Abstract
Blood-brain barrier (BBB) disruption is a common pathological feature of many neurological disorders including stroke and brain trauma, therefore is an important therapeutic target for treatment of these diseases. Basic fibroblast growth factor (bFGF) as a member of FGF superfamily plays critical roles in angiogenesis, neurogenesis, and neuron survival. We recently showed that recombinant bFGF protects against BBB disruption in traumatic brain injury in mice. In this study, we further investigated the mechanisms of recombinant bFGF in BBB protection by measuring the permeability of cultured endothelial cell monolayer induced by oxygen-glucose deprivation and reoxygenation (OGD/R). We found that recombinant bFGF significantly decreased OGD/R-induced permeability of primary human brain microvascular endothelial cell (HBMEC) monolayer and preserved OGD/R-induced decreases of trans-endothelial electrical resistance (TEER). Western blot and immunocytochemistry showed that bFGF significantly rescued OGD/R-induced downregulation of junction proteins ZO-1, occludin, and VE-cadherin. We further show that the BBB protective effect of bFGF is via FGF receptor 1 (FGFR1) activation as FGFR1 inhibitor can block this protection effect. Moreover, we revealed that the BBB protection effect of bFGF is at least partially through rescuing the OGD/R-induced downregulation of sphingosine-1-phosphate receptor 1 (S1PR1) protein, as S1PR1 inhibitor or SIPR1 small interfering RNA blocked the BBB protective effect of bFGF, whereas S1PR1 agonist alone has comparable BBB protection effect of bFGF. These findings will improve our understanding of the protective effect and mechanisms of bFGF on BBB and propose bFGF as a potential therapeutic agent against BBB damage in neurological disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blood-brain barrier (BBB) is a highly selective permeability barrier that separates blood circulation from the brain tissue. BBB is mainly composed of endothelial cells that are connected by tight junction proteins including ZO-1, occludin, and claudin-5 and also adherent junction proteins such as VE-cadherin [1, 2]. Other components of BBB include pericytes and astrocytes. BBB plays a critical role in maintaining brain function by protecting brain tissue from neurotoxins and pathogens in the circulation, in the meantime allowing the passage of small molecules such as water, gas, glucose and amino acids that are crucial for neuronal function [3]. Compelling experimental and clinical evidence has demonstrated that BBB disruption is a critical pathophysiological feature of neurological disorders including stroke and brain trauma [4,5,6]. BBB dysfunction can persist for a few days through the acute and subacute phase of stroke [4], marked by increased permeability and decreased gene expression of junction proteins [7], and significantly contributes to the severe clinical consequences of stroke such as hemorrhagic transformation (HT), referring to the hemorrhage development within ischemic brain tissue, and motor and cognitive function deficits [3, 8, 9]. It is therefore of tremendous importance to elucidate the mechanisms of BBB disruption after stroke, which will benefit the development of therapeutics targeting BBB protection.
Basic fibroblast growth factor (bFGF), also known as FGF2, is a single-chain polypeptide composed of 146 amino acids and a member of the fibroblast growth factor (FGF) superfamily. Numerous studies have demonstrated that bFGF is a multifunctional growth factor involved in the regulation of neuron survival, neurite growth [10], and angiogenesis [11]. The biological activities of bFGF are mediated through its high-affinity binding to its cell surface receptor, FGF receptor 1 (FGFR1) [12]. In addition, the function of bFGF is also modulated by its binding to heparin and cell surface heparan sulfate proteoglycan [13]. Preclinical studies have shown that administration of exogenous bFGF within hours of stroke onset reduces infarct size and promotes the recovery of sensory motor functions [14] potentially by promoting axon spouting and new synapse formation [15]. In the context of BBB, we recently have demonstrated that exogenous recombinant bFGF administration protects against BBB disruption caused by traumatic brain injury (TBI) in mice [16], and the mechanisms may involve regulation of junction protein expressions, and partially through PI3K-Akt-Rac1 pathway, as blocking this pathway partially blocked the BBB protection effect of bFGF, suggesting that multiple signaling pathways may be involved in this protection effect. Sphingosine-1-phosphate (S1P) signaling pathway plays important roles in vascular system, as experimental evidence has demonstrated that S1P receptor (S1PR1) protects the integrity of BBB against various injuries [17, 18]. In this study, we aimed to further investigate the additional mechanisms, including bFGF receptor and S1PR1, of BBB protection by exogenous bFGF in neurological disorders by testing the permeability of an in vitro-cultured primary human brain microvascular endothelial cell (HBMEC) monolayer exposed to oxygen-glucose deprivation and reoxygenation (OGD/R).
