some modifications [19]. Tau-R3 (20 μM) and heparin (16 μM) were incubated with or without genipin (20 μM) at 37°C for 24 h. Then, one drop of the sample was deposited on copper grids (230 mesh, 5-μm aperture, Beijing Zhongjingkeyi Technology Co., China) and allowed to dry at 25°C. After rinsing twice with water, the grids were dyed with 5-μL 1% uranyl acetate. Then, the excess solution was removed using filter paper, and the grids were dried at room temperature (RT). The grids were analyzed using a JEM-1230 transmission electron microscope (JEOL, Tokyo, Japan).
Molecular Docking
Molecular docking studies between genipin and the human Tau protein were examined with SYBYL-X 2.0 software. The three-dimensional (3D) coordinate of the Tau protein (PDB ID: 5O3L) was downloaded from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (PDB) (http://www.rcsb.org/pdb/home/home.do). The 2D structure of genipin was drawn using ChemBioDraw Ultra 14.0 software and then optimized by ChemBio3D Ultra 14.0 software with the MM2 method to obtain the 3D structure. The Surflex-Dock program was used to identify the potential interaction modes between genipin and the human Tau protein. Visualization of the docked conformation was performed by Chimera molecular graphics software (http://www.cgl.ucsf.edu/chimera/) and LIGPLOT (http://www.ebi.ac.uk/thornton-srv/software/LigPlus/).
Cell Culture
The human embryonic kidney 293 (HEK293)/Tau cells were obtained from HEK293 cells (Shanghai Cell Bank of the Chinese Academy of Sciences, Shanghai, China) stably expressing cDNA of the longest human Tau (Tau441) and cultured in DMEM. The SH-SY5Y/Tau cells were obtained from human neuroblastoma cells (SH-SY5Y, Shanghai Cell Bank of the Chinese Academy of Sciences, Shanghai, China) stably expressing Tau441 cDNA and propagated in DMEM mixed with nutrient F12 (25:18, v/v) supplemented with 1% nonessential amino acids, 1% L-glutamine, and 1% sodium pyruvate. Mouse neuroblastoma N2a cells (Shanghai Cell Bank of the Chinese Academy of Sciences, Shanghai, China) overexpressing the Swedish mutant APP (N2a/SweAPP cells) were grown in 50% opti-MEM and 40% DMEM. In addition, these cells were grown with 10% FBS, 100 μg/mL streptomycin, and 100 IU/mL penicillin and incubated in a humidified 5% CO2 atmosphere at 37°C.
Primary Neuron Culture
Primary neuron culture referred to our previous study [20]. Briefly, the hippocampi of newborn 3×Tg-AD mice (JAX order number 3591206, Bar Harbor, ME, USA) were obtained and then were cut into pieces using a scalpel and digested by papain (2 mg/mL) at 37°C for 30 min. The suspension was filtered and centrifuged at 1000 rpm for 5 min. The primary neuronal cells were obtained and seeded in poly-L-lysine (0.1 mg/mL)-coated culture flasks, and were grown in neurobasal medium with 2% B27 supplement, 1% L-glutamine, penicillin, and streptomycin at 37°C in 5% CO2.
Cell Viability Assay
Cell viability was evaluated using a CCK-8 assay. In brief, HEK293/Tau cells, SH-SY5Y/Tau cells, or N2a/SweAPP cells in 96-well plates (1×105 cells/well) were pretreated with various concentrations of genipin (0, 5, 10, 20, 30, and 40 μM) for 24 h. Then, the cells were incubated with CCK-8 solution approximately 2 h after removing the culture supernatant. Finally, the absorption was measured by a microplate reader (BioTek, Vermont, USA) at 450 nm.
Western Blot Analysis
After treatment with genipin (0, 10, 20, and 40 μM) for 24 h, total protein was collected from HEK293/Tau cells, SH-SY5Y/Tau cells, or N2a/SweAPP cells using lysis buffer containing 1% protease and phosphatase inhibitor. Then, the protein concentration was quantitated by a protein assay kit, and the protein was separated by SDS-PAGE. The protein was blotted onto nitrocellulose (NC) membranes (Merch/Millipore, Schwalbach, Germany) and blocked with 5% bovine serum albumin. After hybridization with primary antibodies (1:1000) at 4°C overnight, the NC membranes were washed with PBS three times and hybridized with secondary antibodies (1:5000) at RT for 1 h. Subsequently, the immunoreactive protein was analyzed using an electroluminescence kit (Thermo Fisher Scientific, Waltham, MA, USA).
