Abstract
The pathophysiology of bipolar disorder remains incompletely elucidated. The purinergic receptor, P2X7 (P2X7R), plays a central role in neuroinflammation, the establishment, and maintenance of microglial activation and neuronal damage/death, all characteristics of bipolar disorder pathology. The present study aims to explore the participation of the P2X7R in a preclinical pharmacological model of mania. We analyzed the modulatory effects of the P2X7R antagonist, brilliant blue, on behavior, monoamines, gene expression, serum purine levels, and cell typing in a pharmacological model of mania induced by d-amphetamine (AMPH) in mice. Our results corroborate an association between the P2X7 receptor and the preclinical animal model of mania, as demonstrated by the decreased responsiveness to AMPH in animals with pharmacologically blocked P2X7R. This study further suggests a possible dopaminergic mechanism for the action of P2X7 receptor antagonism. Additionally, we observed increased peripheral levels of adenosine, a neuroprotective molecule, and increased central expression of Entpd3 and Entpd1 leading to the hydrolysis of ATP, a danger signal, possibly as an attempt to compensate for the damage induced by AMPH. Lastly, P2X7R antagonism in the AMPH model was found to potentially modulate astrogliosis. Our results support the hypothesis that P2X7R plays a vital role in the pathophysiology of mania, possibly by modulating the dopaminergic pathway and astrogliosis, as reflected in the behavioral changes observed. Taken together, this study suggests that a purinergic system imbalance is associated with the AMPH-induced preclinical animal model of mania. P2X7R may represent a promising molecular therapeutic target for bipolar disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Emil Kraeplin, a fundamental psychiatrist of the mid-nineteenth century, not only contributed to the nosology of bipolar disorder [1] but also suggested an association between manic symptoms and levels of uric acid in the pathology of the disease, what later was described as being part of the purinergic system [2]. Purinergic signaling molecules include nucleotides and nucleosides, a large family of ectonucleotidases and purinoreceptors [3], and this signaling is implicated in physiological and pathological conditions, with relevant functions in secretion, immune responses, cell death and inflammation [4]. Purinergic pathophysiology, mainly in the form of adenosine 5’-triphosphate (ATP) and adenosine signaling, has been related to numerous medical conditions, especially neurodegenerative and psychiatric disorders, including bipolar disorder [5, 6]. Since the pathophysiology of bipolar disorder remains incompletely elucidated [7], understanding the role of the purinergic system in this disorder may clarify some of its molecular mechanisms.
Notably, the purinergic receptor P2X7 (P2X7R) has a potential role in the pathophysiology of bipolar disorder [8, 9]. P2X7R is an ATP–binding ligand-gated ion channel that is activated by high concentrations of extracellular ATP, what is considered a danger signal, a damage-associated molecular patterns (DAMPs) [10]. The activation of this receptor has a central function at inflammasome formation which leads to increased levels of proinflammatory cytokines, such as interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and interleukin-1beta (IL-1β). It is also crucial for the onset and maintenance of microglial activation and neuronal damage/death, making a critical contribution in mediating neuroinflammation and excitotoxicity [11], conditions that are possibly involved in the bipolar disorder process [12,13,14].
Additionally, it has been shown that the gene coding for P2X7R is located on a susceptibility locus associated with bipolar disorder, with specific polymorphisms associated with the disease [15]. Recently, it was demonstrated a differential 1513A>C P2RX7 polymorphism frequency in BD patients, compared with controls, indicating an enhanced function of the pro-inflammatory P2X7R in BD subjects, further suggesting that a polymorphism in P2X7R gene could be a potential biomarker for the prediction and diagnosis of the disorder [16]. The relationship between this receptor and BD has been also verified in animal models of mania, and blockade/deletion of P2X7R has been shown to decrease d-amphetamine (AMPH)–induced hyperactivity [17, 18], also preventing the establishment of excitotoxicity and proinflammatory states induced by the model [9].
Although these studies have demonstrated the association between P2X7R and bipolar disorder, the mechanism of this association is unclear. The present study aims to explore the modulatory effects of P2X7R on assessments related to behavior, monoamines, gene expression, serum purine levels and cell typing in a preclinical pharmacological model of mania induced by AMPH.
Methods and Materials
Animals
Sixty-four male C57BL/6 mice (aged 6–8 weeks; weight 18–25 g) were used in all experiments. The animal handling pattern and experiment details follow those of a previous study [9]. Animals were maintained in a 12:12 light-dark cycle, with food and water ad libitum. All the experiments were performed during the light phase. The experimental procedures reported in this manuscript were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Brazilian College of Animal Experimentation. The Animal Ethics Committee of the Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil, approved this project under protocol number 15-0192.
Drugs and Treatment
d-amphetamine sulfate salt (AMPH) and all other drugs were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless indicated otherwise. The pharmacological animal model of mania induced by AMPH was adapted from previous studies [9, 19, 20] and performed as follows: mice received intraperitoneal (i.p.) injections of AMPH (2 mg/kg) or vehicle (saline, 0.9% NaCl) once a day for seven consecutive days. Mice also received intraperitoneal (i.p.) injections of the non-selective P2X7R antagonist, Brilliant Blue G (BBG) (45 mg/kg), or vehicle (saline, 0.9% NaCl), 30 min before the AMPH or vehicle injection, during the seven consecutive days. Behavioral tests were not performed between days 1 and 6. On the seventh day, immediately after the last injection, mice were subjected to the open field apparatus. After the behavioral test, the animals were euthanized by an anesthetic overdose of the 12% isoflurane inhalation until cardiorespiratory arrest, according to the guidelines of the Brazilian Conselho Nacional de Controle de Experimentação Animal (CONCEA) euthanasia practice, 2013. The hippocampal and striatal brain structures were dissected for further analysis.
Open Field Test
Locomotor activity was assessed using the open field test, as previously described [9]. Each animal’s behavior was recorded and analyzed using the ANY-Maze video-tracking system (Stoelting Co.). The apparatus was cleaned with ethanol 70% after each trial.
Monoamine and Glutamate Determination
The hippocampal and striatum structures were bilaterally dissected on ice and stored at − 80 °C overnight. Monoamine determination was performed using ultra-high-pressure liquid chromatography–mass spectroscopy (UHPLC-MS) following previous studies [21]. The results were expressed as ng/mg tissue for dopamine, DOPAC and 5-HT and μg/mg tissue for glutamate.
mRNA Analysis
Total RNA from the hippocampus was isolated with TRIzol™ Reagent (Invitrogen™) under the manufacturer’s instructions. The cDNA was synthesized with Reverse Transcription System (Promega) from 2 μg of the total, according to the manufacturer’s instructions. Real-time PCR analysis was performed in the StepOne™ Real-Time PCR Systems Instrument (Applied Biosystems®) using the SYBR® Green amplification System, according to manufacturer’s instructions and respective forward and reverse primers (10 μM) (Table 1). All results were analyzed by the 2-ΔΔCT method, for determination of relative expression data, taking β-actin gene expression as an endogenous control for normalization.
