Abstract
Human neuroblastoma SH-SY5Y cells have been used as an in vitro model for neurodegenerative disorders such as Parkinson’s disease and can be induced to a mature neuronal phenotype through retinoic acid (RA) differentiation. However, mechanisms of RA-induced differentiation remain unclear. Here, we investigate the role of reactive species (RS) on SH-SY5Y neuroblastoma cells under RA differentiation, using the antioxidant Trolox® as co-treatment. We found that RA treatment for 7 days reduced the cell number and proliferative capacity and induced the expression of adult catecholaminergic/neuronal markers such as tyrosine hydroxylase (TH), β-III tubulin, and enolase-2. Evaluation of intracellular RS production by DCFH oxidation assay and quantification of cell non-enzymatic antioxidant activity by TRAP demonstrated that RA increases RS production. Furthermore, mitochondrial NADH oxidation showed to be inhibited under differentiation with RA. Cells subjected to co-treatment with antioxidant Trolox® demonstrated a remaining proliferative capacity and a decrease in the pro-oxidant state and RS production. Besides, antioxidant treatment restores the mitochondrial NADH oxidation. Importantly, Trolox® co-treatment inhibited the appearance of morphological characteristics such as neurite extension and branching, and decreased the expression of TH, β-III tubulin, and enolase-2 after a seven-day differentiation with RA, indicating that RS production is a necessary step in this process. Trolox® also inhibited the phosphorylation of Akt and ERK1/2, which are involved in differentiation and survival, respectively, of these cells. Altogether, these data indicate the presence of a redox-dependent mechanism in SH-SY5Y RA-differentiation process and can be a useful insight to improve understanding of neuronal differentiation signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the most common movement disorder, affecting approximately six million people worldwide [1] and is highly connected to oxidative stress, with increased lipid peroxidation, decreased glutathione, abnormalities in iron homeostasis, and protein aggregation [2]. Cellular models are often used to understand pathways and molecular events of pathological processes. In vitro research emerged as first choice for preliminary studies on the molecular action, cytotoxic and genetic events of neurodegenerative diseases, including PD [1]. The human neuroblastoma SH-SY5Y cell line has been largely used in neuroscience studies, particularly to generate different PD cell models [3–6]. Originally derived as subclone of the neuroblastoma cell line SK-N-SH, SH-SY5Y cells have been frequently used either in an undifferentiated condition or in a neuron-like state after inducing the cells into a functional and morphological neuronal phenotype. Retinoic acid (RA) is a signaling molecule and exerts a major role in neuronal differentiation and patterning [7]. Differentiation with RA has been shown to induce morphological and metabolic changes that confer neuronal-like features [5] and also induces cell cycle arrest, neurite outgrowth, and differentiation into catecholaminergic phenotype [6, 8–10]. Administration of RA in therapeutic protocols for neuroblastoma treatment had promising clinical results [10, 11]. Also, neurons derived from SH-SY5Y treatment with RA for 7 to 10 days express specific neuron markers, such as tyrosine hydroxylase (TH), enolase-2, and β-III tubulin, enabling this model for molecular studies on cell replacement therapy, neuronal differentiation, disease modeling, and drug screening [8, 12].
RA is the main active metabolite of retinol, from the retinoid family of related compounds (which includes vitamin A) and may influence the oxidant status of the cell due to intrinsic redox-active properties [13, 14]. Classically, RA effects are mediated by the nuclear retinoic acid receptors (RAR) and retinoid X receptors (RXR) [9, 10, 14]. More recently, new mechanisms of action triggering cellular events not related to retinoid receptor activation were identified, and these events have been referred to as non-genomic, non-classical, or extra nuclear effects of retinoids [15, 16]. Several studies reported non-genomic actions by retinoids, and some of these are dependent on reactive species (RS) production and redox modifications [17–21]. Although it is known that RAR-/RXR-mediated gene transcription exerts an important role in neuronal differentiation, the necessity of high concentrations (5 to 10 μmol/L) of RA to induce SH-SY5Y differentiation [5] suggests the participation of RAR-/RXR-independent mechanisms.
RA-induced differentiation of SH-SY5Y cells has been related with resistance to oxidants and xenobiotics, possibly due to modulation of RS production and oxidative stress responses [8, 22, 23]. In previous works, it was observed that SH-SY5Y cells expressing Nrf2, a transcription factor responsible for the activation of key genes in antioxidant response and xenobiotic detoxification, enhanced the expression of markers associated to neurite outgrowth, such as neurofilament-M and microtubule-associated protein 2 (MAP2) when treated with RA for up to 4 days [24]. This result indicated that up-regulation of antioxidant defense was essential to RA-induced differentiation in SH-SY5Y cells. Besides, the observation that RA enhances NADPH oxidase expression in SH-SY5Y cells and that pharmacological inhibition of this enzyme inhibits both MAP2 expression and morphological differentiation [25] indicates that RS production may be an essential step in RA-induced neuronal differentiation.
Here, we evaluated the role of RS production and oxidative stress during RA differentiation using the neuroblastoma SH-SY5Y cell line. Using both undifferentiated (proliferative) and differentiated SH-SY5Y cells, we characterized the redox-dependent effects of RA on SH-SY5Y cell viability, proliferation and cell cycle, cellular morphology, expression of specific neuronal proteins, and activation of signaling pathways. Synthetic antioxidant Trolox®, a vitamin E analog, was used to evaluate the effect of oxidative stress during differentiation. Therefore, in the present study, we provide valuable insight into the mechanisms of action of RA on SH-SY5Y cells, specifically on the role of RS and oxidative stress as necessary events taking place in the signaling cascade evoked by RA during neuronal differentiation.
Materials and Methods
Cell Culture and Treatments
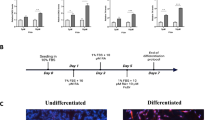
Human SH-SY5Y neuroblastoma cell line was obtained from the European Collection of Cell Cultures (ECACC). Cells were grown in 1:1 mixture of Ham’s F12 and Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 2 mM L-glutamine, 0.28 mg/mL of gentamicin, 250 μg amphotericin B, and 10 % heat inactivated fetal bovine serum (FBS) in a 5 % CO2-humidified incubator at 37 °C. Cells were seeded at an initial density of 104 cells/cm2 in culture dishes for 24 h (TPP Techno Plastic Products AG). Differentiation was induced in 1 % FBS medium by 10 μM all-trans-retinoic acid (RA). The culture medium was replaced every 3 days, and the cells were maintained under differentiation for 7 days. Antioxidant treatment was carried by adding Trolox® (100 μM). Two different control groups were considered for different purposes: proliferating cells cultured in 10 % FBS (proliferative cells) were used as standard for proliferative, undifferentiated cells; and cells exposed to 1 % FBS medium (non-proliferative cells) were used as control in relation to the cells submitted to differentiation with RA 10 μM in 1 % FBS. Besides, a zero time group was used as control of the starting point of differentiation, in which cells were briefly exposed to RA in 1 % FBS and incubation was promptly terminated (Day 0) (Fig. 1). For phosphorylation experiments, cells were changed to free-serum medium for 1 h, followed by pre-incubation of 2 h with Trolox® (AR+T and T groups). After this time, cells were treated with RA or vehicle (Trolox® group) for 0, 15, 30, 45, and 60 min.