Immunofluorescence Staining
After 40 μM genipin treatment for 24 h, HEK293/Tau cells or the primary neuronal cells of 3×Tg-AD mice were fixed with methanol for 10 min on ice. Then, the cells were incubated with 10% goat serum and 0.1% Triton X-100 at RT for 1 h. After incubation with primary antibodies at 4°C overnight, the cells were rinsed with PBS three times and treated with Alexa Fluor-conjugated secondary antibody at RT for 1 h. Then, the secondary antibody was removed, and the cells were rinsed with PBS three times. After the cell nuclei were dyed with DAPI (Invitrogen, Carlsbad, CA) at RT in a dark room, micrographs of cells were visualized using a confocal microscopy (Carl Zeiss, Thornwood, NY, USA).
Statistical Analysis
The data are expressed as the mean ± standard deviation (SD) and were processed by GraphPad Prism 6.0. Significant differences were analyzed using a two-tailed Student’s t-test. When p values are < 0.05, differences were considered to reach statistical significance: * p < 0.05, ** p < 0.01, *** p < 0.001.
Results
Genipin Inhibited Heparin-Induced Fibrillar Tau Aggregation and Interacted with Tau Protein
First, the effect of genipin on fibrillar Tau aggregation was determined by investigating the kinetics of Tau-R3 aggregation using a ThT fluorescence assay. The fluorescence increased with increasing processing time, and the fluorescence of the genipin-treated groups was dose-dependently decreased compared with that of the control group (Fig. 1a). As illustrated in Fig. 1b, a large reduction in the number of Tau filaments was observed in the genipin-treated group in the electron micrographs, which was consistent with the findings of the ThT fluorescence assay. These results demonstrated that genipin effectively inhibited Tau-R3 aggregation in ThT fluorescence and TEM assays.
Effect of genipin on heparin-induced fibrillar Tau aggregation and the latent binding sites of genipin in the Tau protein. a ThT fluorescence time course of Tau-R3 aggregation with genipin (0, 5, 10, 20, and 40 μM). The control in “% of Ctrl” was the fluorescence intensity of the control group at 25 h incubation. b Electron micrographs of Tau-R3 aggregation with genipin (20 μM). Scale bar=500 nm. c Molecular modeling of the interaction between genipin and Tau protein. d LIGPLOT representations of the interactions between genipin and Tau protein. The hydrogen bonds are shown using green dashed lines. Representative results from at least three independent experiments are shown
To find a more desirable binding site of genipin on the Tau protein and to identify its theoretical binding mode, molecular docking-based calculations were performed, as illustrated in Fig. 1c and d. It was demonstrated that genipin interacted with the hydrophobic pocket of the Tau protein and was surrounded by the residues His-362, Gly-367, Asn-368, and Lys-369. The His-362, Gly-367, and Asn-368 residues were involved in the formation of the hydrophobic bond with genipin. Moreover, two hydrogen bonds were found between genipin and Lys-369, and the bond lengths were 2.72 and 2.75 Å (Fig. 1d). These interactions indicated that genipin anchored to the R3 domain of the Tau protein, leading to the obstruction of Tau protein assembly.
Genipin Attenuated Phosphorylated Tau Levels and Protein Kinase Expression in AD Cell Models
Both SH-SY5Y/Tau cells and HEK293/Tau cells overexpress the cDNA of the longest human Tau (Tau441) and were used as cell models to investigate the effects of genipin on Tau phosphorylation. After treatment with genipin for 24 h, cell viability was not affected, which indicated that genipin was not cytotoxic to SH-SY5Y/Tau cells and HEK293/Tau cells even at the maximum dose of 40 μM genipin (Fig. 2a). As shown in Fig. 2b and c, the primary neuronal cells of 3×Tg-AD mice more completely simulate the neuropathology of the disease in humans, which can be further used to evaluate the effect of genipin on Tau phosphorylation inhibition. As shown in Fig. 3, genipin treatment significantly reduced the fluorescence staining of pS404-Tau in HEK293/Tau cells and in the primary neuronal cells of 3×Tg-AD mice. These results suggested that genipin reduced the expression of Tau phosphorylation in the abovementioned kinds of Tau-overexpressing cells and in the primary neuronal cells of 3×Tg-AD mice.