Material and Methods
Reagents and Antibodies
Primary HBMEC was obtained from Cell Systems Corporation (ACBRI376, Kirkland, WA). Recombinant human bFGF was purchased from Sigma (Sigma-Aldrich, St. Louis, MO). Anti-FGFR1 (mouse), anti-pFGFR1 (rabbit), anti-claudin-5 (rabbit), anti-VE-cadherin (rabbit), and anti-S1PR1 (rabbit) antibodies were purchased from Abcam (Cambridge, MA); anti-occludin (rabbit) and anti-ZO-1(mouse) antibodies were purchased from Invitrogen (Carlsbad, CA). S1PR1 inhibitor VPC23019 was purchased from Cayman Chemical (Ann Arbor, MI). S1PR1 activator FTY720 was purchased from Sigma (St. Louis, MO).
Endothelial Monolayer Permeability Assay
Primary HBMEC was cultured in complete growth media EBM-2 (Lonza, Walkersville, MD). The cells were seeded on the inner surface of collagen-coated Transwell inserts (6.5-mm diameter, 0.4-μm pore size polycarbonate filter; Corning, Corning, NY), which were placed in wells of a 24-well plate with complete EBM-2 media. When the monolayer of cells was confluent, confirmed by ensuring that it is impermeable to media, the cells were serum starved for 8 h with EBM media without growth supplement before OGD/R treatment. Normal control group is the monolayer culture without OGD/R treatment. For OGD/R treatment, EBM-2 media were replaced with DMEM without glucose, and the cells were then put in a specialized, humidified chamber (Heidolph, incubator 1000, Brinkmann Instruments, Westbury, NY) at 37 °C, which contained an anaerobic gas mixture (90% N2, 5% H2, and 5% CO2). After 4-h incubation, the cultures were removed from the anaerobic chamber, and the OGD medium was replaced with EBM-2 complete medium without bFGF. The cells were then allowed to recover in a regular incubator for 20 h (reoxygenation) before permeability measurement. For the bFGF-treated group, recombinant bFGF was added to the medium (final concentration 2.5 μM) during OGD/R. After 20-h reoxygenation, media in both upper and lower chambers were removed and replaced with fresh media without supplement. Permeability was measured by adding 0.1 mg/ml of fluorescein isothiocyanate (FITC)-labeled dextran (MW, 70,000; Sigma, St. Louis, MO) to the upper chamber, with lower compartment containing fresh serum-free media. After incubation for 20 min, 100 μl of sample from the lower compartment was measured for fluorescence at excitation 490 nm and emission 520 nm. All independent experiments were performed in duplicate or triplicate.
Trans-Endothelial Electrical Resistance
Trans-endothelial electrical resistance (TEER) of cultured HBMEC monolayer was measured using an EndOhm-6 chamber and an EVOM resistance meter (World Precision Instruments, Sarasota, FL) following the manufacturer’s instructions. Briefly, the 6 mm Transwell containing cultured endothelial cell monolayer was transferred into the EndOhm-6 chamber containing 0.1 M KCl. The culture media within the Transwell were also replaced with 0.1 M KCl. EndOhm cap was then inserted on the top of the chamber and Transwell and then connected with the chamber using a connector cable, and resistance was then measured using the EVOM resistance meter. A blank Transwell containing 0.1 M KCl but without any cells was used as blank control.
Cell Viability Assay
The viability of cultured endothelial cells was assessed by WST assay. Briefly, HBMEC cells were cultured in a 24-well plate in complete EBM-2 medium. After treatment, WST-1 reagent (50 μl) was added to each well (containing 450-μl medium) and the cells were incubated for 2 h at 37 °C. The absorbency at 450 nm was measured against a background blank control using a microplate reader (SpectraMax M5, Molecular Devices) according to the manufacturer’s protocol.
Western Blot
Western blot was performed following our previously published protocol [22]. Briefly, total protein was isolated from cultured endothelial cells using lysis buffer (Cell Signaling, Danvers, MA). Membrane protein was isolated using the Mem-PER™ Plus Membrane Protein Extraction Kit (Thermo Fisher Scientific, Waltham, MA) following the manufacturer’s instructions. The proteins were separated on 4–20% Tris-glycine gel, followed by blotting to nitrocellulose membrane. The membrane was then incubated with primary anti-FGFR1 (1:200, Santa Cruz), anti-pFGFR1 (1:200, Santa Cruz), anti-claudin-5 (1:1000, Invitrogen), anti-occludin (1:1000, Santa Cruz), anti-ZO-1 (1:1000, Abcam), anti-VE-cadherin (1:1000, Abcam), and anti-S1PR1 (1:1000, Abcam) antibodies. After being washed with TBST (250 mM Tris, 27 mM KCl, and 1.37 M NaCl, pH 7.4, 0.1% Tween 20), the membrane was incubated with corresponding horseradish peroxidase-conjugated secondary antibodies and washed with TBST, and immunolabeling was detected by enhanced chemiluminescence (ECL; GE Healthcare) according to the manufacturer’s instruction. Optical density of protein bands was quantified with ImageJ.