Schematic overview of experimental design. Proliferative cells consist in cells under 10 % FBS medium for 48 h with medium replacement after 24 h. Non-proliferative cells consist in 24 h under 1 % FBS medium and Day 0 consist of cells briefly exposed to 1 % FBS medium with RA. The differentiation protocol consisted in reducing medium FBS concentration from 10 to 1 % and then adding RA (10 μM) 24 h later. RA plus Trolox® (100 μM) and Trolox® alone were the other groups to evaluate the role of antioxidant treatment in RA differentiation. The culture medium was replaced every 3 days, and after 7 days under treatment, cells were collected and prepared for the experiments
Parameters of Cellular Viability and Neuronal Morphologic Phenotype
The sulforhodamine B (SRB) incorporation assay was used for determination of cellular density, as it is directly related to cellular protein biomass. This assay relies in the high affinity of SRB to protein components of the cell [26]. The absorbance was determined spectrophotometrically at 515 nm, and results were expressed as percentage of SRB incorporation. Lactate dehydrogenase (LDH) activity in the incubation medium was assessed to estimate cell membrane leakage, using a commercial kit from Labtest SA (MG, Brazil). To evaluate cellular morphology at daily basis, phase-contrast micrographies were taken by using an inverted microscope (Nikon Eclipse TE300) connected to a digital camera.
Flow Cytometry Analyses of Cell Proliferation and Differentiation
To determine the cell cycle state of cells, first was used Click-iT EdU® Alexa Fluor 647 flow cytometry assay kit (Molecular Probes). Briefly, SH-SY5Y cells were cultured in 6-well plates and differentiated for 7 days. After this, the cells were pre-incubated for 3 h with EdU® (5-ethynyl-2′-deoxyuridine) to allow it to incorporate into DNA. The cells then were fixed in 4 % formaldehyde followed by incubation with rabbit anti-tyrosine hydroxylase (Cell Signaling Technology, #9664, 1:800). Cells were analyzed by flow cytometry (BD FACS Calibur flow cytometer, BD Biosciences); data were analyzed by FlowJo (cytometric data analysis and presentation software). Ten thousand cells were analyzed per sample, and data were also reported as the percentage of proliferative (EdU®-positive) and differentiated cells (TH-positive cells). We also performed a cell cycle progression assessment in which differentiated and proliferative cells were stained with PI (propidium iodide) followed by flow cytometry sorting.
Confocal Immunofluorescence Microscopy
Cells were washed with phosphate-buffered saline (PBS) and fixed on chamber slides with 4 % paraformaldehyde (PFA) in 4 °C for 15 min. Samples were sequentially treated with 0.15 % Triton X-100 and 5 % FBS for 1 h and 30 min. Cells were immunostained with specific antibodies, the primary antibodies were utilized as follows: rabbit anti-TH (Cell Signaling Technology, 9664, 1:800), mouse anti-β-III tubulin (Novex, 480011, 1:1000), and fluorescent-labeling Alexa Fluor 488 goat anti-mouse (Molecular Probes, A11001, 1:800) and fluorescent-labeling Alexa Fluor 488 goat anti-rabbit (Molecular Probes, A11008, 1:800). For nuclear staining, Prolong Gold Antifade Reagent with DAPI (Molecular Probes, P36931) was used. Images were taken with an Olympus FluoView 1000 confocal microscope and subsequently analyzed by using Olympus FluoView FV1000 Software, ver. 3.0. Thresholds discriminating between signal and background were selected by utilizing cells that were only stained with secondary antibodies in order to discriminate any false positive result.
Immunoblotting
To perform immunoblot experiments, cells were lysed with 2 % sodium dodecyl sulfate (SDS) and was resolved in standard SDS-polyacrilamide gel electrophoresis and were transferred to an nitrocellulose membrane (Millipore, Bedford) using a Trans-Blot SD Semi-Dry Electrophoretic Transfer Cell (Bio-Rad, Hercules, CA, USA). Protein loading and electroblotting efficiency was verified through Ponceau S staining, and the membrane was washed with TTBS (Tris 100 mM, pH 7.5, 0.9 % NaCl and 0.1 % Tween-20). The membrane was blocked in TTBS containing 5 % of non-fat/skim dry milk for 60 min at room temperature. Primary antibody was incubated overnight at 4 °C and subsequently washed with TTBS. Anti-rabbit or mouse IgG peroxidase-linked secondary antibody was incubated for 2 h at room temperature, and the membrane was washed again with TTBS. Blots were finally developed and immunoreactivity was detected by enhanced chemiluminescence using Supersignal West Pico Chemiluminescent kit (Thermo Scientific). Densitometric analyses were carried using a CCD camera (GE ImageQuant LAS 4000) and the quantitative analysis was performed using Image J. software. Anti-enolase-2 (Cell Signaling Technology, 9664, 1:500), anti-β-actin (Sigma-Aldrich®, A1978, 1:500), anti-phospho-Akt (Thr308) (Cell Signaling Technology, #13038S, 1:1000), anti-Akt (Cell Signaling Technology, #9272S, 1:1000), anti-phospho-ERK1/2 (Thr202/Tyr204) (Cell Signaling Technology, #9101, 1:1000), and anti-ERK1/2 (Cell Signaling Technology, #9102, 1:1000) were used.
Quantitative RT-PCR
Total RNA was extracted from cells according to the manufacturer’s instructions (PureLink® RNA mini kit - 12183018A–Life Technologies™). The cDNA was synthesized with the Super Script III Reverse Transcriptase kit (Invitrogen) using 1 μg of total RNA. Quantitative RT-PCR reactions were performed in triplicate for each sample using the Step One Plus thermocycler (Applied-Biosystems) and Power SYBR® Green PCR Master Mix (4367659, Life Technologies™). Reactions were carried out in 25 μL following manufacturer’s instructions. Primer sequences are as follows: forward sequence (5’CAGAGGCCATCATGTCCCCG3’) and reverse (5’CTTCCCCTCCTTCTCCTCAA3’) for tyrosine hydroxylase; forward sequence (CCATGTTCGTCATGGGTGTGAACCA) and reverse sequence (GCCAGTAGAGGCAGGGATGATGTTG) for endogen gene GAPDH. Quantification was performed using the 2 −ΔΔCT method.
Total Reactive Antioxidant Potential (TRAP)
TRAP was measured and calculated as previously described [27]. Briefly, TRAP represents the non-enzymatic antioxidant capacity of the cells. This is determined by measuring the luminol chemiluminescence intensity of emission induced by thermolysis of 2,2′-azobis (2-amidinopropane) hydrochloride (AAPH) as free radical source. The system was left to stabilize for 2 h, then samples were added and the readings monitored for 60 min in a liquid scintillator counter (Wallac 1409, Perkin–Elmer, Boston, MA, USA). Results were transformed into a percentage, and the area under the curve (AUC) was calculated by utilizing the GraphPad software (SanDiego, CA, USA). Results express the extent by which the non-proteic fraction of the cells inhibits the free radical-derived luminescence.