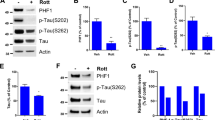
Effect of genipin on the expression of phosphorylated Tau and protein kinase in SH-SY5Y/Tau cells and HEK293/Tau cells. a Adherent SH-SY5Y/Tau cells and HEK293/Tau cells (1×104 cells/well) in a 96-well plate were treated with genipin (0, 5, 10, 20, 30, and 40 μM) for 24 h, and cell viability was analyzed using a CCK-8 assay. b After genipin treatment (0, 10, 20, and 40 μM) for 24 h, the expression of Tau, pT231-Tau, pS396-Tau, and pS404-Tau in SH-SY5Y/Tau cells and HEK293/Tau cells was investigated by Western blot analysis. c The densitometric analysis of data from b. d After genipin treatment (0, 10, 20, and 40 μM) for 24 h, the expression of CDK5 and pY216 GSK-3β in SH-SY5Y/Tau cells and HEK293/Tau cells was investigated by Western blot analysis. e The densitometric analysis of data from d. Representative results from at least three independent experiments are shown
Effect of genipin on Tau phosphorylation in HEK293/Tau cells and primary neuronal cells of 3×Tg-AD mice. a HEK293/Tau cells were treated with genipin (40 μM) for 24 h, and the expression of pS404-Tau was analyzed by immunofluorescence. Scale bar=10 μm. b After genipin treatment (40 μM), primary neuronal cells from 3×Tg-AD mice were stained by dual-color immunofluorescent staining. Scale bar=50 μm. Representative results from at least three independent experiments are shown
CDK5 and GSK-3β are two major Tau kinases and play an important role in the abnormal hyperphosphorylation of the microtubule-associated protein Tau. Thus, the contribution of these two protein kinases to genipin-induced Tau changes was investigated. After treatment with genipin for 24 h, the expression levels of CDK5 and pY216 GSK-3β in SH-SY5Y/Tau cells and HEK293/Tau cells were significantly reduced in a dose-dependent manner (Fig. 2d and e). Thus, the effect of genipin on the inhibition of Tau phosphorylation in SH-SY5Y/Tau cells and HEK293/Tau cells was probably due to a decrease in protein kinase expression.
Genipin Upregulated the Level of Autophagy in SH-SY5Y/Tau Cells and HEK293/Tau Cells
Furthermore, autophagy is also a main pathway for clearing phosphorylated Tau, and Beclin-1 and LC3 are markers of autophagy. As presented in Fig. 4a and b, genipin treatment significantly increased Beclin-1 and LC3 II/LC3 I expression and inhibited p62 expression in SH-SY5Y/Tau cells and HEK293/Tau cells. These results suggested that genipin increased the level of autophagy in SH-SY5Y/Tau cells and HEK293/Tau cells, which might be involved in the decrease in Tau phosphorylation.
Effect of genipin on autophagy level in AD cells. a After genipin treatment (0, 10, 20, and 40 μM) for 24 h, the expression of Beclin-1, LC3 II/LC3 I, and p62 in SH-SY5Y/Tau cells and HEK293/Tau cells was analyzed by Western blot analysis. b The densitometric analysis of data from a. c After genipin treatment (0, 10, 20, and 40 μM) for 24 h, the expression of SIRT1, p-LKB1, LKB1, p-AMPK, AMPK, p-mTOR, mTOR, p-p70S6K, and p70S6K in SH-SY5Y/Tau cells and HEK293/Tau cells was investigated by Western blot analysis. (d) The densitometric analysis of data from c. Representative results from at least three independent experiments are shown
To determine the mechanism by which genipin was able to increase the autophagy level, the expression of the proteins related to the SIRT1/LKB1/AMPK signaling pathway was evaluated by Western blot analysis. After genipin treatment for 24 h, the expression of SIRT1, p-LKB1, and p-AMPK was significantly increased, whereas the phosphorylation of mTOR and p70S6K (the direct substrate of mTOR) was simultaneously decreased in SH-SY5Y/Tau cells and HEK293/Tau cells (Fig. 4c and d). These results indicated that genipin could upregulate autophagy by activating the SIRT1/LKB1/AMPK signaling pathway in SH-SY5Y/Tau cells and HEK293/Tau cells.
Genipin Suppressed Aβ Generation in N2a/SweAPP Cells
In addition to Tau pathology, Aβ accumulation is another primary pathological feature associated with AD. Therefore, the effect of genipin on Aβ production was next evaluated in N2a/SweAPP cells. As shown in Fig. 5a, genipin had no cytotoxicity in N2a/SweAPP cells, even at a maximum concentration of 40 μM genipin. Genipin treatment caused a dose-dependent inhibitory effect on the expression of APP and Aβ in N2a/SweAPP cells (Fig. 5b and c). Additionally, genipin treatment significantly reduced the fluorescence staining of Aβ in N2a/SweAPP cells (Fig. 5d). To further identify the mechanism by which genipin inhibited the expression levels of APP and Aβ, the expression of proteins related to the PERK/eIF2α signaling pathway was measured. As shown in Fig. 5e and f, the expression of p-PERK, p-eIF2α, and BACE1 was dose-dependently decreased after genipin treatment in N2a/SweAPP cells. Overall, these results demonstrated that genipin could inhibit Aβ production by inhibiting BACE1 expression through the PERK/eIF2α signaling pathway in N2a/SweAPP cells.