Blood Serum Purine Level Analysis
Purine levels were determined in the blood serum by high-pressure liquid chromatography (HPLC). Three milliliters of blood was collected by trans-cardiac blood collection into an anticoagulant-free vacuum tube for each mouse, 30 min after the last injection. Immediately after collection, blood samples were centrifuged at 1000×g for 10 min, and the serum was aliquoted, labeled, and stored at − 80 °C until assay. The protocol was performed according to a previously described method [22] with minor modifications. The amounts of purines were measured by absorption at 260 nm. Purine concentrations (ATP, ADP, AMP, adenosine, inosine, xanthine, hypoxanthine and uric acid) are expressed as μM.
Cellular Analysis
The hippocampus was dissociated under enzymatic digestion (2 mg/mL collagenase, 28 U/mL DNAse I in HBSS), filtered (40 μm nylon cell strainer), and aliquoted into 106 cells for subsequent analysis.
Analysis of Cell Death
Subsequently, dissociated hippocampal cells (105) were pelleted and submitted to the analysis of apoptosis and necrosis by annexin V/propidium iodide (PI) staining, according to the manufacturer’s instructions (BD Biosciences). Acquisition of stained cells was performed on a BD FACScalibur flow cytometer (BD Biosciences). The results were analyzed using FlowJo® software. The cells were classified as follows: live (AnnexinV−/PI−), early apoptotic (AnnexinV+/PI−), late apoptotic (AnnexinV+/PI+) or necrotic (AnnexinV−/PI+).
Cell Typing of P2X7R Immunocontent
The hippocampal cell typing of P2X7R immunocontent was performed by staining with specific antibodies and flow cytometry analysis. For intracellular staining, we first performed the incubation with staining buffer (2% FBS in PBS) containing extracellular anti-mouse P2X7R-FITC conjugated antibody (Sigma-Aldrich) followed by fixation with 1% PFA and a permeabilization step with permeabilizing buffer (2% FBS, 0.1% Triton-X 100 in PBS). The cells were then stained with either GFAP-660 conjugated antibody (ThermoFisher) or NeuN (Millipore) primary antibody followed by incubation with anti-mouse APC secondary antibody (eBioscience, USA). For the surface staining, cells were incubated with staining buffer (2% FBS in PBS) containing extracellular anti-mouse P2X7R-FITC conjugated antibody (Sigma Andrich) plus CD11b-660 conjugated antibody (ThermoFisher). Three different staining combinations were used: P2X7R-FITC/GFAP-660; P2X7R-FITC/NeuN-APC and P2X7R-FITC/CD11b-660. A secondary antibody or isotype control was used as a non-specific binding control, when appropriate. At the end of the incubation period, the cells were washed twice and immediately analyzed using a FACScalibur flow cytometer (BD Biosciences, USA) and FlowJo®, LLC software. The results are expressed as percentage of positive cells on viable cells and as mean fluorescence intensity (MFI) when appropriated.
Statistical Analysis
Results were analyzed using two-way analysis of variance (ANOVA) or repeated two-way ANOVA. The model includes two fixed factors; AMPH administration used to induce the animal model of mania and the administration of the P2X7R antagonist, BBG. Tukey’s multiple comparison post hoc test or Sidak post hoc test was performed to identify differences. A complete description of all ANOVA analyses for the entire study has been provided (Supplementary Table 1). Correlations between continuous variables were assessed using Pearson correlation test. Significance was set at p < 0.05. Statistical analyses and graphs were made using the Statistical Package for the Social Sciences (SPSS) version 20.0 for Windows and the GraphPad Prism software version 7.0 for Windows (GraphPad, San Diego, CA, USA), respectively. Data are presented as means ± standard error of mean (SEM).
Results
P2X7R and Hyperlocomotion Induced by AMPH
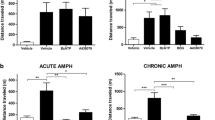
Figure 1 shows the animals’ behavior after pharmacological antagonism of P2X7R in a preclinical animal model of mania. In the locomotor activity parameter (Fig. 1a, Supplementary Table 1), two-way ANOVA revealed a significant main effect for P2X7R antagonism (F(1,29) = 10.09, p = 0.003), a main effect for AMPH treatment (F(1,29) = 103.5, p < 0.0001) and a significant interaction between both factors (F(1,29) = 8.803, p = 0.006). As expected, post hoc analysis indicated that AMPH increased locomotor activity, compared with the control group (p < 0.0001). However, when BBG and AMPH were co-administered, there was a significant decrease in locomotion, when compared with AMPH group (p < 0.001), indicating that non-selective P2X7R pharmacological blockade reduces the behavioral action of AMPH.