Intracellular Reactive Species Production (DCFH-DA)
Intracellular reactive species production was measured by the DCFH-DA oxidation assay, as previously described [28]. This technique is based on the capability of DCFH to be oxidized to highly fluorescent dichlorofluorescein (DCF) in the presence of reactive species (RS) and can be used as an index to quantify the overall RS production in cells. Briefly, SH-SY5Y cells were seeded in 96-well plates and 100 μM DCFH-DA dissolved in medium containing 1 % FBS was added in each well. Cells were incubated for 2 h in order to allow cellular incorporation, and DCF fluorescence was read at 37 °C in a fluorescence plate reader (Spectra Max M2, Molecular Devices, USA) with an emission wavelength of 535 nm and an excitation wavelength of 485 nm. The results were expressed as percentage of DCF fluorescence in relation to untreated control.
Assessment of Mitochondrial Activity by NADH Oxidase Assay
The NADH segment comprises the electron transport from Complex I to Complex IV. The mitochondria from SH-SY5Y cells were isolated according to Voss et al. (1961) [29]. For complete lysis of the mitochondria, several freeze-thaw cycles were applied. The membrane fragments were used to evaluate enzymatic activity of NADH oxidase segment through spectrophotometrical assay as described by Singer (1974) [30]. Briefly, NADH oxidase activity was measured at 28 °C in phosphate buffer 80 mmol/L, pH 7.4, EDTA 50 μmol/L, NADH 0.2 mmol/L, and 0.1 mg/mL of mitochondrial protein in a total volume of 1.0 mL. The reaction was initiated by the addition of the substrate and was followed by the decrease in absorbance at 340 nm.
Protein Assay
Total protein was quantified by Lowry assay and used to normalize all data [31].
Statistical Analysis
Results were expressed as the mean ± SEM of three independent experiments (n = 3). Data were analyzed by a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Data were analyzed by using GraphPad software® (San Diego, CA; version 5.00). Differences were considered to be significant when ∗p < 0.05, ∗∗ p < 0.01, or ∗∗∗ p < 0.001 and between groups (#p < 0.05, ##p < 0.01, or ###p < 0.001).
Results
Effects of Antioxidant Treatment on Morphologic Differentiation, Cell Viability, and Proliferation
SH-SY5Y cells were differentiated for 7 days with RA in the presence or absence of the antioxidant Trolox®. Proliferative (grown at 10 % FBS) and non-proliferative (grown at 1 % FBS) cells were used as controls for differentiation, while cells under RA at day zero (brief addition of RA in 1 % FBS and termination at time zero of differentiation) were used to compare with the effects of RA at 7 days of differentiation. The neuroblastoma SH-SY5Y cell line includes at least two morphologically and biochemically distinct phenotypes: neuroblastic (N-type) and substrate adherent (S-type) (Fig. 2a, c). Proliferative cells present typical rounded cell bodies and few short projections. In non-proliferative cells, increased formation of neurite projections, that were generally small, was observed. Prolonged exposure to RA (7 days) caused substantial morphologic differentiation, with a considerable proportion of neuroblastic (N-type) cells being differentiated into a neuronal phenotype by extending long neuritic processes, whereas the S-type cells did not undergo apparent morphological changes (Fig. 2g, h). Besides the length of neurites, cells differentiated with RA also presented a higher number of elongations and branching and evident fewer cells/area than observed in other treatments. Co-treatment with Trolox® led to a smaller proportion of the N-type cells undergoing differentiation, and the apparent number of S-type cells increased compared to cells treated with RA (Fig. 2i, j). Cells treated only with Trolox® presented significantly increased cells/field (Fig. 2k, l), and although many of them had minor neuritic projections, we observed a significantly higher proportion of cells displaying morphologic characteristics resembling proliferative or apoptotic states. Cells maintained in 1 % FBS without RA for 7 days presented extensive cell death and detachment and were not evaluated in further assays (not shown).
Parameters of cellular viability and neuronal morphologic phenotype. a–l Effect of antioxidant co-treatment with Trolox® on morphologic differentiation induced by RA. SH-SY5Y cells are mainly neuroblastic N-type (arrows), but some substrate-adherent S-type cells appear (arrowheads) and present morphological alterations after differentiation, with neurite elongation and branching. Scale bars represent 30 μm (20×) and 15 μm (40×). m Cellular protein biomass assessed by SRB incorporation. n LDH leakage after RA, RA+Trolox®, and Trolox® treatment for 7 days. Data are mean ± S.E.M. from three independent experiments. Analyzed by one-way ANOVA followed by post hoc Tukey’s test, triple asterisk indicates p < 0.05 vs control 10 % and triple octothorpe indicates p < 0.05 vs respective groups
In order to understand the effect of antioxidant treatment on RA-induced neurodifferentiation, we performed assays to evaluate parameters of cell viability and cytotoxicity, proliferation and cell cycle progression. First, we assessed SRB incorporation and medium LDH activity to evaluate protein biomass and viability/cytotoxicity. As expected, non-proliferative cells had a decreased rate of SRB incorporation compared to proliferative cells, and RA treatment for 7 days further decreased this effect (Fig. 2m). This effect is probably related to the decrease in the number of proliferating and undifferentiated cells observed along the differentiation treatment with RA. Antioxidant treatment with Trolox®, however, had no significant effect on RA-induced SRB incorporation. Cells subjected to Trolox® alone for 7 days displayed a similar SRB incorporation to proliferative cells. As SRB incorporation is related to the protein biomass and cultured cells undergoing differentiation may present less protein biomass than in proliferative or undifferentiated conditions, we assessed LDH activity in the incubation medium to detect cytosolic leakage. We did not observe significant differences between groups, except for cells treated with Trolox® in the presence or absence of RA, which presented a small increase in medium LDH activity (Fig. 2n). A positive control group for cytotoxicity with Triton X-100 1 % was used, and medium LDH activity was approximately 100-fold higher than in proliferative cells (not shown).
Next, we determined the number of cells incorporating EdU® to evaluate the proportion of cells under active mitotic activity in each treatment. An equal number of cells from each group was analyzed, and the number of cells incorporating EdU® was decreased in non-proliferative cells compared to proliferative cells (Fig. 3a, b). Differentiation with RA substantially decreased the number of EdU®-incorporating cells and this effect was not inhibited by co-treatment with Trolox®. Treatment with Trolox® alone also decreased EdU®-incorporation in relation to the proliferative cell control, but not to the extent of RA treatment (Fig. 3a, b). FACS analysis of cell cycle by PI staining revealed that differentiation with RA increased the proportion of cells in G0/G1 phase and decreased the amount of cells in G2/M phase (Fig. 3c). Co-treatment with Trolox® induced an increase in sub-G1 phase cells and also decreased the proportion of G0/G1 cells (Fig. 3c). Trolox® alone increased the number of cells in G2/M phase and reduced the amount in G0/G1 phase. Altogether, these results are suggestive that RA-induced changes in proliferation and cell cycle progression during the process of differentiation are inhibited by antioxidant treatment, which causes cell cycle arrest with cell death as possible consequence.