Effect of genipin on Aβ generation in N2a/SweAPP cells. a Adherent N2a/SweAPP cells (1×104 cells/well) in 96-well plates were treated with genipin (0, 5, 10, 20, 30, and 40 μM) for 24 h, and cell viability was analyzed using a CCK-8 assay. b After genipin treatment (0, 10, 20, and 40 μM) for 24 h, the expression of APP and Aβ in N2a/SweAPP cells was investigated by Western blot analysis. c The densitometric analysis of data from b. d N2a/SweAPP cells were treated with genipin (40 μM) for 24 h, and the level of pS404-Tau was measured by immunofluorescence. Scale bar=10 μm. e After genipin treatment (0, 10, 20, and 40 μM) for 24 h, the expression of p-PERK, PERK, p-eIF2α, eIF2α, and BACE1 in N2a/SweAPP cells was investigated by Western blot analysis. f The densitometric analysis of data from e. Representative results from at least three independent experiments are shown
Discussion
At present, a large number of phase 3 clinical trials have failed to demonstrate benefits of potential treatments, and effective treatments are still lacking for AD. Novel drugs and therapies for AD are urgently needed for the aging population around the world. Genipin is an active ingredient isolated from GFE that exerts anticancer, anti-inflammatory, antidiabetic, and neuroprotective effects [10]. Recent investigations have proposed that genipin has protective effects against Aβ-induced cytotoxicity and tunicamycin (a specific ER stress inducer)-induced cytotoxicity in Neuro2a cells [21, 22]. However, the effects of genipin on Aβ and Tau pathology in AD models have not been reported to date. In the present study, the inhibitory effects of genipin on Tau hyperphosphorylation and Aβ generation were evaluated, and the involved mechanisms were also revealed. The confirmed results of this study will further provide a reference and basis for genipin as a potential drug or nutraceutical for preventing AD-related pathology.
In AD, the formation of NFTs and the accumulation of senile plaques in the brain are the main pathological characteristics [23]. In the “pre-tangle” phase of neurofibrillary degeneration, the abnormal phosphorylation, aggregation, and proteolysis of the Tau protein have been confirmed to be early and important aspects of the pathogenesis of AD [24]. Tau abnormally phosphorylates and aggregates and subsequently forms NFTs, which cause synapse loss and axonal transport damage, resulting in mitochondrial and cytoskeletal dysfunction [25]. Thus, reducing Tau hyperphosphorylation or aggregation is considered a potential neuroprotective strategy. The current therapeutic agents related to Tau pathology include LMTX (a novel stabilized reduced form of methylthioninium, a Tau aggregation inhibitor), salsalate (Tau acetylation inhibitor), and TPI-287 (Taxol-derived compound, a microtubule-stabilizing drug) in clinical trials [26]. In this study, the inhibitory effect of genipin on Tau-R3 aggregation was evidenced by a ThT fluorescence assay and TEM (Fig. 1a and b). Based on the molecular docking results, genipin anchored at the R3 domain of the Tau protein, leading to an inhibitory effect on Tau aggregation (Fig. 1c and d). From the above results, genipin might have a beneficial effect on Tau pathology-related neurodegenerative disorders.
Moreover, the effect of genipin on attenuating Tau phosphorylation was found in Tau-overexpressing cells and 3×Tg-AD mouse primary neuron cells (Fig. 2 and 3). These results are consistent with a previous study that claimed that geniposide can reduce Tau phosphorylation by approximately 30% in a streptozotocin-induced AD rat model [12]. Thus, genipin, an aglycon of geniposide, may effectively regulate Tau phosphorylation. CDK5 and GSK-3β are the two main protein kinases that can phosphorylate Tau sites, including Thr231, Ser396, and Ser404, and participate in the abnormal hyperphosphorylation of the microtubule-associated protein Tau, subsequently leading to AD[27]. For example, the memory of AD mice was improved by CDK5 silencing [28]. LDC8, a small molecule, protected neurons and their processes in zebrafish models by inhibiting CDK5 and GSK-3β [29]. In the present study, the expression of CDK5 and pY216 GSK-3β was decreased in SH-SY5Y/Tau cells and HEK293/Tau cells after genipin treatment (Fig. 2d and e), which may be responsible for downregulating the level of Tau phosphorylation.