Modulatory effects of P2X7R on locomotor behavior in the AMPH-induced model of mania. Effect of chronic i.p. administration of the non-selective P2X7R antagonist, BBG (45 mg/kg, once a day, 7 days), on locomotor behavior, in a mouse model of mania induced by chronic (2 mg/kg, i.p., once a day, 7 days) AMPH treatment. (a) Total distance traveled (m). (b) Distance traveled by time. Except for the first 10 min, the co-treatment of BBG and AMPH caused a decrease in the distance traveled, compared with the AMPH group, significant decreasing at times 1200–1800 s, 1800–2400 s, 2400–3000 s (p < 0.0001, p < 0.0001, and p < 0.001 respectively). AMPH was able to increase locomotion significantly at all times, in comparison with the control group (at all times: p < 0.0001, except 0–600 s: p < 0.01) and the BBG group (for all times: p < 0.0001, except 0–600 s: p < 0.001). The group co-treated with AMPH and BBG had a significant increase in locomotion, compared with the control groups (for all times: p < 0.001, except 600–1200 s: p < 0.01, 1200–1800 s: p < 0, p < 0.01) and BBG (for all times: p < 0.001, except 600–1200 s: p < 0.05, 1200–1800 s: p < 0.01). (c) Mean speed (m/s). (d) Mean speed by time. Except for the first 10 min, co-treatment of BBG and AMPH causes a decrease in mean velocity, compared with the AMPH-only group, (p < 0.01, p < 0.0001, p < 0.0001, and p < 0.01, respectively). The AMPH was able to increase mean speed significantly at all times compared with the control group (at all times: p < 0.0001, except 0–600 s: p < 0.05) and the BBG group (for all time: p < 0.0001 except 0–600 s: p < 0.01). The group that was co-treated with AMPH and BBG had a significant increase in mean velocity, compared with control groups, at times 0–600 s, 600–1200 s, 1800–2400 s, 2400–3000 s (p < 0.01, p < 0.05, p < 0.05, p < 0.01, and p < 0.01 respectively) and BBG (for all time: p < 0.001, except 600–1200 s, 1200–1800 s, 1800–2400 s: p < 0.01). (e) Total time immobile (s). (f) Time immobile by time. We observed a decrease in immobility time in the AMPH group compared with the control group (at all times: p < 0.001, except 600–1200 s: p < 0.05) and in comparison with the BBG group at the times 600–1200 s (p < 0.05), 1200–1800 s, 1800–2400 s, 2400–3000 s (p < 0.0001 for such comparisons). (g) Total distance traveled for the first 5 min of the test (m). (h) Rearing (number of presses). Two-way ANOVA and repeated measure two-way ANOVA followed by Tukey’s multiple comparison post hoc test: *p < 0.05, *p < 0.01, ***p < 0.001, ****p < 0.0001 comparisons to saline/saline group. Data expressed as means ± SEM of eight to nine animals per group. AMPH, d-amphetamine; BBG, Brilliant Blue G
Similarly, for the mean speed parameter, two-way ANOVA revealed a significant effect of P2X7R antagonism (F(1,29) = 11.23, p = 0.002) and AMPH treatment (F(1,29) = 77.61, p < 0.0001) (Fig. 1c, Supplementary Table 1). Indeed, post hoc analysis showed that treatment with AMPH causes a significant increase in the mean speed of the animals, when compared with the control group (p < 0.0001). Co-administration of BBG and AMPH resulted in a significantly decreased mean speed, compared with the AMPH group (p < 0.01).
In contrast, the total immobility time was significantly lower in the group of AMPH-treated animals than in the control group (p < 0.0001) (Fig. 1e, Supplementary Table 1). Co-treatment with AMPH and BBG also caused a decrease in the immobility time of the animals, compared with the control group (p < 0.001); however, even though this immobility time was shorter than that of the control group, there was no statistical difference. When observing locomotion, the mean speed and the immobility time of the animals (Fig. 1b, d, f), 60 min for analysis were deemed adequate (representing 30 min after the last injection the main point of analysis).
An additional point of analysis focused on the distance traveled during the first 5 min of behavior, to evaluate the exploration of the animal in its new environment [23]; this analysis did not show any statistical difference between the groups (Fig. 1g, Supplementary Table 1). The vertical behavior of the animals in the same first 5 minutes, as determined by the number of rearing (according to previous studies [24]), increased in the group of animals treated with AMPH, in comparison with control animals (p < 0.01); a decrease in rearing was observed when animals were co-treated with BBG and AMPH (p < 0.05) (Fig. 1h, Supplementary Table 1).
P2X7R Is Involved in the Hyperlocomotion Induced by AMPH via a Dopaminergic-Dependent Mechanism
Figure 2 shows the effects of P2X7R blockade on the monoamine levels in the model of mania induced by AMPH treatment. With regard to the dopamine levels in the striatum, although two-way ANOVA revealed an effect with AMPH treatment (F(1,30) = 4.77, p = 0.036), post hoc analysis did not show any difference (data not shown, Supplementary Table 1). Two-way ANOVA revealed a significant effect of both P2X7R blockade (F(1,27) = 9.802, p = 0.004) and AMPH treatment (F(1,27) = 7.44, p = 0.011) (Fig. 2a, Supplementary Table 1), on hippocampal DOPAC levels. Post hoc analysis demonstrated a significant increase in DOPAC levels following treatment with AMPH, compared with the control group (p < 0.05), which was prevented by co-treatment with BBG and AMPH (p < 0.01). In the striatum, we observed an effect of the antagonism of P2X7R on DOPAC levels (F(1,24) = 10.14, p = 0.004) and of AMPH treatment (F(1,24) = 15.09, p = 0.0007) (Fig. 2b, Supplementary Table 1). Post hoc analysis demonstrated a pattern opposite than that of the hippocampus, with a decrease in the striatum DOPAC levels following treatment with AMPH, compared with the control group (p < 0.001). The dopamine levels in the hippocampus were below detectable levels, and analysis was not possible (data not shown).
Modulatory effects of P2X7R on monoamines response in the the AMPH-induced model of mania. Effect of chronic i.p. administration of the non-selective P2X7R antagonist BBG (45 mg/kg, once a day, 7 days) on the monoamine response, in a mouse model of mania induced by chronic (2 mg/kg, i.p., once a day, 7 days) AMPH treatment. Tissues were dissected one hour after the last injection. (a) DOPAC determination in the hippocampus and (b) striatum. (c) 5-HT determination in the hippocampus and (d) striatum. (e) Glutamate determination in the hippocampus and (f) striatum. (g) Correlation between hippocampal DOPAC levels and total distance traveled (m). Two-way ANOVA followed by Tukey’s multiple comparison post hoc test: *p < 0.05, *p < 0.01, ***p < 0.001 for comparisons to saline/saline group. Pearson correlation test. Data expressed as means ± SEM of eight to nine animals per group. AMPH, d-amphetamine; BBG, Brilliant Blue G. DOPAC, 3,4-dihydroxyphenylacetic acid; 5-HT, 5-hidroxitriptamina
With regards to serotonin levels in the hippocampus (Fig. 2c, Supplementary Table 1), post hoc analysis indicates a significant decrease in the AMPH-treated group when compared with the control group (p < 0.001), similarly as in the group co-treated with AMPH and BBG (p < 0.05). In the same way in striatum, post hoc analysis showed a significant decrease in the AMPH treated group, when compared with the control group (p < 0.001) as well as in the group co-treated with AMPH and BBG (p < 0.05) (Fig. 2d, Supplementary Table 1).
Similarly, in the hippocampus, we observed a decrease in glutamate levels in the AMPH-treated group and in the BBG/AMPH group, when compared with the control group (p < 0.0001 for both analyses, Fig. 2e, Supplementary Table 1). For glutamate levels in the striatum, post hoc analysis showed a decrease in striatum glutamate levels in the AMPH group and in the BBG/AMPH group, compared with the control group (p < 0.0001 for both analyses, Fig. 2f, Supplementary Table 1).