Flow cytometry analyses of cell proliferation and differentiation. a Cell proliferation was assessed by flow cytometry to detect and count cells incorporating EdU®. b Quantification of EdU® incorporation in cells treated with RA, RA+Trolox®, and Trolox® for 7 days, with respective controls. c Cell cycle analysis of SH-SY5Y cells was performed with detection and sorting of cells with different profiles of incorporated PI. p < 0.05 G0/G1 phase RA vs RA+T; p < 0.001 SubG1 phase control 10 % vs RA+T; p < 0.001 SubG1 phase RA vs RA+T
Effects of Antioxidant Treatment on Neuronal Markers Expression
Tyrosine hydroxylase (TH) is considered a marker of SH-SY5Y cell differentiation into an adult catecholaminergic phenotype, and all treatments increased the proportion of cells containing TH as seen in FACS analysis (Fig. 3b, quantification at Fig. 4a). The proportion of TH-positive, differentiated cells was decreased by co-treatment with Trolox® (Figs. 3b and 4a). The quantification of TH mRNA by RT-PCR confirmed these observations (Fig. 4b). Enolase-2, another neuronal specific protein [8], was evaluated by WB and followed a similar pattern (Fig. 4c). We also evaluated the effect of antioxidant treatment on β-III tubulin and TH by confocal immunofluorescence microscopy which allows observing the expression of these neuronal markers along with the differentiation into the neuronal morphologic phenotype (Fig. 4d). All of the treatments increased the content of TH in the same overall pattern as previously seen, with the cells treated with RA for 7 days showing the largest increase in TH immunostaining along with neurite projection and branching per cell body (Fig. 4d13–d16). Co-treatment with Trolox® decreased TH immunostaining along with neurite formation and branching (Fig. 4d17–d20). A similar pattern of neuronal marker expression in response to RA and Trolox® was observed with β-III tubulin (Fig. 4). Cells treated with Trolox® alone also showed a substantial increase in β-III tubulin immunofluorescence, but probably due to the higher number of cells per field.
Effect of antioxidant treatment on expression of mature neuron markers induced by RA differentiation. a Mean values quantification of flow cytometry analysis of tyrosine hydroxylase positive cells. Data are mean ± S.E.M. from three independent experiments. b Quantification of mRNA expression for catecholaminergic marker tyrosine hydroxylase (TH) after RA differentiation. c Western blot analysis of neuron specific marker enolase-2. Values are expressed as mean ± SEM of three independent experiments (n = 3). Statistical difference compared to control was determined by one-way ANOVA followed by Tukey’s post hoc test (single asterisk indicates different to control 10 % p < 0.05; triple asterisk indicates p < 0.001; octothorpe indicates difference between groups p < 0.05). d Confocal immunofluorescence microscopy of SH-SY5Y cells after RA, RA+Trolox®, and Trolox® treatment for 7 days with respective controls. Tyrosine hydroxylase (green fluorescence), DAPI (nuclei), and β-III tubulin (red fluorescence) are shown. Scale bars represent 10 μm
Effects of RA on Intracellular RS Production and Mitochondrial Activity in SH-SY5Y Cells
As our results indicated that antioxidant treatment inhibits the action of RA on neuronal differentiation, we next investigated the intracellular reactive species production in SH-SY5Y cells treated with RA. The first assay performed was the monitoring of DCFH oxidation, a technique used to analyze the overall production of intracellular RS (Fig. 5a). Proliferative and non-proliferative cells did not present differences in basal RS production. However, RA induces a significant increase in DCFH oxidation rate and this effect was partially inhibited by co-treatment with Trolox®. The second assay used was the total reactive antioxidant potential (TRAP), which gives an indication of the non-enzymatic antioxidant capacity of the cells against RS produced by an artificial generation system. The production of RS by the TRAP system is enhanced by homogenates from cells treated with RA (Fig. 5b), which correlates to the increase in RS production found by DCFH-DA assay. Homogenates from cells co-treated with Trolox® present a significant reversion of this effect, indicating that Trolox® inhibits the pro-oxidant effect of RA. We next evaluated the rate of NADH oxidation by isolated mitochondria, as mitochondrial activity is one of the main sources of RS production in aerobic cells (Fig. 5c). Proliferative cells generally demonstrate higher mitochondrial activity that is related to their increased metabolic demand, and our results confirmed a reduced mitochondrial activity in non-proliferative cells compared to proliferative cells. Cells exposed to RA demonstrated a similar profile of mitochondrial oxidative activity to non-proliferative cells, but co-treatment with Trolox® partially restored the rate of NADH oxidation. Interestingly, Trolox alone® significantly enhanced the rate of NADH oxidation, which may be related to the increased proliferation rate observed in this group earlier. These results, altogether, are suggestive that the increased RS production observed in cells treated with RA may be a consequence of impaired NADH oxidation by mitochondria, as altered mitochondrial activity is a common source of oxidative stress in cells.
Effect of antioxidant co-treatment on reactive species production, non-enzymatic antioxidant profile, and mitochondrial NADH oxidation in cells differentiated with RA. a DCF fluorescence monitoring after SH-SY5Y RA, RA+Trolox®, and Trolox® treatment for 7 days and respective controls. b The total antioxidant potential (TRAP) evaluation was performed and the area under curve values obtained from kinetic monitoring are demonstrated. Values are expressed as mean ± SEM of three independent experiments (n = 3). c SH-SY5Y cells mitochondrial function was analyzed through NADH oxidase assay. Mitochondria were isolated from cells after RA, RA+Trolox®, and Trolox® treatments for 7 days, with respective controls. Statistical difference compared to control was determined by one-way ANOVA followed by Tukey’s post hoc test (single asterisk indicates different to control 10 % p < 0.05; triple asterisk indicates p < 0.001; octothorpe indicates difference between groups p < 0.05)
Effect of Antioxidant Treatment on Akt and ERK1/2 Phosphorylation by RA in SH-SY5Y Cells
In previous reports, RA was observed to induce non-genomic, rapid (up to 60 min) activation of Akt and ERK1/2 in SH-SY5Y cells, and neuronal differentiation and cell survival were shown to be dependent on this effect [9, 32]. To investigate whether RS induced by RA influence protein kinases phosphorylation, we evaluated the effect of co-treatment with Trolox®. Cells were pre-incubated with Trolox® for 2 h and then RA was added for different periods. Increases in Akt and ERK1/2 phosphorylation are observed in different periods of RA incubation; Akt phosphorylation was increased 45 min after RA addition while ERK1/2 phosphorylation was enhanced in cells briefly exposed to RA (day zero) and also 15 min later (Fig. 6). The extent of Akt and ERK1/2 phosphorylation with RA was reduced in the presence of Trolox®, thus indicating the participation of RA-induced RS in the mechanism of activation of these protein kinases.