Immunocytochemistry
Cultured neurons were washed with cold PBS (pH 7.4) and fixed with 4% paraformaldehyde for 30 min. The cells were then washed with PBS containing 0.1% Tween and further incubated with 5% FBS for 1 h. Next, the cells were incubated with primary antibodies against VE-cadherin (rabbit monoclonal; 1:200; Abcam) and S1PR1 (rabbit polyclonal; 1:200; Abcam) at 4 °C overnight. After PBS washing, the cells were incubated with goat anti rabbit IgG conjugated with FITC (1:150; Jackson ImmunoResearch), respectively, for 1 h at room temperature. Vectashield mounting medium containing DAPI (for nucleic staining) (Vector Laboratory, Burlingame, CA) was used to coverslip the immunocytochemic slides. Immunostaining was analyzed using a fluorescence microscope (Olympus BX51).
Real-Time PCR
Quantitative real-time PCR (RT-PCR) was used to measure S1PR1 messenger RNA (mRNA) levels in cultured HBMEC following the standard protocols. Briefly, total RNA was extracted using miRNeasy kit (Qiagen) following the manufacturer’s instructions and quantified spectrophotometrically at 260 nm using a NanoDrop 2000C spectrophotomer (Thermo Scientific); the purity of RNAs was assessed by the ratio of the absorbance at 260 and 280 nm (A260/280) >1.80. One hundred nanograms of total RNAs was reverse transcribed into complementary DNA (cDNA) using random primers and M-MLV reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. S1PR1 mRNA levels were tested using specific S1PR1 primer and TaqMan Gene Expression Assays in triplicates (ABI 7500HT, Applied Biosystems). Each reaction was performed with 50 ng of cDNA. B2M was used as an internal control housing keeping gene. Changes in gene expression (fold change) were determined using the 2−ΔΔCt method with normalization to B2M.
Small interfering RNA-Mediated Gene Silencing
S1PR1 small interfering RNA (siRNA) and control siRNA were obtained from Santa Cruz Biotechnology. siRNA transfection was conducted following the manufacturer’s instruction. Briefly, HBMEC was cultured on six-well plates or Transwell for 2 days. siRNA duplex and siRNA Transfection Reagent (Santa Cruz) were separately diluted with Transfection Medium (Santa Cruz) and then mixed together and incubated at room temperature for 40 min. The cells were washed once with siRNA Transfection Medium and then incubated with mixed siRNA and siRNA Transfection Reagent for 5 h at 37 °C. The cells on six-well plates were collected for Western blot to detect S1PR1 protein level, and cells in Transwell underwent OGD/R treatment and tested for permeability.
Statistical Analysis
Results were expressed as mean ± SEM. The number of samples was six per group for endothelial monolayer permeability assays and four for western blot experiments. Multiple comparisons were evaluated by one-way ANOVA followed by Tukey–Kramer’s tests between all groups. P < 0.05 was considered statistically significant.
Results
Recombinant Basic Fibroblast Growth Factor Reduces Oxygen-Glucose Deprivation and Reoxygenation-Induced Endothelial Monolayer Permeability Through Upregulating FGF Receptor 1 Activity
To examine the effect of recombinant bFGF treatment on OGD/R-induced endothelial monolayer permeability, cultured HBMEC monolayer in Transwells was subjected to 4-h OGD followed by 24-h reoxygenation. Recombinant bFGF (2.5 μM) was used to treat the cells during OGD/R, and then, the permeability of FITC-dextran (70 kDa) was measured. Our results show that OGD/R significantly increased HBMEC monolayer permeability (2.8 ± 0.25-fold of normal control), while treatment with recombinant bFGF significantly reduced the permeability compared to OGD/R (1.6 ± 0.24-fold of normal control), indicating the protective effect of bFGF on OGD/R-induced endothelial monolayer permeability (Fig. 1a).
Recombinant bFGF reduces OGD/R-induced endothelial monolayer permeability through upregulating FGFR1 activity. Cultured HBMEC in Transwells was subjected to 4-h OGD/R followed by 24-h reoxygenation. Recombinant bFGF (2.5 μM) or bFGF combined with FGFR1 inhibitor PD173074 was used to treat endothelial monolayer during OGD/reoxygenation. The monolayer integrity was then measured by FITC-dextran permeability and TEER. FGFR1 activation was measured by Western blot for FGFR1 and pFGFR1 protein levels. a Permeability of FITC-dextran (70 kDa). b TEER measurement. c Representative image of Western blot for FGFR1 and pFGFR1. d Quantification of FGFR1 and pFGFR1 protein levels. e Endothelial viability after OGD/R and bFGF treatment measured by WTS assay (n = 6, *p < 0.05 vs normal control, #p < 0.05 vs OGD/R group, & p < 0.05 vs OGD/R + bFGF group)
To further validate the effect of recombinant bFGF on endothelial monolayer integrity, we tested the TEER using the EndOhm chamber and EVOM resistance meter. We show that OGD/R significantly deceased the TEER of cultured HBMEC monolayer compared to normal control (0.39 ± 0.07-fold of normal control), whereas bFGF treatment significantly rescued the TEER compared to GOD/R condition (0.83 ± 0.09-fold of normal control) (Fig. 1b), further proving the protective effect of bFGF on endothelial monolayer integrity.