Hippocampal DOPAC Levels and Total Distance Traveled (m) Are Positively Correlated
Pearson’s correlation test demonstrated a significant positive correlation between hippocampal DOPAC levels and total distance traveled (m) in mice (r = 0.592; p = 0.0006) (Fig. 2g), confirming the dopaminergic involvement in hyperlocomotion.
Hippocampal Entpd3 Gene Expression Is Increased by AMPH, and P2X7R Blockade Can Prevent This Expression
Figure 3 shows the analysis of gene expression in total RNA isolated from mice hippocampi. With regard to the relative expression of P2rx7 (Fig. 3a), tyrosine hydroxylase (Th) (Fig. 3b), the dopamine transporter (DAT) (Fig. 3c) and ecto-5′-nucleotidase (Nt5e) (Fig. 3f), we did not observe any statistical differences between groups. However, we could see an interaction of AMPH and BBG in order to increase in the relative expression of ectonucleoside triphosphate diphosphohydrolase 1 (Entpd1) (F(1,10)= 6.13, p = 0.03, Fig. 3d, Supplementary Table 1). Also, there was statistically significant increase in the relative expression of ectonucleoside triphosphate diphosphohydrolase 3 (Entpd3) after AMPH treatment (F(1,10) = 8.37, p = 0.013, Fig. 3e, Supplementary Table 1) in comparison with the control group, as shown by post hoc analysis (p < 0.05), which was prevented by pharmacological blockade of P2X7R.
Analysis of gene expression in total RNA isolated from hippocampi of mice. Effect of chronic i.p. administration of the non-selective P2X7R antagonist BBG (45 mg/kg, once a day, 7 days) on gene expression response, in a mouse model of mania induced by chronic (2 mg/kg, i.p., once a day, 7 days) AMPH treatment. Tissue was dissected 30 min after the last injection. Analysis of the relative expression of (a) P2rx7, (b) Th, (c) DAT, (d) Entpd1, (e) Entpd3 and (f) Nt5e. β-Actin expression was used as an internal control for normalization of expression levels. Two-way ANOVA followed by Tukey post hoc test: *p < 0.05 for comparisons to saline/saline group. Data expressed as means ± SEM of five to seven animals per group. AMPH, d-amphetamine; BBG, Brilliant Blue G; RQ, relative quantification; P2rx7, purinergic receptor P2X7; TH, tyrosine hydroxylase; DAT, dopamine transporter; Entpd1, ectonucleoside triphosphate diphosphohydrolase 1; Entpd3, ectonucleoside triphosphate diphosphohydrolase 3; Nt5e, ecto-5′-nucleotidase
Serum Purine Levels and P2X7R Antagonism
Figure 4 displays the serum purine levels in the animal model of mania and after P2X7R antagonism. We did not observe any significant difference in serum ATP levels (Fig. 4a, Supplementary Table 1). For serum ADP levels, two-way ANOVA revealed a significant interaction between the effects of P2X7R antagonism and AMPH treatment (F(1,20) = 25.22; p < 0.0001) (Fig. 4b, Supplementary Table 1). We also found an effect of P2X7R antagonism (F(1,20) = 33.07, p < 0.0001) and AMPH treatment (F(1,20) = 38.92, df = 1; p < 0.0001) on ADP levels. Post hoc analysis indicated that co-treatment with AMPH and BBG was able to increase serum ADP levels, in comparison with the control group (p < 0.0001). With regard to serum AMP levels, we found an effect of AMPH treatment (F(1,20) = 8.806, p = 0.007) and a decrease in serum AMP levels in the group that was co-treated with BBG and AMPH, compared with controls (p < 0.05) (Fig. 4c, Supplementary Table 1).
Measurement of blood serum purine levels (μM) by high-pressure liquid chromatography (HPLC). Effect of chronic i.p. administration of the non-selective P2X7R antagonist BBG (45 mg/kg, once a day, 7 days) on serum purine levels, in a mouse model of mania induced by chronic (2 mg/kg, i.p., once a day, 7 days) AMPH treatment. Trans-cardiac blood was collected for each mouse 30 min after the last injection. (a) ATP levels. (b) ADP levels. (c) AMP levels. (d) Adenosine levels. (e) Inosine levels. (c) Xanthine levels. (d) Hypoxanthine levels. (e) Uric acid levels. Two-way ANOVA followed by Sidak post hoc test: *p < 0.05, *p < 0.01, ***p < 0.001 for comparisons to saline/saline group. Data expressed as means ± SEM of five to seven animals per group. AMPH, d-amphetamine; BBG, Brilliant Blue G
Two-way ANOVA revealed an effect of P2X7R antagonism (F(1,20) = 6.339, p = 0.0204) and AMPH treatment (F(1,20) = 16.53, p = 0.0006) on serum adenosine levels (Fig. 4d, Supplementary Table 1). Post hoc analyses showed that AMPH and co-treatment with BBG and AMPH were able to increase serum adenosine levels, both in comparison with the control group (p < 0.001 and p < 0.05 respectively). No significant differences were found in serum inosine (Fig. 4e), xanthine (Fig. 4f), or hypoxanthine (Fig. 4g) levels. Lastly, for serum acid uric levels, we found an effect of AMPH treatment (F(1,18) = 54.03, p < 0.0001) (Fig. 4h, Supplementary Table 1). Post hoc analyses showed that AMPH and the co-administration of BBG and AMPH were both able to decrease serum uric acid levels, in comparison with controls (p < 0.01 and p < 0.001, respectively).
Effect of P2X7R Antagonism on Immunocontent
There was no statistical difference between groups when total apoptosis and necrosis were compared, and the range for all groups was under 15% (data not shown). AMPH treatment had a significant effect on percentage of cell expressing and the expression (MFI) of P2X7R (F(1,17) = 14.28, p = 0.0015 and F(1,17) = 17.07, p = 0.0007, respectively). Post hoc analysis demonstrated a decrease in the number of cells expressing P2X7R (Fig. 5a) as well as a decrease in P2X7R expression in the hippocampal cell population (MFI) (Fig. 5b).