Effect of antioxidant treatment on Akt and ERK1/2 phosphorylation by RA in SH-SY5Y cells. Cells were pre-incubated for 2 h with Trolox® (AR+T and T groups) and then RA was added (Trolox group). Cells were harvested at 0, 15, 30, 45, and 60 min after RA addition and Akt and ERK1/2 phosphorylation were evaluated by western blot. Mean quantitative values are depicted as a percentage of control and b fold-induction
Discussion
For many years, several attempts to transplant neurons and restore the dopaminergic transmission have been performed [12]. The nervous system involves a complex and intrinsic cellular signaling, and our understanding of the molecular mechanisms involved in cell cycle regulation and neuronal differentiation has markedly increased over recent years. In order to elucidate the mechanisms of neuronal differentiation and maturation, different cell models have been used in mechanistic studies. One of these models is the human neuroblastoma SH-SY5Y cell line, which is typically locked in an early neuronal differentiation stage, characterized by low levels of neuronal markers. Upon exposure to appropriate differentiation conditions, SH-SY5Y cells can be driven toward differentiated, mature, neuronal phenotypes. In this report, we explored the role of RS during in vitro neurodifferentiation, demonstrating through differentiation of SH-SY5Y cells with RA and the antioxidant Trolox®, that this process is actually dependent on RS production and oxidative stress.
The effects of RA in SH-SY5Y neurodifferentiation are well documented, which include attenuation of proliferative rate and extension of neuritic processes [4]. Nonetheless, proper evaluation of cell cycle progression, cytotoxicity, and cell number are often underestimated, as these parameters rarely are evaluated simultaneously in the same work. In our experiments, we observed considerable changes in cell number and viability, and these features seemed to be conditioned by the concentration of FBS in the medium (which is the major stimulator of proliferation) together with RA, which is the main inducer of biochemical and morphological differentiation in this model. Here, we confirm that long-term exposure to RA markedly reduced proliferation as the cell number decreases, EdU® incorporation (indicative of proliferation) decreases, SRB incorporation (indicative of total cell protein biomass) decreases, and percentage of cells at G0/G1 increases. The SRB incorporation assay, commonly used to assess cell viability, gives an accurate measure of total protein biomass, which is a parameter more closely related to the number of cells under a given condition. Thus, the decrease in SRB incorporation promoted by RA is a consequence of the inhibition in proliferative capacity, but is not related to cytotoxicity/cell death. However, cells co-treated with RA and Trolox® also displayed decreased SRB incorporation, but increased LDH activity in the medium and this indicates that the decrease in protein biomass is a consequence of cell death. Importantly, cells treated with RA did not show any alterations in morphology or medium LDH activity suggestive of cytotoxicity. However, the co-treatment with Trolox® reduced the proportion of cells under G0/G1 by RA and led to a significant increase in cells at sub-G1 phase, which is associated to cells undergoing apoptotic processes. These observations contribute to the suggestion that antioxidant treatment inhibits the modifications in cell cycle by RA and this process results in cell death. Although LDH activity is commonly associated to necrotic death while cells under sub-G1 state are more associated to apoptosis, these two types of cell death are not mutually exclusive of each other and may take place together in heterogeneous populations of cells. Previous studies demonstrated that RA induction of cellular differentiation is preceded by cell cycle arrest, with a higher percentage of cells in G1 and a reduction of cells in S phase after RA treatment [4, 12]. Our data are consistent with that, as in our model RA treatment maintained SH-SY5Y cells in a post mitotic state, with higher percentage of cells at G0/G1 state, and here we observed that antioxidant treatment did not reverse these cells to proliferative state, as EdU® incorporation was not altered. Altogether, these observations suggest that RS production by RA is a necessary step to drive cells into the differentiated phenotype and that the changes in cell cycle induced by RA may result in cell death if RS production is inhibited.
The capacity of RA to induce differentiation is a well-established protocol in neuroscience research [10]. Here, we confirmed that RA induced morphological alterations to cells, with extension of neuritic processes in N-type cells to form a typical neuronal network phenotype. Importantly, RA induced biochemical changes that are commonly associated to the adult catecholaminergic phenotype, including increased expression of TH, β-III tubulin, and enolase-2, considered markers associated with neurogenesis and neural differentiation [33–35]. Trolox® decreased both the morphological changes and the expression of such markers, indicating that RA-induced differentiation of SH-SY5Y cells requires a pro-oxidant state to take place. In previous works, some evidence that changes in the redox state were required for neurodifferentiation by RA were observed. The induction and activation of Nrf2, which up-regulates the transcription of antioxidant enzymes and is considered the major transcription factor in cellular antioxidant response, was demonstrated to be a necessary step in neurite formation and induction of MAP2, a marker of neuritogenesis, in SH-SY5Y cells treated for 4 days with RA [24]. In cells treated with RA for up to 96 h, Nrf2 was shown to exert a prominent role in the promotion of the survival of SH-SY5Y cells against RA cytotoxicity, which suggested that RA induced a pro-oxidant environment during differentiation, causing oxidative stress [36]. Here, we investigated if RS production and consequent oxidative stress induced by RA were necessary events in the full differentiation of SH-SY5Y by evaluating the expression of biochemical markers of adult neuronal phenotype, such as TH (considered the gold standard for catecholaminergic neurons) and enolase-2, as well as β-III tubulin.
Exposure of SH-SY5Y cells to RA substantially increased the level of RS and induced a pro-oxidant state since day zero of incubation, and we found this observation important for several reasons concerning the possible mechanisms of RA action on neuronal differentiation. RA is a byproduct of vitamin A (retinol) metabolism, and it is generally considered to act through the activation of nuclear steroid receptors and retinoid receptors of the RAR/RXR subfamilies. These changes in RS production and pro-oxidant environment occurred well before protein expression changes are likely to have developed and so alternative mechanisms for RA action must be considered. Non-genomic or extra nuclear effects of retinoids have shown that RA can modulate signaling pathways independently of retinoid nuclear receptor activation and gene transactivation [14, 37, 38]. RS production by RA was previously demonstrated to result in increased activity of the antioxidant enzyme catalase [16] and also cause the non-genomic stimulation of MEK1/2-ERK1/2-caspase-3 pathway [15], all events related to oxidative stress responses. Once we observed that RA was creating a pro-oxidant effect in cells and possibly acting through non-genomic mechanisms, we analyzed the phosphorylation of Akt and ERK1/2. Both Akt and ERK1/2 are cell survival pathways and also regulate the differentiation and proliferation. In previous works, the phosphorylation/activation of Akt and ERK1/2 was observed in SH-SY5Y differentiation by RA [9, 32]. Differentiation was completely blocked using inhibitors of PI3K/Akt pathway; however, the use of ERK1/2 inhibitors had no effect on cellular differentiation, although led to cell death [10]. Activation of Akt was demonstrated to be mediated by RAR through a non-classical mechanism of interaction with PI3K [11], confirming the involvement of non-genomic mechanisms of RA action in protein kinase signaling. In our study, brief treatment with RA was able to induce the generation of RS, leading to a pro-oxidant environment that was associated to the increased phosphorylation of Akt and ERK1/2, suggesting that non-genomic actions by RA involving RS production are essential in SH-SY5Y neuronal differentiation. We observed that pre-treatment with Trolox® caused a reduction in Akt and ERK1/2 phosphorylation induced by RA addition. It is important to note that Trolox® alone also increased ERK1/2 phosphorylation in such short periods of incubation and this effect may be related to the increased number of cells in this group. Non-proliferative SH-SY5Y cells, which were exposed only to FBS 1 %, did not show changes in RS production, indicating that serum deprivation did not contribute to the pro-oxidant state observed with RA. Redox-dependent activation of protein kinases including members of the MAPK family and the PI3K/Akt pathway were observed in other cell types and with different pro-oxidant agents, including retinoids [19, 21, 39, 40].