Autophagy, a lysosome-dependent process, also called autophagic flux, is also a main pathway for clearing Tau phosphorylation. In the process of autophagy, Beclin-1 plays a central role in the early phase, while cytosolic LC3-I combines with phosphatidylethanolamine and then produces membrane-bound LC3-II [30]. p62 is a multiubiquitin chain-binding protein and is involved in protein degradation through the ubiquitin-proteasome system or autophagy pathway [31]. Indeed, the autophagic substrate p62 directly interacts with LC3 and is sequestered into autophagosomes for degradation. Therefore, measuring the decline in p62 is used to further evaluate autophagic flux [32]. In this study, the findings showed that genipin increased the expression levels of Beclin-1 and LC3 II/LC3 I and simultaneously reduced the expression of p62 in SH-SY5Y/Tau cells and HEK293/Tau cells (Fig. 4a and b). LC3-II plays an essential role in the formation of autophagosomes, and LC3-II expression is closely related to the number of autophagosomes [33, 34]. These results indicated that genipin could enhance autophagosome formation and then interacted with p62 for degradation. Furthermore, it is known that the mechanistic target of rapamycin (mTOR) is able to sense intracellular nutrients and growth factors, and regulates autophagy. AMPK is considered a crucial intracellular energy sensor and important checkpoint of mTOR activity and autophagy, and the activation of AMPK is mainly regulated by the upstream kinase LKB1 [35, 36]. LKB1 can phosphorylate AMPK with the deacetylation of its lysine residue by SIRT1 [37]. The p70S6K is located downstream of the mTOR signal transduction pathway and plays an important role in the regulation of cell cycle, growth, and survival [38]. Our experimental results suggested that genipin was able to activate autophagy via the SIRT1/LKB1/AMPK signaling pathway and simultaneously inhibited the phosphorylation of mTOR and its downstream p70S6K in SH-SY5Y/Tau cells and HEK293/Tau cells (Fig. 4c and d). Some previous studies suggested that genipin can induce autophagy through the p53-DRAM signaling pathway in gastric cancer treatment or via the PI3K/AKT/mTOR signaling pathway in oral squamous cell carcinoma treatment [39, 40]. Herein, genipin was also confirmed to induce autophagy, which may further be involved in regulating Tau phosphorylation.
mTOR signaling is currently considered one of the most promising targets in autophagy-related AD treatment [41, 42]. In addition to Tau phosphorylation, Aβ accumulation is another typical pathological feature of AD, and Aβ is neurotoxic to brain cells. Senile plaques are composed mostly of Aβ, which is derived from APP [43]. Previous studies have reported that genipin protects neuronal cells against the cytotoxicity mediated by ER stress [22]. In this study, the dose-dependent inhibitory effect of genipin on the expression of APP and Aβ was shown in N2a/SweAPP cells (Fig. 5b and c). It has been reported that PERK may increase the phosphorylation of eIF2α, leading to the promotion of BACE1 translation and further accelerating Aβ generation [44]. Here, our results demonstrated that genipin treatment could efficiently reduce the Aβ level by decreasing BACE1 expression via the PERK/eIF2α signaling pathway in N2a/SweAPP cells (Fig. 5e and f).
In summary, genipin bound to Tau and inhibited the heparin-induced formation of Tau fibrils, and the underlying impacts of genipin in AD cell models have been intensively investigated. As summarized in Fig. 6, genipin reduced Tau phosphorylation probably by downregulating the expression of CDK5 and GSK-3β, and activated mTOR-dependent autophagy via the SIRT1/LKB1/AMPK signaling pathway in Tau-overexpressing cells. Moreover, genipin could reduce Aβ by suppressing BACE1 expression through the PERK/eIF2α signaling pathway in N2a/SweAPP cells. Therefore, all these findings demonstrated that genipin could effectively attenuate both Tau phosphorylation and Aβ production. This study provides guidance for further illuminating the molecular mechanism of genipin, and highlights the potential application of genipin for the prevention and treatment of AD.