Since bFGF normally functions through its receptor FGFR1, we tested the effect of bFGF treatment on FGFR1 activation by measuring the phosphorylation of FGFR1. Cultured HBMEC was subjected to 4-h OGD followed by 4-h reoxygenation, and then, the protein levels of FGFR1 and phosphorylated FGFR1 (p-FGFR1) were examined by Western blot. The results show that total FGFR1 protein level was not changed by OGD/R or OGD/R plus bFGF, whereas the level of p-FGFR1 was significantly decreased by OGD/R, and this decrease was significantly rescued by bFGF treatment (Fig. 1c, d), suggesting that bFGF may function through promoting FGFR1 activation in endothelial monolayer after OGD/R injury.
To test whether FGFR1 activation is involved in the protective effect of bFGF for endothelial monolayer integrity, we treated the cultured HBMEC monolayer with bFGF combined with a specific FGFR1 inhibitor, PD173074 (50 nM), and then measured and compared the permeability and TEER with bFGF treatment alone. Our results show that PD173074 blocked the protective effect of bFGF on endothelial monolayer integrity, as the permeability of bFGF and PD173074 co-treated group is significantly higher than bFGF-treated group (Fig. 1a). Furthermore, the TEER of bFGF and PD173074 co-treated group is significantly decreased compared to bFGF-alone-treated group (Fig. 1b). These results suggest that the protective effect of bFGF on endothelial monolayer integrity is mediated by FGFR1 activation.
To test whether the protective effect of bFGF on endothelial monolayer integrity is simply due to protection against OGD/R-induced cytotoxicity, we further assessed the effect of bFGF treatment on the viability of cultured HBMEC. HBMEC was subjected to 4-h OGD followed by 20-h reoxygenation. Recombinant bFGF (2.5 μM) was used to treat endothelial cells during OGD/R. Cell viability was assessed using WST assay normalized by cell number counting. Our results show that OGD/R did not significantly change the viability of endothelial cells, and bFGF did not significantly change the cell viability either (Fig. 1e), suggesting that the protective effect of bFGF against OGD/R-induced endothelial monolayer permeability is not through increasing cell viability.
Recombinant Basic Fibroblast Growth Factor Rescues Oxygen-Glucose Deprivation and Reoxygenation-Induced Downregulation of Endothelial Junction Proteins
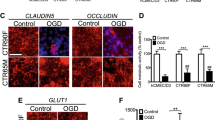
Since endothelial tight junction and adherent junction proteins are important regulators of endothelial paracellular permeability, we next examined the effect of bFGF treatment on endothelial junction protein expression after OGD/R. After 4-h OGD followed by 20-h reoxygenation, total protein levels of tight junction proteins claudin-5, occludin, and ZO-1 and adherent junction protein VE-cadherin were examined by Western blot. Our results show that OGD/R significantly reduced the protein levels of occludin, ZO-1, and VE-cadherin. Recombinant bFGF treatment significantly rescued the expression of these proteins (Fig. 2a–d). Claudin-5 protein level was not significantly changed by either OGD/R or bFGF.
Recombinant bFGF ameliorates OGD/R-induced downregulation of endothelial junction proteins. After exposure to 4-h OGD followed by 20-h reoxygenation, endothelial junction proteins including tight junction proteins claudin-5, occludin, and ZO-1 and adherent junction protein VE-cadherin were measured by Western blot for total proteins or isolated membrane fraction. a Representative image of western blot for total junction proteins. b–d Quantification of the relative total protein levels of occludin, ZO-1, and VE-cadherin. e Representative image of Western blot for membrane junction proteins. NaK-ATPase was used as membrane internal marker. f–h Quantification of the relative membrane protein levels of occludin, ZO-1, and VE-cadherin. i Immunostaining for VE-cadherin protein in cultured endothelial cells after OGD/R and bFGF treatment. Nuclei were stained using DAPI (n = 4, *p < 0.05 vs normal control, #p < 0.05 vs OGD/R group)
Since adjacent endothelial cells are mainly connected by membrane-localized junction proteins, we further tested the membrane localization of these junction proteins in cultured HBMEC after 4-h OGD and 20-h reoxygenation. Membrane protein was isolated from HBMEC and then subjected to Western blot. NaK-ATPase was used as a membrane internal marker. Our results show that OGD/R significantly reduced the protein levels of occludin, ZO-1, and VE-cadherin in the membrane, while bFGF21 treatment significantly rescued these protein levels (Fig. 2e–h). Claudin-5 protein level in the membrane was not significantly changed by either OGD/R or bFGF. These results suggest that bFGF may protect against OGD/R-induced BBB disruption through rescuing the junction protein expression and localization on cell membrane.
We further performed immunocytochemistry to test the membrane localization of VE-cadherin in HBMEC after 4-h OGD and 20-h reoxygenation. Our results show that VE-cadherin is predominantly localized on endothelial membrane. OGD/R significantly decreased the membrane localization of VE-cadherin, while bFGF treatment significantly rescued the membrane localization of VE-cadherin. Furthermore, the FGFR1 inhibitor PD173074 (50 nM) significantly blocked the effect of bFGF on recovering membrane localization of VE-cadherin (Fig. 2i), indicating that this effect is mediated by FGFR1 activation.