Long-term exposure of SH-SY5Y cells to RA maintained the level of RS in a pro-oxidant state for 7 days. Retinoids are known to have redox-related properties and influence the oxidant status of the cell [19, 22, 41, 42]. Previous studies showed that RA treatment for 7 days in SH-SY5Y cells alters the redox status of the cell [43], and activates antioxidant enzymes that contribute to endogenous cellular defense (e.g., catalase, superoxide dismutase, and glutathione peroxidase) [22]. RA co-treatment with Trolox® significantly decreased RS production and the pro-oxidant environment generated by RA. The TRAP assay, in which the production of RS (mainly peroxyl radicals) by a synthetic free radical generator system is monitored in the presence of samples depleted from their enzymatic activities, showed that homogenates from cells incubated with RA were able to enhance RS production by this system; however, in DFCH oxidation assay, which is performed with intact living cells, RA also enhances RS production. These assays altogether indicate that both enzymatic and non-enzymatic mechanisms may be involved in RA-induced RS production in our experimental conditions. RA-induced differentiation is therefore dependent on the rapid production and long-term maintenance of RS and pro-oxidant environment, suggesting a redox-dependent mechanism of differentiation. Nonetheless, the exact mechanisms by which RA modulates RS production during neuronal differentiation remain to be better understood. Retinoids and RA itself have been demonstrated to influence the cell redox state by several mechanisms, acting as antioxidants or pro-oxidants in different contexts. Beyond creating a pro-oxidant status in the cell, another consistent feature of exposure to RA is the presence of changes in cellular bioenergetics [23, 44] and mitochondria impairment in response to oxidative stress [45]. Recent evidence indicates that mitochondria might be a key effector in RA-induced differentiation of SH-SY5Y cells [44]. Accordingly, the well-established pharmacological use of RA to treat neuroblastoma is based in its ability to induce cell cycle arrest and apoptosis through Bcl-2 activation [46]. Increased mitochondrial membrane potential, levels of cytochrome c oxidase, MnSOD, and also increased bioenergetic reserve capacity occur after RA-induced differentiation [23, 44]. Furthermore, RA induces the expression of glycolytic pathway proteins, probably due to the increase in energy required for neurodifferentiation [22].
Here, we observed that cells submitted to differentiation with RA decreased their rate of mitochondrial NADH oxidation and this was partially reversed by Trolox®. Importantly, mitochondrial NADH oxidation was significantly decreased in all treatments carried in medium with decreased serum (1 % FBS), if compared to proliferative cells, maintained in 10 % FBS. This result is expected due to the presence of oxidizable energetic substrates in FBS, such as glucose; nevertheless, Trolox® also stimulated NADH oxidation in cells maintained in 1 % FBS for 7 days in the absence of RA. These results, altogether, are suggestive that changes in mitochondrial activity in order to adapt the bioenergetic requirements of the cells to the conditions of serum deprivation are a necessary step prior to RA addition in this model of neuronal differentiation. It is known that differentiated cells in a post mitotic state depend primarily on mitochondria to meet their energetic requirements [23, 44], and we found that long-term treatment with RA maintained mitochondrial NADH oxidation decreased. The reduced mitochondrial activity is likely to be associated with the observed increase in RS production by RA, since oxidative stress can result from cellular respiratory changes that lead to the leakage of electrons from donor redox centers to molecular oxygen [45, 47, 48]. Schneider et al. observed that RA treatment actually increased the mitochondrial capacity and bioenergetic reserve along 5 days of differentiation; importantly, in that study, cells at the day zero of differentiation—therefore maintained 24 h in 1 % FBS before RA addition—were considered as undifferentiated cells, compared to cells under 5-day differentiation with RA [23]. Here, we also observed a significant increase in mitochondrial NADH oxidation rate by cells differentiated with RA for 7 days compared to day zero, although cells maintained at 10 % FBS presented much higher rates of NADH oxidation. It was suggested that differentiation by RA increased the cell resistance against oxidative stress [23], but our findings indicate that oxidative stress is actually an essential step of differentiation. Corroborating this, we observed that RA co-treatment with Trolox® reduced the level of oxidative stress and consequently mitochondrial activity was restored; besides, and more surprisingly, treatment with Trolox® alone substantially improved mitochondrial function, consistent with low levels of RS and the continued proliferative status of the cell. This result suggests that maintaining mitochondrial activity at low levels during differentiation may be an adaptation to the pro-oxidant effect of RA, as treatment with Trolox® for 7 days in 1 % FBS without RA-stimulated mitochondrial activity to levels above proliferative cells. Previous works by us and others demonstrated that Nrf2 is an essential step in RA-induced differentiation [24, 36] and the role of Nrf2 activity on mitochondrial activity during RA-induced neuronal differentiation remains to be better understood. Besides, Trolox® is able to exert specific effects in mitochondrial NADH oxidation due to its intrinsic ability to scavenge RS generated by intracellular metabolism. It was previously observed that, when added alone to cultured cells, Trolox® induces mitochondrial thiol reduction, mitochondrial filamentation and, importantly, increased expression of fully assembled mitochondrial complex I, as well as enhanced citrate synthase activity [49].
In conclusion, findings from this study demonstrate that the pro-oxidant effects of RA are required for the differentiation of SH-SY5Y cells into an adult neuronal phenotype, expressing markers of mature catecholaminergic neurons such as TH, enolase-2, and β-III tubulin. Moreover, RA-differentiation induced cell cycle withdrawn and reduced mitochondrial NADH oxidation. Co-treatment with Trolox® was able to block the neuronal differentiation, maintaining cells into a proliferative state but ultimately leading to cell death after 7 days, probably due to the serum deprivation. Our results confirm previous findings showing that the antioxidant defense system was essential for RA-induced neuronal differentiation in SH-SY5Y cells, but also highlight that the generation of RS and oxidative stress is an essential step in this process. Since RS and oxidative stress affect the activity of several regulatory pathways through redox-dependent protein modulation, this study may bring new insights on the understanding of neuronal differentiation signaling.
