Abstract
Glial cell line-derived neurotrophic factor (GDNF), which belongs to transforming growth factor β superfamily, plays important roles in glioma pathogenesis. Gdnf mRNA is aberrantly increased in glioma cells, but the underlying transcription mechanism is unclear. Here, we found that although the base sequence in the promoter region of the gdnf gene was unchanged in glioma cells, there were significant changes in the methylation level of promoter region I (P < 0.05) in both high- and low-grade glioma tissues. However, the methylation degree in promoter region II was notably decreased in low-grade glioma tissue compared to normal brain tissue (P < 0.05), and the demethylation sites were mainly located in the enhancer region. Conversely, methylation was markedly increased in high-grade glioma tissue (P < 0.05), and the sites with decreased methylation level were mainly located in the silencer region. The binding capacities of several transcriptional factors, such as activating protein 2, specificity protein 1, ETS-related gene 2, and cAMP response element binding protein, which specifically bind to regions with altered methylation status decreased along with the pathological grade of glioma, and the differences between high-grade glioma and normal brain tissue were significant (P < 0.05). The results suggest that changes in transcriptional factor binding capacity are due to changes in promoter region methylation and might be the underlying mechanism for aberrantly high gdnf expression in glioma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glial cell line-derived neurotrophic factor (GDNF) was originally isolated, purified, and cloned from rat B49 glial cell line by Lin et al. [1] in 1993. It belongs to the transforming growth factor family β (TGF-β) and exerts protective effects in many cell types as an important bioactive nutritional factor [2]. In humans, the gdnf gene is mapped to chromosome 5p12-p13.1 and is a single-copy gene comprised of two promoters and five exons. Early studies suggested that GDNF was merely a type of growth-promoting factor that influenced the survival and differentiation of dopaminergic neurons in the embryonic mesocerebrum [3, 4]. Later, it was shown that GDNF showed nutritional and protective effects in the periphery including sympathetic, parasympathetic, sensory, and motor neurons. Recent studies have demonstrated that GDNF acts as a potent proliferation-promoting factor in glioma cells [5]. Gdnf levels are significantly higher in human glioma cells compared to normal glial cells, and GDNF has been shown to participate in tumor cell metastasis [6].
In recent years, the mechanisms underlying the proliferation- and metastasis-promoting effects of GDNF have been studied thoroughly, but few groups have investigated the mechanism underlying the abnormally high expression of the gdnf gene in glioma cells. Markedly increased expression of any gene is usually the result of mutation or epigenetic modification [7]. Methylation in DNA promoter region is one of the most important epigenetic modifications, which regulates gene expression through the changes in the methylation level of DNA promoter region [8, 9]. We previously found that the DNA copy number and coding sequence of gdnf were not altered in glioma cells; therefore, we hypothesized that the abnormally high expression might be due to the change of methylation modification in its promoter region. Specifically, it was reported that promoter region methylation of the gene closely related with glioma can affect the ability of transcription factors to bind to its promoter region.
To test the above hypothesis and clarify the methylation status of the gdnf promoter region and transcription factor binding in glioma, we compared the methylation status of promoter regions I and II of gdnf in glioma and normal brain tissues using bisulfite sequencing PCR (BSP). We also employed chromatin immunoprecipitation (ChIP) coupled with PCR to detect the binding of four transcription factors [activating protein 2 (AP-2), specificity protein 1 (SP1), gene 2 (ERG-2), and cAMP response element binding protein (CREB)], to the gdnf promoter region in glioma and normal brain tissues. Our results provide evidence for altered methylation and transcription factor binding in glioma and shed light on the mechanism of aberrantly high gdnf expression in glioma.
Materials and Methods
Tissue Source
Six human brain tissue samples (collected from patients with acute brain trauma given with intracranial decompression) and 12 glioma tissue samples (World Health Organization grades 1–4) acquired from the Affiliated Hospital of Suzhou University were used for BSP, real-time PCR, enzyme-linked immunosorbent assay (ELISA), and single-nucleotide polymorphism (SNP) analysis. Biopsied glioma tissues from patients who had not undergone cancer therapy were randomly sampled. Samples in pathological grades 1–2 and 3–4 were classified as low- and high-grade glioma groups, respectively.