A proposed model showing the molecular mechanism of the efficiency of genipin for AD treatment. Genipin reduced Tau phosphorylation by downregulating the expression of CDK5 and GSK-3β, and induced mTOR-dependent autophagy via the SIRT1/LKB1/AMPK signaling pathway. Moreover, genipin reduced Aβ levels by inhibiting BACE1 expression via the PERK/eIF2α signaling pathway
Abbreviations
- AD :
-
Alzheimer’s disease
- Aβ :
-
amyloid-beta
- ThT :
-
Thioflavin T
- DMEM :
-
Dulbeco’s modified Eagle’s medium
- FBS :
-
fetal bovine serum
- CCK :
-
cell counting kit
- MAP-2 :
-
microtubule-associated protein-2
- CDK5 :
-
cyclin-dependent kinase 5
- GSK-3β :
-
glycogen synthase kinase-3β
- LC 3 :
-
microtubule-associated protein II light chain 3
- SIRT1 :
-
silent information regulator of transcription 1
- LKB1 :
-
liver kinase B1
- AMPK :
-
adenosine monophosphate-activated protein kinase
- mTOR :
-
mechanistic target of rapamycin
- p70S6K :
-
p70 ribosomal protein S6 kinase
- APP :
-
amyloid precursor protein
- PERK :
-
protein kinase RNA-like endoplasmic reticulum kinase
- eIF2α :
-
eukaryotic translation initiation factor-2α
- BACE1 :
-
β-secretase 1
References
Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q, Orr ME (2018) Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 17(6):e12840. https://doi.org/10.1111/acel.12840
Sebastián-Serrano Á, de Diego-García L, Díaz-Hernández M (2018) The neurotoxic role of extracellular tau protein. Int J Mol Sci 19(4). https://doi.org/10.3390/ijms19040998
Nowacek A, Kosloski LM, Gendelman HE (2009) Neurodegenerative disorders and nanoformulated drug development. Nanomedicine (London) 4(5):541–555. https://doi.org/10.2217/nnm.09.37
Long JM, Holtzman DM (2019) Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179(2):312–339. https://doi.org/10.1016/j.cell.2019.09.001
Hara Y, McKeehan N, Fillit HM (2019) Translating the biology of aging into novel therapeutics for Alzheimer disease. Neurology 92(2):84–93. https://doi.org/10.1212/wnl.0000000000006745
Congdon EE, Wu JW, Myeku N, Figueroa YH, Herman M, Marinec PS, Gestwicki JE, Dickey CA et al (2012) Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy 8(4):609–622. https://doi.org/10.4161/auto.19048
Gauthier S, Feldman HH, Schneider LS, Wilcock GK, Frisoni GB, Hardlund JH, Moebius HJ, Bentham P et al (2016) Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet 388(10062):2873–2884. https://doi.org/10.1016/s0140-6736(16)31275-2
Serenó L, Coma M, Rodríguez M, Sánchez-Ferrer P, Sánchez MB, Gich I, Agulló JM, Pérez M et al (2009) A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol Dis 35(3):359–367. https://doi.org/10.1016/j.nbd.2009.05.025
Lovestone S, Boada M, Dubois B, Hüll M, Rinne JO, Huppertz HJ, Calero M, Andrés MV et al (2015) A phase II trial of tideglusib in Alzheimer’s disease. J Alzheimers Dis 45(1):75–88. https://doi.org/10.3233/jad-141959
Shanmugam MK, Shen H, Tang FR, Arfuso F, Rajesh M, Wang L, Kumar AP, Bian J et al (2018) Potential role of genipin in cancer therapy. Pharmacol Res 133:195–200. https://doi.org/10.1016/j.phrs.2018.05.007
Yang N, Liu YY, Hao WY (2010) Effects of crude extract of Cape Jasmine on learning and memory function in experimental dementia animal model. Chin J Rehabil Theory Pract 16(04):308–310. https://doi.org/10.3969/j.issn.1006-9771.2010.04.003
Gao C, Liu Y, Jiang Y, Ding J, Li L (2014) Geniposide ameliorates learning memory deficits, reduces tau phosphorylation and decreases apoptosis via GSK3β pathway in streptozotocin-induced alzheimer rat model. Brain Pathol 24(3):261–269. https://doi.org/10.1111/bpa.12116
Lv C, Wang L, Liu X, Yan S, Yan SS, Wang Y, Zhang W (2015) Multi-faced neuroprotective effects of geniposide depending on the RAGE-mediated signaling in an Alzheimer mouse model. Neuropharmacology 89:175–184. https://doi.org/10.1016/j.neuropharm.2014.09.019
Zhang Y, Xia Z, Liu J, Yin F (2015) Cell signaling mechanisms by which geniposide regulates insulin- degrading enzyme expression in primary cortical neurons. CNS Neurol Disord Drug Targets 14(3):370–377. https://doi.org/10.2174/1871527314666141229110156