Effect of P2X7R modulation on immunocontent. Effect of chronic i.p. administration of the non-selective P2X7R antagonist BBG (45 mg/kg, once a day, 7 days) on hippocampal cellular analysis, in a mouse model of mania induced by chronic (2 mg/kg, i.p., once a day, 7 days) AMPH treatment. Tissue was dissected 30 min after the last injection. Hippocampal P2X7R immunocontent represented in (a) (%) and (b) MFI. Neuronal marker, NeuN immunocontent represented in (c) (%) and (d) (MFI) and (e) co-staining of NeuN and P2X7R (%). Astrocyte marker, GFAP immunocontent represented in (f) (%) and (g) (MFI) and (h) co-staining of GFAP and P2X7R (%). Microglial marker, CD11b immunocontent represented in (i) (%) and (j) (MFI) and (k) co-staining of CD11b and P2X7R (%). Two-way ANOVA followed by Sidak post hoc test: *p < 0.05, *p < 0.01, **p < 0.001 for comparisons to saline/saline group. Data expressed as means± SEM of four to seven animals per group. AMPH, d-amphetamine; BBG, brilliant blue G; GFAP, Glial fibrillary acidic protein; %, percentage of positive cells on viable cells; MFI, mean fluorescence intensity
In addition, we observed an effect of AMPH treatment on the percentage of cells expressing the neuronal marker NeuN and total expression (MFI) (F(1,18) = 7.414, 0.014 and F(1,17) = 5.908, p = 0.026, respectively). An interaction between AMPH and P2X7 blockade was also observed in the NeuN MFI analysis (F(1,17) = 9.739, p = 0.006) (Fig. 5d). Post hoc analysis indicated an increase in the percentage of cells expressing NeuN in the group that received both BBG and AMPH group, compared with the BBG group (Fig. 5c). Regarding the total expression (MFI) of NeuN, co-treatment with BBG and AMPH increased the immunocontent in relation to controls (Fig. 5d). Double-staining for NeuN and P2X7R demonstrated a significant effect of AMPH treatment (F(1,16) = 12.13, p = 0.003) and a difference between the group that received both BBG and AMPH, compared with the control group (Fig. 5e).
Interestingly, double-staining for P2X7R and the astrocyte marker, GFAP, demonstrated a significant interaction between both treatments, BBG and AMPH (F(1,15) = 4.79, p = 0.044), and an effect for each treatment in comparison with the respective control (F(1,15) = 6.50, p = 0.022 and F(1,15) = 5.22, p = 0.037, respectively). Post hoc analysis showed increased double-staining in the co-treated BBG and AMPH group, compared with the control group (Fig. 5h). We did not find any significant statistical difference, in any analysis, for the microglial marker, CD11b (Fig. 5 i, j, k).
Discussion
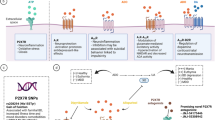
Firstly, our results corroborate the previously demonstrated association between the P2X7 receptor and an animal model of mania, as shown by the decreased responsiveness to AMPH in animals in which P2X7R was pharmacologically blocked (Fig. 6). However, in this study, we were able to further investigate the mechanisms by which P2X7 receptor antagonism mediates behavioral outcomes. We previously demonstrated that blockade and deletion of P2X7R interferes negatively in the establishment of the animal model of mania [9]. Here, we observed a decrease in hyperlocomotion in animals that were co-treated with BBG and AMPH, compared with animals treated with AMPH alone, in agreement with previous results [17, 18]. We chose to use BBG, a non-selective antagonist of P2X7R, due to its ability to cross the blood-brain barrier [25], facilitating peripheral administration. The dose of 45 mg/kg, via i.p, is widely used and has already been shown to achieve a brain bioavailability of about 200 nM, which is within the effective range of BBG for P2X7R antagonism [26]. BBG treatment resulted in a partial blockade of the receptor, observed as decreased locomotor activity compared with the AMPH group; it was not able to revert this behavior to control levels, as previously reported for the intracerebroventricular administration of the selective antagonist, A438079 [9]. This is probably due to the ability of BBG to also antagonize the P2X1, 2, 4 and 5 receptors (Revised in [11]), which may decrease its action on the P2X7 receptor. Nevertheless, the use of knockout mice for P2X7R confirmed the selective participation of P2X7R in the modulation of the animal model of mania [9]; thus, this BBG working model is quite effective and useful for understanding the involvement of the P2X7 receptor in the AMPH-induced mania model.
Scheme of the P2X7 purinergic receptor involvement in the pathophysiology of mania. In this study, we further demonstrate the mechanisms by which P2X7 receptor antagonism mediates behavioral outcomes in a preclinical model of mania. Firstly, we have replicated that the pharmacological animal model of mania induced by AMPH treatment leads to increase in DOPAC levels in hippocampus, decrease in 5-HT and glutamate levels and is able to establish neuroinflammation and oxidative stress in both hippocampus and striatum 1[9]. We also demonstrated that AMPH leads to increase Entpd3 expression in hippocampus and blood adenosine levels. Ultimately those central and peripheric AMPH effects are reflected in the hyperlocomotion, the main behavioral outcome considered for the mania-like phenotype. Interestingly, we have demonstrated that the pharmacological blockade of P2X7R by BBG, the P2X7R antagonist, prevents the hyperlocomotion promoted by AMPH. Molecularly, we showed that P2X7R antagonism prevented the increases in DOPAC and Entp3 levels. Also was able to prevent the establishment of neuroinflammation and oxidative stress 1[9]. Lastly, we demonstrated that the co-treatment of AMPH and BBG is able to modulate astrogliosis, what we hypothesize promotes brain repair (including neuroinflammation and oxidative stress) and reducing the neurological impairment induced by AMPH. Also, the co-treatment is able to increase the expression of Entpd1, an efficient ATP and ADP hydrolyzer, possibly with the purpose of maintain the brain homeostasis. Altogether, these P2X7R antagonism effects demonstrates that the blockade of this receptor interferes negatively in the establishment of this pharmacological animal model of mania, in the last instance suggesting the P2X7R to be a promising new molecular target for bipolar disorder. AMPH, d-amphetamine; BBG, Brilliant Blue G; P2X7R, Purinergic Receptor P2X7; Entpd1, ectonucleoside triphosphate diphosphohydrolase 1; Entpd3, ectonucleoside triphosphate diphosphohydrolase 3, DOPAC, 3,4-dihydroxyphenylacetic acid; 5-HT, 5-hidroxitriptamina; Glu, glutamate
A key finding of this study was the induction of increased levels of the dopamine metabolite, DOPAC, by AMPH, and the prevention of this increase by the P2X7R antagonist in the hippocampus. The action of AMPH on dopaminergic levels is already well defined, as well as the increase of this neurotransmitter in episodes of mania in patients [27]; and for this reason, this molecule is used to induce animal models of mania. High levels of the dopamine metabolite DOPAC in the hippocampus of AMPH-treated animals may reflect an earlier dopamine peak, which has been rapidly metabolized to DOPAC. Many studies indicate that the nucleus accumbens-hippocampus pathway acts on the release of dopamine to locomotor activity [28]. Thus, the AMPH-induced increase in this pathway in the hippocampus could be responsible for the hyperlocomotion observed, as confirmed by the positive correlation between hippocampal DOPAC levels and total distance traveled. In turn, BBG treatment prevented the increases in DOPAC levels and hyperlocomotion that were induced by AMPH, suggesting a possible role for the P2X7 receptor in the dopaminergic mechanism reflected in the animals’ behavior. On the other hand, the same profile was not found in the striatum, in agreement with a previous study reporting an increase in locomotion in euthymic patients after administration of AMPH, without any increase in dopamine levels in this structure [29].