References
Alberio T, Lopiano L, Fasano M (2012) Cellular models to investigate biochemical pathways in Parkinson’s disease. FEBS J 279(7):1146–1155. doi:10.1111/j.1742-4658.2012.08516.x
Hattori N, Wanga M, Taka H, Fujimura T, Yoritaka A, Kubo S, Mochizuki H (2009) Toxic effects of dopamine metabolism in Parkinson’s disease. Parkinsonism and Related Disorders 15:S35–S38
Agholme L, Lindstrom T, Kagedal K, Marcusson J, Hallbeck M (2010) An in vitro model for neuroscience: differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. Journal of Alzheimer’s disease: JAD 20(4):1069–1082
Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Ceña V, Gallego C, Comella JX (2000) Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem 75(3):991–1003
Filograna R, Civiero L, Ferrari V, Codolo G, Greggio E, Bubacco L, Beltramini M, Bisaglia M (2015) Analysis of the catecholaminergic phenotype in human SH-SY5Y and BE(2)-M17 neuroblastoma cell lines upon differentiation. PLoS One 10(8):e0136769. doi:10.1371/journal.pone.0136769
Korecka JA, van Kesteren RE, Blaas E, Spitzer SO, Kamstra JH, Smit AB, Swaab DF, Verhaagen J et al (2013) Phenotypic characterization of retinoic acid differentiated SH-SY5Y cells by transcriptional profiling. PLoS One 8(5):e63862. doi:10.1371/journal.pone.0063862
Maden M (2007) Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci 8(10):755–765. doi:10.1038/nrn2212
Cheung YT, Lau WK, Yu MS, Lai CS, Yeung SC, So KF, Chang RC (2009) Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 30(1):127–135. doi:10.1016/j.neuro.2008.11.001
Miloso M, Villa D, Crimi M, Galbiati S, Donzelli E, Nicolini G, Tredici G (2004) Retinoic acid-induced neuritogenesis of human neuroblastoma SH-SY5Y cells is ERK independent and PKC dependent. J Neurosci Res 75:241–252
Qiao J, Paul P, Lee S, Qiao L, Josifi E, Tiao JR, Chung DH (2012) PI3K/AKT and ERK regulate retinoic acid-induced neuroblastoma cellular differentiation. Biochem Biophys Res Commun 424(3):421–426. doi:10.1016/j.bbrc.2012.06.125
Masia S, Alvarez S, de Lera AR, Barettino D (2007) Rapid, nongenomic actions of retinoic acid on phosphatidylinositol-3-kinase signaling pathway mediated by the retinoic acid receptor. Mol Endocrinol 21(10):2391–2402
Li W, Chen S, Li JY (2015) Human induced pluripotent stem cells in Parkinson’s disease: a novel cell source of cell therapy and disease modeling. Prog Neurobiol. doi:10.1016/j.pneurobio.2015.09.009
Klamt F, Dal-Pizzol F, Roehrs R, de Oliveira RB, Dalmolin R, Henriques JAP, de Andrades HHR, de Paula Ramos ALL et al (2003) Genotoxicity, recombinogenicity and cellular preneoplasic transformation induced by vitamin a supplementation. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 539(1–2):117–125. doi:10.1016/s1383-5718(03)00155-4
Gelain DP, Moreira JC (2008) Evidence of increased reactive species formation by retinol, but not retinoic acid, in PC12 cells. Toxicology in vitro: an international journal published in association with BIBRA 22(3):553–558. doi:10.1016/j.tiv.2007.11.007
Zanotto-Filho A, Cammarota M, Gelain DP, Oliveira RB, Delgado-Canedo A, Dalmolin RJ, Pasquali MA, Moreira JC (2008) Retinoic acid induces apoptosis by a non-classical mechanism of ERK1/2 activation. Toxicology in vitro: an international journal published in association with BIBRA 22(5):1205–1212. doi:10.1016/j.tiv.2008.04.001
Pasquali MA, Gelain DP, Zanotto-Filho A, de Souza LF, de Oliveira RB, Klamt F, Moreira JC (2008) Retinol and retinoic acid modulate catalase activity in Sertoli cells by distinct and gene expression-independent mechanisms. Toxicology in vitro: an international journal published in association with BIBRA 22(5):1177–1183. doi:10.1016/j.tiv.2008.03.007
Klamt F, Dal-Pizzol F, Roehrs R, de Oliveira RB, Dalmolin R, Henriques JA, de Andrades HH, de Paula Ramos AL et al (2003) Genotoxicity, recombinogenicity and cellular preneoplasic transformation induced by vitamin A supplementation. Mutat Res 539(1–2):117–125
Dal-Pizzol F, Klamt F, Dalmolin RJ, Bernard EA, Moreira JC (2001) Mitogenic signaling mediated by oxidants in retinol treated Sertoli cells. Free Radic Res 35(6):749–755
Gelain DP, Cammarota M, Zanotto-Filho A Jr, de Oliveira RB, Dal-Pizzol F, Izquierdo I, Bevilaqua LRM, Moreira JC (2006) Retinol induces the ERK1/2-dependent phosphorylation of CREB through a pathway involving the generation of reactive oxygen species in cultured Sertoli cells. Cell Signal 18(10):1685–1694. doi:10.1016/j.cellsig.2006.01.008
Moreira JC, Dal-Pizzol F, Rocha AB, Klamt F, Ribeiro NC, Ferreira CJ, Bernard EA (2000) Retinol-induced changes in the phosphorylation levels of histones and high mobility group proteins from Sertoli cells. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica [et al] 33(3):287–293
Gelain DP, Moreira JC, Bevilaqua LR, Dickson PW, Dunkley PR (2007) Retinol activates tyrosine hydroxylase acutely by increasing the phosphorylation of serine40 and then serine31 in bovine adrenal chromaffin cells. J Neurochem 103(6):2369–2379. doi:10.1111/j.1471-4159.2007.04935.x
de Bittencourt Pasquali MA, de Ramos VM, Albanus RD, Kunzler A, de Souza LH, Dalmolin RJ, Gelain DP, Ribeiro L et al (2014) Gene expression profile of NF-kappaB, Nrf2, glycolytic, and p53 pathways during the SH-SY5Y neuronal differentiation mediated by retinoic acid. Mol Neurobiol. doi:10.1007/s12035-014-8998-9
Schneider L, Giordano S, Zelickson BR, Johnson MS, Benavides GA, Ouyang X, Fineberg N, Darley-Usmar VM et al (2011) Differentiation of SH-SY5Y cells to a neuronal phenotype changes cellular bioenergetics and the response to oxidative stress. Free Radic Biol Med 51(11):2007–2017. doi:10.1016/j.freeradbiomed.2011.08.030
Zhao F, Wu T, Lau A, Jiang T, Huang Z, Wang XJ, Chen W, Wong PK et al (2009) Nrf2 promotes neuronal cell differentiation. Free Radic Biol Med 47(6):867–879. doi:10.1016/j.freeradbiomed.2009.06.029
Nitti M, Furfaro AL, Cevasco C, Traverso N, Marinari UM, Pronzato MA, Domenicotti C (2010) PKC delta and NADPH oxidase in retinoic acid-induced neuroblastoma cell differentiation. Cell Signal 22(5):828–835. doi:10.1016/j.cellsig.2010.01.007
Vichai V, Kirtikara K (2006) Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 1(3):1112–1116. doi:10.1038/nprot.2006.179