Liu Z, Zhang Y, Liu J, Yin F (2017) Geniposide attenuates the level of Aβ(1-42) via enhancing leptin signaling in cellular and APP/PS1 transgenic mice. Arch Pharm Res 40(5):571–578. https://doi.org/10.1007/s12272-016-0875-9
Zhang Y, Yin F, Liu J, Liu Z, Guo L, Xia Z, Zidichouski J (2015) Geniposide attenuates insulin-deficiency-induced acceleration of β-amyloidosis in an APP/PS1 transgenic model of Alzheimer’s disease. Neurochem Int 89:7–16. https://doi.org/10.1016/j.neuint.2015.04.002
Zhang Y, Yin F, Liu J, Liu Z (2016) Geniposide attenuates the phosphorylation of tau protein in cellular and insulin-deficient APP/PS1 transgenic mouse model of Alzheimer’s disease. Chem Biol Drug Des 87(3):409–418. https://doi.org/10.1111/cbdd.12673
Yamazaki M, Chiba K, Yoshikawa C (2009) Genipin suppresses A23187-induced cytotoxicity in neuro2a cells. Biol Pharm Bull 32(6):1043–1046. https://doi.org/10.1248/bpb.32.1043
Cai N, Chen J, Bi D, Gu L, Yao L, Li X, Li H, Xu H et al (2020) Specific degradation of endogenous tau protein and inhibition of tau fibrillation by tanshinone IIA through the ubiquitin–proteasome pathway. J Agric Food Chem 68(7):2054–2062. https://doi.org/10.1021/acs.jafc.9b07022
Bi D, Yao L, Lin Z, Chi L, Li H, Xu H, Du X, Liu Q et al (2021) Unsaturated mannuronate oligosaccharide ameliorates β-amyloid pathology through autophagy in Alzheimer’s disease cell models. Carbohydr Polym 251:117124. https://doi.org/10.1016/j.carbpol.2020.117124
Tanaka M, Yamazaki M, Chiba K (2009) Neuroprotective action of genipin on tunicamycin-induced cytotoxicity in neuro2a cells. Biol Pharm Bull 32(7):1220–1223. https://doi.org/10.1248/bpb.32.1220
Yamazaki M, Sakura N, Chiba K, Mohri T (2001) Prevention of the neurotoxicity of the amyloid beta protein by genipin. Biol Pharm Bull 24(12):1454–1455. https://doi.org/10.1248/bpb.24.1454
Armstrong RA (2009) The molecular biology of senile plaques and neurofibrillary tangles in Alzheimer's disease. Folia Neuropathol 47(4):289–299
Šimić G, Babić Leko M, Wray S, Harrington C, Delalle I, Jovanov-Milošević N, Bažadona D, Buée L et al (2016) Tau protein hyperphosphorylation and aggregation in Alzheimer’s disease and other tauopathies, and possible neuroprotective strategies. Biomolecules 6(1):6. https://doi.org/10.3390/biom6010006
Hashiguchi M, Saito T, Hisanaga S, Hashiguchi T (2002) Truncation of CDK5 activator p35 induces intensive phosphorylation of Ser202/Thr205 of human tau. J Biol Chem 277(46):44525–44530. https://doi.org/10.1074/jbc.M207426200
Gao Y, Tan L, Yu JT, Tan L (2018) Tau in Alzheimer’s disease: mechanisms and therapeutic strategies. Curr Alzheimer Res 15(3):283–300. https://doi.org/10.2174/1567205014666170417111859
Jayapalan S, Natarajan J (2013) The role of CDK5 and GSK3B kinases in hyperphosphorylation of microtubule associated protein tau (MAPT) in Alzheimer’s disease. Bioinformation 9(20):1023–1030. https://doi.org/10.6026/97320630091023
Posada-Duque RA, López-Tobón A, Piedrahita D, González-Billault C, Cardona-Gomez GP (2015) p35 and Rac1 underlie the neuroprotection and cognitive improvement induced by CDK5 silencing. J Neurochem 134(2):354–370. https://doi.org/10.1111/jnc.13127
Reinhardt L, Kordes S, Reinhardt P, Glatza M, Baumann M, Drexler HCA, Menninger S, Zischinsky G et al (2019) Dual inhibition of GSK3β and CDK5 protects the cytoskeleton of neurons from neuroinflammatory-mediated degeneration in vitro and in vivo. Stem Cell Rep 12(3):502–517. https://doi.org/10.1016/j.stemcr.2019.01.015
Zheng S, Han F, Shi Y, Wen L, Han D (2017) Single-prolonged-stress-induced changes in autophagy-related proteins Beclin-1, LC3, and p62 in the medial prefrontal cortex of rats with post-traumatic stress disorder. J Mol Neurosci 62(1):43–54. https://doi.org/10.1007/s12031-017-0909-x
Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, Elazar Z (2010) LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J 29(11):1792–1802. https://doi.org/10.1038/emboj.2010.74
Sahani MH, Itakura E, Mizushima N (2014) Expression of the autophagy substrate SQSTM1/p62 is restored during prolonged starvation depending on transcriptional upregulation and autophagy-derived amino acids. Autophagy 10(3):431–441. https://doi.org/10.4161/auto.27344
Peidu J (2014) Autophagy and human diseases. Cell Res 24:69–79. https://doi.org/10.1038/cr.2013.161