We also evaluated serotonin and glutamate levels, which are also essential neurotransmitters for bipolar disorder [30]. The pharmacological mania model decreases the levels of both serotonin and glutamate in both the hippocampal and striatum structures. In contrast to the modulation of dopaminergic signaling, treatment with BBG did not alter this effect. Some evidence has suggested a decrease in glutamate levels in patients with bipolar disorder, as well as a reduction of neuronal integrity in bipolar disorder patients (as demonstrated by neuroimaging studies [31]). However, a study that used chronic administration of MDMA (3,4-methylenedioxymethamphetamine), a drug with a similar action to that of AMPH, demonstrated peaks of dopamine and serotonin in rodents, followed by selective depletion of serotonergic neurons [32], which could explain the decrease in the levels of serotonin induced by the treatment with AMPH observed herein. The results of the present study indicate that the action of the P2X7R antagonist in this animal model involved the dopaminergic and non-serotoninergic or even the glutamatergic system.
To further investigate the interplay between purinergic and dopaminergic signaling in the hippocampus of animals, we evaluated the gene expressions of critical participants of both systems. Interestingly we found increased Entpd3 relative expression following AMPH treatment, in comparison with the control, which was prevented by pharmacological P2X7R blockade. The Entpd3 enzyme is an ectonucleotidase expressed in multiple regions of the brain, primarily in neuronal cells and can hydrolyze ATP very efficiently [33]. This action has fundamental importance in the complex microenvironment presented by the nervous system, since high levels of ATP in the extracellular environment can establish neuroinflammation [34] and ultimately induce neuronal death [35]. This could be viewed as a compensatory attempt to reverse the damage caused by the mania model induced by AMPH, where increased expression of Entpd3 would augment the hydrolysis of ATP. It is possible that the co-treatment with BBG prevented the increase in Entpd3 expression due to its ability to decrease the inflammatory and excitotoxicity mechanisms induced by AMPH [9].
ENTPD1 protein is also known as CD39 and it is present in the surface of endothelial cells of central nervous system and more predominantly in microglial cells [36] hydrolyzing ATP and ADP at the same proportion [37]. Our results indicate that the co-treatment of BBG and AMPH is able to increase the expression of this gene, what could lead to an increase of ATP and ADP hydrolyzation by microglial cells in order to maintain homeostasis and protect brain tissue. However, more studies are needed in order to confirm our hypothesis. Also, apparently, the changes in the gene expressions of tyrosine hydroxylase and the dopamine transporter cannot be explained by the modulation of dopaminergic signaling, although further studies are needed to define this point.
To understand the ectonucleotidase-mediated pattern of response to the mania model, we collected blood from the animals to evaluate the general purinergic profile at 30 min after treatment. Interestingly, P2X7R antagonism together with AMPH treatment increased serum adenosine, a molecule known to be neuroprotective and anti-inflammatory [38]. Adenosine is also involved in the regulation of important CNS mechanisms, such as modulation of glutamatergic and dopaminergic neurotransmission [39] and it was described being decreased in BD euthymic patients in comparison with controls [40]. This response, which occurred at the peak of hyperlocomotion, which mimics an episode, may indicate an overall compensatory mechanism as an attempt to reverse the damage caused by the mania model, which (when taken together with other data) is reflected in the observed behavioral outcome of the antimanic effect [41]. This different profile of adenosine levels observed in patients and at the animal model is probably due the euthymic state versus the mania state, which clearly leads to different outcomes. It is possible that blocking P2X7R alone allows accumulation of adenosine since, once this receptor is blocked, it leads to ATP production due to impaired of purine degradation. This result may also be a compensatory attempt to reverse the damage caused by the AMPH-induced mania model.
In contrast, we demonstrated that AMPH treatment decreased uric acid levels. It is well accepted that subjects with bipolar disorder have higher uric acid levels than healthy controls; indeed, there is evidence that uric acid may constitute a biomarker in bipolar disorder [42]. Initially, our results seemed to be in disagreement with data reported in the literature; however, it is possible that this difference occurs due to the timepoint of analysis employed, since our data are collected at the behavioral peak of the animal model, which is often not possible to evaluate in studies with patients. Furthermore, following accumulation of adenosine, uric acid may be reasonably expected to decrease, since it is the end product of the purine catabolism [43]. As such, these data support the hypothesis of a purinergic system imbalance in the animal model of mania.
Lastly, in order to identify which hippocampal cellular type was involved in the neuroprotection against AMPH, we performed cell typing analysis using cellular immunostaining. P2X7R immunocontent was increased in the animals treated with the receptor antagonist BBG, which was not maintained when animals were co-treated with BBG and AMPH. Antagonist-induced receptor expression been previously demonstrated in the literature [44], leading to hypotheses of a possible “inverse agonism” [45]. However, we suggest that our results demonstrate a compensatory response to seven consecutive days of receptor antagonism; the neuronal marker, NeuN, was increased only in the hippocampus of animals co-treated with the P2X7R antagonist and AMPH. We also found an increase in NeuN and P2X7R co-staining in the same group, compared with the BBG group. P2X7R neuronal expression and activation have been widely associated with neurotoxicity and neuronal damage [46], although this receptor has also been considered to display dual function in both cell death and cell growth/proliferation [47]; however, it is not known how the same receptor mediates these opposing effects [48]. Since our results did not demonstrate significant cell death in any of the treatment groups, we suggest that these results suggest a potential neuroprotective effect of P2X7R antagonism (reviewed by [49]).
Interestingly, we found a significant increase in double-staining for GFAP and P2X7R in the group that was co-treated with BBG and AMPH, compared with all other groups. Astrogliosis is known to handle brain stress and restore homeostasis by limiting tissue damage [50]. In other words, astrogliosis plays a critical role in neuroprotection when neural tissue is damaged [51]. Chronic administration of AMPH has been previously shown to increase GFAP levels in the rat hippocampus, but the density of astrocytes is concomitantly reduced in bipolar disorder, suggesting a deficient astroglial function, which constitutes a negative prognosis for episodes and pathological evolution (reviewed by [52]). Overall, our results suggest that P2X7R antagonism in the AMPH model could modulate astrogliosis, potentially promoting brain repair and reducing the neurological impairment induced by AMPH.