Recombinant Basic Fibroblast Growth Factor Rescues Oxygen-Glucose Deprivation and Reoxygenation-Induced Downregulation of Sphingosine-1-Phosphate Receptor 1 Protein Expression via FGF Receptor 1 Activation
S1PR1 has been reported to regulate the expression of endothelial junction proteins [19]. We then tested whether bFGF treatment can change the S1PR1 protein expression. HBMEC was subjected to 4-h OGD followed by 4-h reoxygenation, and bFGF was added during OGD and reoxygenation. Total S1PR1 protein levels were measured by Western blot. Our results show that OGD/R significantly reduced S1PR1 protein levels, while bFGF treatment significantly rescued S1PR1 protein expression (Fig. 3a, b). Furthermore, the specific FGFR1 inhibitor PD173074 (50 nM) significantly blocked the upregulation effect of bFGF on S1PR1 protein expression (Fig. 3a, b).
Recombinant bFGF rescues OGD/R-induced S1PR1 downregulation via FGFR1 activation. HBMEC was subjected to 4-h OGD followed by 4-h reoxygenation; bFGF or bFGF combined with PD173074 was treated during OGD/R. The intracellular protein levels of S1PR1 were measured by Western blot, immunocytochemistry, and RT-PCR. a Representative image of Western blot for S1PR1. b Quantification of S1PR1 protein levels. c Immnocytochemistry for measurement of S1PR1 protein. Nuclei were stained using DAPI. d RT-PCR to measure the gene transcription of S1PR1. (n = 4, *p < 0.05 vs normal control, #p < 0.05 vs OGD/R group, &p < 0.05 vs OGD/R + bFGF group)
Moreover, we performed immunocytochemistry to examine S1PR1 protein expression in HBMEC after OGD/R and bFGF treatment. We show that OGD/R significantly reduced S1PR1 protein expression in HBMEC compared to normal condition, while bFGF treatment significantly rescued S1PR1 protein expression. Additionally, PD173074 significantly blocked the effect of bFGF in rescuing S1PR1 protein expression (Fig. 3c). These results suggest that bFGF can rescue OGD/R-induced S1PR1 downregulation, via FGFR1 activation.
We further examined the mRNA expression of S1PR1 gene by RT-PCR in cultured HBMEC after 4-h OGD followed by 4-h reoxygenation, with or without bFGF treatment. Our results show that OGD/R significantly decreased S1PR1 mRNA levels, but bFGF treatment only slightly increased (no significance) S1PR1 mRNA compared to the OGD/R group (Fig. 3d). These data suggest that bFGF may rescue S1PR1 protein expression mainly through increasing protein translation, but not gene transcription.
The Protective Effect of Recombinant Basic Fibroblast Growth Factor Against Oxygen-Glucose Deprivation and Reoxygenation-Induced Endothelial Monolayer Permeability Is Dependent on Sphingosine-1-Phosphate Receptor 1 Activation
We next examined whether S1PR1 is required for the protective effect of bFGF on endothelial monolayer integrity. We first used a specific inhibitor of S1PR1, VPC23019 (final concentration 40 nM) [20], to treat the HBMEC monolayer in combination with bFGF, and then measured the FITC-dextran permeability and TEER. Our results show that bFGF significantly decreased OGD/R-induced HBMEC monolayer permeability, while VPC23019 significantly reversed this effect when co-treated with bFGF (Fig. 4a). Moreover, bFGF significantly rescued OGD/R-induced decrease in TEER, whereas VPC23019 also significantly blocked this effect (Fig. 4b). VPC23019 alone did not significantly change the permeability and TEER of the endothelial monolayer.
The protective effect of recombinant bFGF on endothelial monolayer permeability is through S1PR1-dependent mechanisms. To examine whether S1PR1 is required for the protective effect of bFGF on endothelial monolayer integrity, we used a specific inhibitor of S1PR1, VPC23019, and also S1PR1 siRNA to treat the cultured endothelial monolayer in combination with bFGF and then measured the endothelial monolayer permeability and TEER. We also used an S1PR1 agonist, FTY720, to treat endothelial monolayer and compared its effect with bFGF. a Permeability of FITC-dextran in cultured HBMEC monolayers after OGD/R treated with bFGF or bFGF combined with VPC23019. b TEER measurement of HBMEC monolayers after OGD/R treated with bFGF or bFGF combined with VPC23019. c Representative Western blot image and quantification diagram showing the effect of S1PR1 siRNA in silencing S1PR1 protein expression. d Permeability of FITC-dextran in cultured HBMEC monolayers after OGD/R treated with bFGF or bFGF combined with S1PR1 siRNA or control siRNA. e Permeability of FITC-dextran in cultured HBMEC monolayers after OGD/R treated with bFGF or FTY720 or bFGF + FTY720. f TEER measurement of HBMEC monolayer after OGD/R treated with bFGF or FTY720 or bFGF + FTY720 (n = 6, *p < 0.05 vs normal control, #p < 0.05 vs OGD/R group, &p < 0.05 vs OGD/R + bFGF group)
There are limitations for chemical inhibitors of S1PR1 in that they might have off-target effect; we therefore further validated the involvement of S1PR1 in bFGF protection effect on endothelial monolayer integrity using siRNA-mediated gene silencing. Western blot showed that S1PR1 siRNA significantly decreased S1PR1 protein level compared to normal cells or control siRNA-transfected cells (Fig. 4c). In HBMEC monolayer permeability assay in Transwells, S1PR1 siRNA transfection significantly reversed the protection effect of bFGF on FITC-dextran extravasation, compared to control siRNA (Fig. 4d).