Narendra D, Kane LA, Hauser DN, Fearnley IM, Youle RJ (2010) p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy 6(8):1090–1106. https://doi.org/10.4161/auto.6.8.13426
Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC (2004) The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A 101(10):3329–3335. https://doi.org/10.1073/pnas.0308061100
Ganesan R, Hos NJ, Gutierrez S, Fischer J, Stepek JM, Daglidu E, Krönke M, Robinson N (2017) Salmonella Typhimurium disrupts Sirt1/AMPK checkpoint control of mTOR to impair autophagy. PLoS Pathog 13(2):e1006227. https://doi.org/10.1371/journal.ppat.1006227
Lan F, Cacicedo JM, Ruderman N, Ido Y (2008) SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem 283(41):27628–27635. https://doi.org/10.1074/jbc.M805711200
Couch FJ, Wang X-Y, Wu G-J, Qian J, Jenkins RB, James CD (1999) Localization of <em>PS6K</em> to chromosomal region 17q23 and determination of its amplification in breast cancer. Cancer Res 59(7):1408–1411
Kim BR, Jeong YA, Kim DY, Kim JL, Jeong S, Na YJ, Yun HK, Park SH et al (2020) Genipin increases oxaliplatin-induced cell death through autophagy in gastric cancer. J Cancer 11(2):460–467. https://doi.org/10.7150/jca.34773
Wei M, Wu Y, Liu H, Xie C (2020) Genipin induces autophagy and suppresses cell growth of oral squamous cell carcinoma via PI3K/AKT/MTOR pathway. Drug Des Devel Ther 14:395–405. https://doi.org/10.2147/dddt.S222694
Uddin MS, Stachowiak A, Mamun AA, Tzvetkov NT, Takeda S, Atanasov AG, Bergantin LB, Abdel-Daim MM et al (2018) Autophagy and Alzheimer’s disease: from molecular mechanisms to therapeutic implications. Front Aging Neurosci 10:04. https://doi.org/10.3389/fnagi.2018.00004
Sun YX, Ji X, Mao X, Xie L, Jia J, Galvan V, Greenberg DA, Jin K (2014) Differential activation of mTOR complex 1 signaling in human brain with mild to severe Alzheimer’s disease. J Alzheimers Dis 38(2):437–444. https://doi.org/10.3233/jad-131124
Nilsson P, Loganathan K, Sekiguchi M, Matsuba Y, Hui K, Tsubuki S, Tanaka M, Iwata N et al (2013) Aβ secretion and plaque formation depend on autophagy. Cell Rep 5(1):61–69. https://doi.org/10.1016/j.celrep.2013.08.042
Rozpedek W, Markiewicz L, Diehl JA, Pytel D, Majsterek I (2015) Unfolded protein response and PERK kinase as a new therapeutic target in the pathogenesis of Alzheimer’s disease. Curr Med Chem 22(27):3169–3184. https://doi.org/10.2174/0929867322666150818104254
Acknowledgements
The authors thank the Instrumental Analysis Center of Shenzhen University (Xili Campus) for their assistance in our experiments.
Availability of Data and Material
All data is real and guarantees the validity of experimental results.
Funding
This work was supported financially by National Natural Science Foundation of China (31871734), National Key R&D Program of China (2018YFD0901106), National Natural Science Foundation of China (31970366), Guangdong Natural Science Foundation (2018A0303130054 and 2018A030313507), the Science and Technology Innovation Commission of Shenzhen (JCYJ20190808141415052, JCYJ20180507182405562, JCYJ20180305124211995 and JCYJ20180305125619343), National Key Project for Synthetic Biology (SQ2018YFA090029).
Author information
Authors and Affiliations
Contributions
Meiting Li, Nan Cai, Liang Gu, Hui Li, and Zhangli Hu conceived and designed the project. Meiting Li, Nan Cai, Liang Gu, Lijun Yao, and Decheng Bi performed majority of the experiments. Meiting Li, Nan Cai, Weishan Fang, Zhijian Lin, and Hong Xu performed data analyzes. Meiting Li, Nan Cai, Xu Xu, Weishan Fang, Zhijian Lin, Hong Xu, Hui Li, and Zhangli Hu wrote the manuscript. Xu Xu, Meiting Li, Nan Cai, and Yan Wu revised the paper. Xu Xu supervised the paper.
Corresponding author
Ethics declarations
Compliance with Ethical Standards
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 2480 kb).
Rights and permissions
About this article
Cite this article
Li, M., Cai, N., Gu, L. et al. Genipin Attenuates Tau Phosphorylation and Aβ Levels in Cellular Models of Alzheimer’s Disease. Mol Neurobiol 58, 4134–4144 (2021). https://doi.org/10.1007/s12035-021-02389-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02389-8