Some limitations of this study should be considered. Although there is a demand for better animal models in psychiatry [53], the most well-established animal model of mania is the administration of AMPH in both mice and rats, to simulate the increase in dopamine levels in patients during manic episodes [27]. The construct and predictive validities of the model have been proven, correlating findings in animals with clinical data and biochemistry observed in patients [54]. Our sample size was small for some specific experiments, and this may have limited our power to detect a significant result in this particular groups. Also, we have used isoflurane as the euthanasia method and this anesthetic is known to either open the blood-brain barrier [55] and increase adenosine levels [56]; however, since all the mice from all groups had the same exposition to this drug, we do not correlate our results to this event, even because increased adenosine levels were only observed in AMPH group, demonstrating a clear separated effect. Furthermore, we were unable to establish the specific mechanisms mediating the participation of P2X7R in the dopaminergic response induced by AMPH or the astrogliosis induced by co-treatment with BBG and AMPH. As such, our results suggest some pathways of relevance, but more studies are needed to characterize the involvement of P2X7R in the dopaminergic response induced by AMPH and the mechanism of astrogliosis.
In conclusion, our results add new information that supports the hypothesis that the P2X7R plays a role in the pathophysiology of mania, being important for the establishment of the pharmacological animal model of mania by potentially mediate the dopaminergic pathway, astrogliosis and important purinergic mediators. Finally, this study suggests the P2X7R to be a promising new molecular target for bipolar disorder.
References
Mondimore FM (2005) Kraepelin and manic-depressive insanity: an historical perspective. Int Rev Psychiatry 17(1):49–52
Kraeplin E Manic-Depressive Insanity and Paranoia. 1921, Edinburgh
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50(3):413–492
Baroja-Mazo A, Barberà-Cremades M, Pelegrín P (2013) The participation of plasma membrane hemichannels to purinergic signaling. Biochim Biophys Acta 1828(1):79–93
Cheffer A, Castillo ARG, Corrêa-Velloso J, Gonçalves MCB, Naaldijk Y, Nascimento IC, Burnstock G, Ulrich H (2018) Purinergic system in psychiatric diseases. Mol Psychiatry 23(1):94–106
Burnstock G (2017) Purinergic signalling: therapeutic developments. Front Pharmacol 8:661
Bobo WV (2017) The diagnosis and management of bipolar I and II disorders: clinical practice update. Mayo Clin Proc
Backlund L, Nikamo P, Hukic DS, Ek IR, Träskman-Bendz L, Landén M, Edman G, Schalling M et al (2011) Cognitive manic symptoms associated with the P2RX7 gene in bipolar disorder. Bipolar Disord 13(5-6):500–508
Gubert C et al (2016) Role of P2X7 Receptor in an animal model of mania induced by D-amphetamine. Mol Neurobiol 53(1):611–620
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82(4):1013–1067
Sluyter R (2017) The P2X7 Receptor. Adv Exp Med Biol 1051:17–53
Rao JS, Harry GJ, Rapoport SI, Kim HW (2010) Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry 15(4):384–392
Stertz L, Magalhães PV, Kapczinski F (2013) Is bipolar disorder an inflammatory condition? The relevance of microglial activation. Curr Opin Psychiatry 26(1):19–26
Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yücel M, Gama CS et al (2011) Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 35(3):804–817
McQuillin A, Bass NJ, Choudhury K, Puri V, Kosmin M, Lawrence J, Curtis D, Gurling HM (2009) Case-control studies show that a non-conservative amino-acid change from a glutamine to arginine in the P2RX7 purinergic receptor protein is associated with both bipolar- and unipolar-affective disorders. Mol Psychiatry 14(6):614–620
Gubert C, Andrejew R, Jacintho Moritz CE, Dietrich F, Vasconcelos-Moreno MP, Dos Santos BTMQ, Fijtman A, Kauer-Sant'Anna M et al (2019) Bipolar disorder and 1513A>C P2RX7 polymorphism frequency. Neurosci Lett 694:143–147
Bhattacharya A, Wang Q, Ao H, Shoblock JR, Lord B, Aluisio L, Fraser I, Nepomuceno D et al (2013) Pharmacological characterization of a novel centrally permeable P2X7 receptor antagonist: JNJ-47965567. Br J Pharmacol 170(3):624–640
Csölle C, Andó RD, Kittel Á, Gölöncsér F, Baranyi M, Soproni K, Zelena D, Haller J et al (2013) The absence of P2X7 receptors (P2rx7) on non-haematopoietic cells leads to selective alteration in mood-related behaviour with dysregulated gene expression and stress reactivity in mice. Int J Neuropsychopharmacol 16(1):213–233
Engel T, Gomez-Villafuertes R, Tanaka K, Mesuret G, Sanz-Rodriguez A, Garcia-Huerta P, Miras-Portugal MT, Henshall DC et al (2012) Seizure suppression and neuroprotection by targeting the purinergic P2X7 receptor during status epilepticus in mice. FASEB J 26(4):1616–1628
Frey BN, Valvassori SS, Réus GZ, Martins MR, Petronilho FC, Bardini K, Dal-Pizzol F, Kapczinski F et al (2006) Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychiatry Neurosci 31(5):326–332
Hows ME, Lacroix L, Heidbreder C, Organ AJ, Shah AJ (2004) High-performance liquid chromatography/tandem mass spectrometric assay for the simultaneous measurement of dopamine, norepinephrine, 5-hydroxytryptamine and cocaine in biological samples. J Neurosci Methods 138(1-2):123–132
Voelter W, Zech K, Arnold P, Ludwig G (1980) Determination of selected pyrimidines, purines and their metabolites in serum and urine by reversed-phase ion-pair chromatography. J Chromatogr 199:345–354
Li CR, Huang GB, Sui ZY, Han EH, Chung YC (2010) Effects of 6-hydroxydopamine lesioning of the medial prefrontal cortex on social interactions in adolescent and adult rats. Brain Res 1346:183–189
Macêdo DS, Medeiros CD, Cordeiro RC, Sousa FC, Santos JV, Morais TA, Hyphantis TN, McIntyre R et al (2012) Effects of alpha-lipoic acid in an animal model of mania induced by D-amphetamine. Bipolar Disord 14(7):707–718
Kimbler DE, Shields J, Yanasak N, Vender JR, Dhandapani KM (2012) Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS One 7(7):e41229
Donnelly-Roberts DL, Jarvis MF (2007) Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol 151(5):571–579
Berk M et al (2007) Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand Suppl 434:41–49