To further validate the roles of S1PR1 in the protective effect of bFGF on endothelial monolayer integrity, we used a widely used S1PR1 agonist FTY720 [21] (final concentration 50 nM) to treat cultured HBMEC monolayer alone or in combination with bFGF. Our results show that FTY720 alone significantly reduced OGD/R-induced HBMEC monolayer permeability, to a degree comparable to bFGF treatment, while co-treatment with bFGF and FTY720 did not further reduce the permeability (Fig. 4e). Moreover, FTY720 alone also significantly rescued OGD/R-induced decrease of TEER, to a degree comparable to bFGF treatment, and bFGF and FTY720 co-treatment did not further increase the TEER (Fig. 4f). Overall, these data suggest that the protective effect of recombinant bFGF against OGD/R-induced endothelial monolayer permeability is at least partially through S1PR1 activation.
Discussion
In this study, using cultured HBMEC, we demonstrated that exogenous recombinant bFGF protects against OGD/R-induced disruption of endothelial monolayer integrity. We found that (1) bFGF significantly reduced OGD/R-induced HBMEC monolayer permeability to FITC-dextran and reversed OGD/R-induced decrease of TEER, via FGFR1 activation, (2) bFGF significantly rescued OGD/R-induced downregulations of tight junction proteins occludin and ZO-1 and adherent junction protein VE-cadherin, (3) bFGF significantly recued OGD/R-induced downregulation of S1PR1 protein expression measured, and (4) the protective effect of bFGF on OGD/R-induced endothelial monolayer permeability is dependent on upregulation of S1PR1 demonstrated by both S1PR1 inhibitor and activator.
BBB damage significantly contributes to the pathophysiological progression of neurodegeneration diseases including stroke and brain trauma, thus is an important target for therapeutics development against these diseases. Our recent study has demonstrated that recombinant bFGF protects against BBB disruption caused by TBI in mice [16], potentially through regulation of junction protein expressions and partially through PI3K-Akt-Rac1 pathway. As a continuous study, here we aimed to explore the additional mechanisms of bFGF in BBB protection. We clearly validated the BBB protection effect of bFGF using OGD/R-induced endothelial monolayer permeability and revealed that this protective effect is mediated through FGFR1 activation and S1PR1 upregulation by recombinant bFGF. These findings suggest that bFGF may have BBB protection effect in a wide range of neurological disorders including stroke and brain trauma, and this protection effect may be mediated through multiple mechanisms.
bFGF plays its physiological functions through membrane-bound FGFRs, a family of tyrosine kinase receptors including FGFR1, FGFR2, FGFR3, and FGFR4. FGFR1–3 are predominantly localized in the central nervous system, whereas FGFR4 is mainly expressed in the lung and kidney [22,23,24]. Although bFGF can also signal through FGFR2 and FGFR3, existing studies up to date suggest that FGFR1 might be the primary mediator for bFGF signaling [25, 26]. However, there is little direct evidence proving the indispensable role of FGFR1 in bFGF signaling in the central nervous system. For example, bFGF has been demonstrated to be essential for cell proliferation and neurogenesis during cortex development, and bFGF gene regulation is synchronized with that of FGFR1, suggesting their functional correlation [27]. A very recent study showed that bFGF protects the integrity of blood-spinal cord barrier (BSCB) after contusive brain injury in the rats, and the interaction of FGFR1 with Cavolin-1 may play important roles in this process [28]. In this current study, we found that bFGF can rescue the activity of FGFR1 ameliorated by OGD/R treatment. Moreover, the specific inhibitor of FGFR1, PD173804, blocked the protective effect of bFGF in OGD/R-induced endothelial monolayer permeability. These results demonstrate the role of FGFR1 as a mediator of bFGF signaling.