Rabelo TK, Zeidan-Chulia F, Vasques LM, dos Santos JP, da Rocha RF, Pasquali MA, Rybarczyk-Filho JL, Araujo AA et al (2012) Redox characterization of usnic acid and its cytotoxic effect on human neuron-like cells (SH-SY5Y). Toxicology in vitro: an international journal published in association with BIBRA 26(2):304–314. doi:10.1016/j.tiv.2011.12.003
Wang HaJ JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27(5/6):612–616
Voss DO, Campello AP, Bacila M (1961) The respiratory chain and the oxidative phosphorylation of rat brain mitochondria. Biochem Biophys Res Comm 4(1):48–51
Singer TP (1974) Determination of the activity of succinate, NADH, choline, and α-glycerophosphate dehydrogenases. Methods Biochem Anal 22:123–175
LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193
Lopez-Carballo G, Moreno L, Masia S, Perez P, Barettino D (2002) Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol Chem 277(28):25297–25304. doi:10.1074/jbc.M201869200
Lehmann IT, Bobrovskaya L, Gordon SL, Dunkley PR, Dickson PW (2006) Differential regulation of the human tyrosine hydroxylase isoforms via hierarchical phosphorylation. J Biol Chem 281(26):17644–17651
Lestanova Z, Bacova Z, Kiss A, Havranek T, Strbak V, Bakos J (2015) Oxytocin increases neurite length and expression of cytoskeletal proteins associated with neuronal growth. J Mol Neurosci. doi:10.1007/s12031-015-0664-9
Graser S, Mentrup B, Schneider D, Klein-Hitpass L, Jakob F, Hofmann C (2015) Overexpression of tissue-nonspecific alkaline phosphatase increases the expression of neurogenic differentiation markers in the human SH-SY5Y neuroblastoma cell line. Bone 79:150–161. doi:10.1016/j.bone.2015.05.033
de Miranda RV, Zanotto-Filho A, de Bittencourt Pasquali MA, Klafke K, Gasparotto J, Dunkley P, Gelain DP, Moreira JC (2015) NRF2 mediates neuroblastoma proliferation and resistance to retinoic acid cytotoxicity in a model of in vitro neuronal differentiation. Mol Neurobiol. doi:10.1007/s12035-015-9506-6
Canon E, Cosgaya JM, Scsucova S, Aranda A (2004) Rapid effects of retinoic acid on CREB and ERK phosphorylation in neuronal cells. Mol Biol Cell 15(12):5583–5592. doi:10.1091/mbc.E04-05-0439
Aggarwal S, Kim SW, Cheon K, Tabassam FH, Yoon JH, Koo JS (2006) Nonclassical action of retinoic acid on the activation of the cAMP response element-binding protein in normal human bronchial epithelial cells. Mol Biol Cell 17(2):566–575
Gelain DP, de Bittencourt Pasquali MA, Caregnato FF, Fonseca Moreira JC (2011) Vitamin A (retinol) up-regulates the receptor for advanced glycation endproducts (RAGE) through p38 and Akt oxidant-dependent activation. Toxicology 289(1):38–44. doi:10.1016/j.tox.2011.07.008
Zanotto-Filho A, Cammarota M, Gelain DP, Oliveira RB, Delgado-Canedo A, Dalmolin RJS, Pasquali MAB, Moreira JCF (2008) Retinoic acid induces apoptosis by a non-classical mechanism of ERK1/2 activation. Toxicol in Vitro 22(5):1205–1212. doi:10.1016/j.tiv.2008.04.001
Gelain DP, Pasquali MA, Caregnato FF, Castro MA, Moreira JC (2012) Retinol induces morphological alterations and proliferative focus formation through free radical-mediated activation of multiple signaling pathways. Acta Pharmacol Sin 33(4):558–567. doi:10.1038/aps.2011.202
de Bittencourt Pasquali MA, Gelain DP, Zeidan-Chulia F, Pires AS, Gasparotto J, Terra SR, Moreira JC (2013) Vitamin A (retinol) downregulates the receptor for advanced glycation endproducts (RAGE) by oxidant-dependent activation of p38 MAPK and NF-kB in human lung cancer A549 cells. Cell Signal 25(4):939–954
da Frota Junior ML, Pires AS, Zeidan-Chulia F, Bristot IJ, Lopes FM, de Bittencourt Pasquali MA, Zanotto-Filho A, Behr GA et al (2011) In vitro optimization of retinoic acid-induced neuritogenesis and TH endogenous expression in human SH-SY5Y neuroblastoma cells by the antioxidant Trolox. Mol Cell Biochem 358(1–2):325–334. doi:10.1007/s11010-011-0983-2
Xun Z, Lee DY, Lim J, Canaria CA, Barnebey A, Yanonne SM, McMurray CT (2012) Retinoic acid-induced differentiation increases the rate of oxygen consumption and enhances the spare respiratory capacity of mitochondria in SH-SY5Y cells. Mech Ageing Dev 133(4):176–185. doi:10.1016/j.mad.2012.01.008
Strom J, Xu B, Tian X, Chen QM (2015) Nrf2 protects mitochondrial decay by oxidative stress. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. doi:10.1096/fj.14-268904
Niizuma H, Nakamura Y, Ozaki T, Nakanishi H, Ohira M, Isogai E, Kageyama H, Imaizumi M et al (2006) Bcl-2 is a key regulator for the retinoic acid-induced apoptotic cell death in neuroblastoma. Oncogene 25(36):5046–5055. doi:10.1038/sj.onc.1209515
Wang X, Yu S, Wang CY, Wang Y, Liu HX, Cui Y, Zhang LD (2015) Advanced glycation end products induce oxidative stress and mitochondrial dysfunction in SH-SY5Y cells. In vitro cellular & developmental biology Animal 51(2):204–209. doi:10.1007/s11626-014-9823-5
Moran M, Moreno-Lastres D, Marin-Buera L, Arenas J, Martin MA, Ugalde C (2012) Mitochondrial respiratory chain dysfunction: implications in neurodegeneration. Free Radic Biol Med 53(3):595–609. doi:10.1016/j.freeradbiomed.2012.05.009
Distelmaier F, Valsecchi F, Forkink M, van Emst-de Vries S, Swarts HG, Rodenburg RJ, Verwiel ET, Smeitink JA et al (2012) Trolox-sensitive reactive oxygen species regulate mitochondrial morphology, oxidative phosphorylation and cytosolic calcium handling in healthy cells. Antioxid Redox Signal 17(12):1657–1669. doi:10.1089/ars.2011.4294
Acknowledgments
This work was supported by the Brazilian funds CNPq (401260/2014-3, 400437/2013-9, 443514/2014-3, 401368/2012-2 and 303227/2015-0), CAPES, FAPERGS (2299-2551/14-6), and Propesq-UFRGS. The authors thank Mr. Henrique Biehl for their technical assistance at the CME.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kunzler, A., Zeidán-Chuliá, F., Gasparotto, J. et al. Changes in Cell Cycle and Up-Regulation of Neuronal Markers During SH-SY5Y Neurodifferentiation by Retinoic Acid are Mediated by Reactive Species Production and Oxidative Stress. Mol Neurobiol 54, 6903–6916 (2017). https://doi.org/10.1007/s12035-016-0189-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0189-4