Peleg-Raibstein D, Feldon J (2006) Effects of dorsal and ventral hippocampal NMDA stimulation on nucleus accumbens core and shell dopamine release. Neuropharmacology 51(5):947–957
Anand A, Verhoeff P, Seneca N, Zoghbi SS, Seibyl JP, Charney DS, Innis RB (2000) Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. Am J Psychiatry 157(7):1108–1114
Kugaya A, Sanacora G (2005) Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr 10(10):808–819
Haarman BC et al (2016) Volume, metabolites and neuroinflammation of the hippocampus in bipolar disorder - a combined magnetic resonance imaging and positron emission tomography study. Brain Behav Immun 56:21–33
López-Muñoz F et al (2014) Ecstasy (3,4-methylenedioxymethamphetamine, MDMA): pharmacological, clinical, and criminological aspects. Trastornos Adictivos 6(1):22
Zimmermann H (2000) Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedeberg's Arch Pharmacol 362(4-5):299–309
Adinolfi E, et al. (2017) The P2X7 receptor: a main player in inflammation. Biochem Pharmacol
Di Virgilio F (1998) ATP as a death factor. Biofactors 8(3-4):301–303
Bulavina L, Szulzewsky F, Rocha A, Krabbe G, Robson SC, Matyash V, Kettenmann H (2013) NTPDase1 activity attenuates microglial phagocytosis. Purinergic Signal 9(2):199–205
Robson SC, Sévigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2(2):409–430
Cekic C, Linden J (2016) Purinergic regulation of the immune system. Nat Rev Immunol 16(3):177–192
Boison D (2008) Adenosine as a neuromodulator in neurological diseases. Curr Opin Pharmacol 8(1):2–7
Gubert C, Jacintho Moritz CE, Vasconcelos-Moreno MP, Quadros Dos Santos BTM, Sartori J, Fijtman A, Kauer-Sant'Anna M, Kapczinski F et al (2016) Peripheral adenosine levels in euthymic patients with bipolar disorder. Psychiatry Res 246:421–426
Machado-Vieira R (2012) Purinergic system in the treatment of bipolar disorder: uric acid levels as a screening test in mania. J Clin Psychopharmacol 32(5):735–736
Bartoli F, Carrà G, Clerici M (2017) Update on bipolar disorder biomarker candidates: what about uric acid/adenosine hypothesis? Expert Rev Mol Diagn 17(2):105–106
Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V (2016) Regulation of uric acid metabolism and excretion. Int J Cardiol 213:8–14
Morton RA, Baptista-Hon DT, Hales TG, Lovinger DM (2015) Agonist- and antagonist-induced up-regulation of surface 5-HT3 A receptors. Br J Pharmacol 172(16):4066–4077
Milligan G, Bond RA, Lee M (1995) Inverse agonism: pharmacological curiosity or potential therapeutic strategy? Trends Pharmacol Sci 16(1):10–13
Rodrigues RJ, Tomé AR, Cunha RA (2015) ATP as a multi-target danger signal in the brain. Front Neurosci 9:148
Di Virgilio F (2016) P2RX7: a receptor with a split personality in inflammation and cancer. Mol Cell Oncol 3(2):e1010937
Khadra A, Tomić M, Yan Z, Zemkova H, Sherman A, Stojilkovic SS (2013) Dual gating mechanism and function of P2X7 receptor channels. Biophys J 104(12):2612–2621
Miras-Portugal MT, Sebastián-Serrano Á, de Diego García L, Díaz-Hernández M (2017) Neuronal P2X7 receptor: involvement in neuronal physiology and pathology. J Neurosci 37(30):7063–7072
Pekny M, Wilhelmsson U, Pekna M (2014) The dual role of astrocyte activation and reactive gliosis. Neurosci Lett 565:30–38
Sofroniew MV (2014) Astrogliosis. Cold Spring Harb Perspect Biol 7(2):a020420
Peng L, Li B, Verkhratsky A (2016) Targeting astrocytes in bipolar disorder. Expert Rev Neurother 16(6):649–657
Logan RW, McClung CA (2016) Animal models of bipolar mania: the past, present and future. Neuroscience 321:163–188
Valvassori SS, Rezin GT, Ferreira CL, Moretti M, Gonçalves CL, Cardoso MR, Streck EL, Kapczinski F et al (2010) Effects of mood stabilizers on mitochondrial respiratory chain activity in brain of rats treated with d-amphetamine. J Psychiatr Res 44(14):903–909
Tétrault S, Chever O, Sik A, Amzica F (2008) Opening of the blood-brain barrier during isoflurane anaesthesia. Eur J Neurosci 28(7):1330–1341
Kim M, Ham A, Kim JY, Brown KM, D'Agati VD, Lee HT (2013) The volatile anesthetic isoflurane induces ecto-5'-nucleotidase (CD73) to protect against renal ischemia and reperfusion injury. Kidney Int 84(1):90–103
Acknowledgments
CG and RA were recipients of scholarships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). CEJM is recipient of scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES). PVSM is supported by a CNPq productivity fellowship. Currently CG is recipient of a Post-Doctoral Fellowship (PDE–Pos Doutorado no Exterior) from CNPq–Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, of the Ministry of Science, Technology, Innovation, Communication of Brazil and a University of Melbourne Early Career Researcher Award.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001. This work was also supported by grants from the National Science and Technology Institute for Translational Medicine (INCT-TM) (Project 573671/2008-7), INCT for excitotoxicity and neuroprotection (INCT-EN) (Project 465671/2014-4), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Project No 303264/2013-6), and Fundo de Incentivo à Pesquisa–Hospital de Clínicas de Porto Alegre (FIPE-HCPA). The funding agencies did not have any role in study design, data collection and analysis, the decision to publish, or manuscript preparation. Dr. Kapczinski reports personal fees from Daiichi sankyo, personal fees from Janssen-Cilag, grants from Stanley Medical Research Institute 07TGF/1148, grants from INCT-CNPq 465458/2014-9, grants from Canada Foundation for Innovation-CFI, outside the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental procedures reported in this manuscript were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Brazilian College of Animal Experimentation. The Animal Ethics Committee of the Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil, approved this project under protocol number 15-0192.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 196 kb)
Rights and permissions
About this article
Cite this article
Gubert, C., Andrejew, R., Leite, C.E. et al. P2X7 Purinergic Receptor Is Involved in the Pathophysiology of Mania: a Preclinical Study. Mol Neurobiol 57, 1347–1360 (2020). https://doi.org/10.1007/s12035-019-01817-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01817-0