Sphingosine-1-phosphate (S1P) signaling pathway, which is mediated by S1P receptors (S1PR), plays important regulatory roles in vascular system including angiogenesis, vascular stability, and permeability [17, 18]. Experimental evidence has demonstrated that S1PR1 protects the integrity of BBB against various injuries. For example, S1PR1 activation is required for the protective effect of activated protein C (APC) on endothelial barrier integrity upon systemic inflammation [29]. A recent study shows that S1PR1 is involved in the protective effect of artesunate, a traditional anti-malaria drug, on BBB integrity in subarachnoid hemorrhage in rats [20]. A more direct evidence arose from the finding that selective S1PR1 agonist protects against intracerebral hemorrhage by enhancing BBB integrity, as well as reducing brain infiltration of lymphocytes, neutrophil, and microglia [19]. Here, we found that recombinant bFGF rescues OGD/R-induced downregulation of S1PR1 protein expression, and the involvement of S1PR1 in the BBB protection effect of bFGF was further confirmed using multiple approaches including S1PR1 inhibitor, siRNA silencing, and agonist. These data strongly support the role of S1PR1 in the protection of BBB integrity. It is worth noting that this S1PR1-dependent mechanism may be just part of the mechanisms underlying the BBB protective effect of bFGF, as S1PR1 inhibitor and gene silencing did not completely abolish the BBB protection effect of bFGF in our study. Other mechanisms may also be involved in bFGF-mediated BBB protection, such as the PI3K-Akt-Rac1 pathway as we demonstrated in our recent report on TBI-induced BBB disruption [16]. These signaling molecules could serve as potential targets in developing therapeutics against BBB disruption in neurological disorders.
There are a few caveats in this study. First, we did not test the roles of other bFGF receptors, i.e., FGFR2 and FGFR3, in the BBB protection effect of bFGF. These factors may also play important roles in the pathophysiological function of bFGF, which warrants further investigation in the future. Second, this is only an in vitro study to prove the protective effect of bFGF on OGD/R-induced BBB damage and possible mechanisms. This effect has to be further validated in in vivo animal stroke models, which will be investigated in our future study.
In summary, in this study, we clearly demonstrated that exogenous recombinant bFGF treatment protected against OGD/R-induced HBMEC monolayer permeability through rescuing the expression of endothelial junction proteins, and this effect is dependent on FGFR1 activation and S1PR1 upregulation. These findings, combined with our recent report of bFGF protection for BBB in TBI [16], provide solid evidence for the protective effect of bFGF in neurological disorders including stroke and brain trauma, which may facilitate the development of bFGF as a therapeutic agent for these neurological disorders.
BBB, blood-brain-barrier; bFGF, basic fibroblast growth factor; BSCB, blood-spinal cord barrier; FGFR1, FGF receptor 1; FITC, fluorescein isothiocyanate; HBMEC, human brain microvascular endothelial cell; HT, hemorrhagic transformation; OGD/R, oxygen-glucose deprivation/reoxygenation; RT-PCR, quantitative real-time PCR; S1PR1, sphingosine-1-phosphate receptor 1; TEER, trans-endothelial electrical resistance
Change history
20 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12035-022-02939-8
References
Stamatovic SM, Johnson AM, Keep RF, Andjelkovic AV (2016) Junctional proteins of the blood-brain barrier: new insights into function and dysfunction. Tissue Barriers 4:e1154641. doi:10.1080/21688370.2016.1154641
Weiss N, Miller F, Cazaubon S, Couraud PO (2009) The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta 1788:842–857. doi:10.1016/j.bbamem.2008.10.022
Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV (2015) Establishment and dysfunction of the blood-brain barrier. Cell 163:1064–1078. doi:10.1016/j.cell.2015.10.067
Kassner A, Merali Z (2015) Assessment of blood-brain barrier disruption in stroke. Stroke 46:3310–3315. doi:10.1161/STROKEAHA.115.008861
Prakash R, Carmichael ST (2015) Blood-brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr Opin Neurol 28:556–564. doi:10.1097/WCO.0000000000000248
Alluri H, Wiggins-Dohlvik K, Davis ML, Huang JH, Tharakan B (2015) Blood-brain barrier dysfunction following traumatic brain injury. Metab Brain Dis 30:1093–1104. doi:10.1007/s11011-015-9651-7
Fernandez-Lopez D, Faustino J, Daneman R, Zhou L, Lee SY, Derugin N, Wendland MF, Vexler ZS (2012) Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat. J Neurosci 32:9588–9600. doi:10.1523/JNEUROSCI.5977-11.2012
Khatri R, McKinney AM, Swenson B, Janardhan V (2012) Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 79:S52–S57. doi:10.1212/WNL.0b013e3182697e70
Jin R, Yang G, Li G (2010) Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis 38:376–385. doi:10.1016/j.nbd.2010.03.008
Abe K, Saito H (2001) Effects of basic fibroblast growth factor on central nervous system functions. Pharmacol Res 43:307–312. doi:10.1006/phrs.2000.0794
Rosen L (2000) Antiangiogenic strategies and agents in clinical trials. Oncologist 5(Suppl 1):20–27
Nugent MA, Iozzo RV (2000) Fibroblast growth factor-2. Int J Biochem Cell Biol 32:115–120
Faham S, Hileman RE, Fromm JR, Linhardt RJ, Rees DC (1996) Heparin structure and interactions with basic fibroblast growth factor. Science 271:1116–1120
Kawamata T, Alexis NE, Dietrich WD, Finklestein SP (1996) Intracisternal basic fibroblast growth factor (bFGF) enhances behavioral recovery following focal cerebral infarction in the rat. J Cereb Blood Flow Metab 16:542–547. doi:10.1097/00004647-199607000-00003
Kawamata T, Dietrich WD, Schallert T, Gotts JE, Cocke RR, Benowitz LI, Finklestein SP (1997) Intracisternal basic fibroblast growth factor enhances functional recovery and up-regulates the expression of a molecular marker of neuronal sprouting following focal cerebral infarction. Proc Natl Acad Sci U S A 94:8179–8184
Wang ZG, Cheng Y, Yu XC, Ye LB, Xia QH, Johnson NR, Wei X, Chen DQ et al (2015) bFGF protects against blood-brain barrier damage through junction protein regulation via PI3K-Akt-Rac1 pathway following traumatic brain injury. Mol Neurobiol. doi:10.1007/s12035-015-9583-6
Ephstein Y, Singleton PA, Chen W, Wang L, Salgia R, Kanteti P, Dudek SM, Garcia JG et al (2013) Critical role of S1PR1 and integrin beta4 in HGF/c-Met-mediated increases in vascular integrity. J Biol Chem 288:2191–2200. doi:10.1074/jbc.M112.404780
Gaengel K, Niaudet C, Hagikura K, Lavina B, Muhl L, Hofmann JJ, Ebarasi L, Nystrom S et al (2012) The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell 23:587–599. doi:10.1016/j.devcel.2012.08.005
Sun N, Shen Y, Han W, Shi K, Wood K, Fu Y, Hao J, Liu Q et al (2016) Selective sphingosine-1-phosphate receptor 1 modulation attenuates experimental intracerebral hemorrhage. Stroke. doi:10.1161/STROKEAHA.115.012236
Zuo S, Ge H, Li Q, Zhang X, Hu R, Hu S, Liu X, Zhang JH et al (2016) Artesunate protected blood-brain barrier via sphingosine 1 phosphate receptor 1/phosphatidylinositol 3 kinase pathway after subarachnoid hemorrhage in rats. Mol Neurobiol. doi:10.1007/s12035-016-9732-6
Nacer A, Movila A, Baer K, Mikolajczak SA, Kappe SH, Frevert U (2012) Neuroimmunological blood brain barrier opening in experimental cerebral malaria. PLoS Pathog 8:e1002982. doi:10.1371/journal.ppat.1002982
Ford-Perriss M, Abud H, Murphy M (2001) Fibroblast growth factors in the developing central nervous system. Clin Exp Pharmacol Physiol 28:493–503
Matakidou A, El Galta R, Rudd MF, Webb EL, Bridle H, Eisen T, Houlston RS (2007) Further observations on the relationship between the FGFR4 Gly388Arg polymorphism and lung cancer prognosis. Br J Cancer 96:1904–1907. doi:10.1038/sj.bjc.6603816
Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, Czaja MJ, Bartz R et al (2016) Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. doi:10.1016/j.kint.2016.05.019
Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, Yayon A, Linhardt RJ, Mohammadi M (2000) Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol Cell 6:743–750
Xuan YH, Huang BB, Tian HS, Chi LS, Duan YM, Wang X, Zhu ZX, Cai WH et al (2014) High-glucose inhibits human fibroblast cell migration in wound healing via repression of bFGF-regulating JNK phosphorylation. PLoS One 9:e108182. doi:10.1371/journal.pone.0108182
Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM (2000) Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci 20:5012–5023
Ye LB, Yu XC, Xia QH, Yang Y, Chen DQ, Wu F, Wei XJ, Zhang X et al (2016) Regulation of caveolin-1 and junction proteins by bFGF contributes to the integrity of blood-spinal cord barrier and functional recovery. Neurotherapeutics. doi:10.1007/s13311-016-0437-3
Feistritzer C, Riewald M (2005) Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood 105:3178–3184. doi:10.1182/blood-2004-10-3985
Acknowledgements
This study was in part supported by the National Natural Science Foundation of China 81470999 (X.L.) and AHA Scientist Development Grant (SDG) 15SDG25550035 (Z.Y.).
Author information
Authors and Affiliations
Contributions
LL, ZY, QW, KQ, and ZC performed the study and analyzed the data. XL and ZY designed the experiment, analyzed the data, and wrote the paper. JX and XW helped in data analysis and paper writing. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All animal experiments were performed following the protocols approved by the Massachusetts General Hospital Animal Care and Use Committee in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Availability of Data and Materials
The dataset supporting the conclusions of this article is included within the article.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lin, L., Wang, Q., Qian, K. et al. bFGF Protects Against Oxygen Glucose Deprivation/Reoxygenation-Induced Endothelial Monolayer Permeability via S1PR1-Dependent Mechanisms. Mol Neurobiol 55, 3131–3142 (2018). https://doi.org/10.1007/s12035-017-0544-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0544-0