Cell Culture
Human U251 glioma cell line were cultured at 37 °C in a humidified 5 % CO2 atmosphere in Dulbecco’s modified Eagle’s medium (Gibco, Shanghai Invitrogen Biotechnology Co., Ltd., China) supplemented with 10 % (v/v) fetal bovine serum (Gibco). Exponentially growing cells were used for the following experiments. Then, the cells were incubated in 0, 1, 5, 10, and 20 μmol/l 5-aza-CR (Sigma-Aldrich, St. Louis, MO) for 48 h (37 °C, 5 % CO2) to detect its demethylation effect.
RNA Extraction and Reverse Transcription
Total RNA was extracted from tissue samples, and its concentration and purification were measured using ultraviolet spectrophotometry. Reverse transcription was performed according to the M-MLV Operating Instructions from Fermentas (Burlington, ON, Canada). Briefly, the procedures are as follows: the reaction system consisted of 20 μl in an RNase-free PCR tube that included 1 μg RNA, 1 μl Oligo (dT) primer (0.5 μg/μl), 1 μl random primer (0.2 μg/μl), and 10 μl diethylpyrocarbonate water. The components were mixed and heated to 65 °C for 10 min then rapidly transferred to an ice bath to cool before adding 4 μl 5× reaction buffer, 0.5 μl RiboLock™ Ribonuclease inhibitor (20 U/μl), 2 μl dNTP mix (10 mM), and 1 μl reverse Tra Ace. The mixture was heated to 42 °C for 60 min, then 70 °C for 10 min. The acquired cDNAs were either stored at −20 °C or immediately used for PCR after determination for the reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Real-Time PCR
Real-time PCR primers were designed for gdnf and GAPDH (Table 1). Serial dilutions of plasmids (pMD19T, Takara, Shanghai, China) containing segments of interest were used to generate the standard curve, and the linear correlation for each dilution was confirmed. The copy number of the plasmids containing segments of interest was calculated according to the following formula: the copy number of the plasmids in per microliter = plasmid concentration (in nanogram per microliter) × 10−9 × 6.02 × 1023/molecular weight of the target fragment (in gram per mole). The PCR mixture was prepared using 2× SYBR Green PCR Master Mix (Shanghai R&S Biotechnology Co., Ltd., Shanghai, Jiangsu, China) according to sample number and parallel number. The reaction system was as follows: 10 μl 2× SYBR Green PCR Master Mix, 1 μl primer mix, 1 μl template, and 8 μl RNase-free water. Then, each aliquot was transferred to PCR tubes and briefly centrifuged before undergoing quantitative real-time PCR on an Eppendorf Realplex PCR instrument (Hamburg, Germany) according to the following protocol: 95.0 °C, 2 min; 95.0 °C, 15 s, 59.0 °C, 20 s; 72.0 °C, 20 s; 40 cycles. After PCR, product specificity was determined with melting curve analysis. Threshold cycle (Ct) values were calculated according to PCR reaction curves, and relative quantitative analysis was performed according to gdnf (copy/microliter)/hGDNF (copy/microliter) method.
Enzyme-Linked Immunosorbent Assay
GDNF protein level was measured by biotin double-antibody sandwich ELISA (Shanghai Yifeng Biotechnology Co., Ltd., Shanghai, Jiangsu, China) using a GDNF antibody that did not cross-react with TGFs, BDGF, or NGF. Standards (0, 0.75, 1.5, 3, 6, and 12 ng/ml) mixed with biotin-labeled antibody were added into wells of 96-well plate in triplicate. In standard wells, 50 μl standard and 50 μl streptomycin–horseradish peroxidase (HRP) were added; in sample wells, 40 μl sample, 10 μl anti-GDNF antibody, and 50 μl streptomycin–HRP were sequentially added into each well. Next, the plate was sealed with a membrane, gently agitated to mix, and then incubated at 37 °C for 60 min. After the membrane was removed, the solution was removed, and the plate was dried by patting. The plate was washed with 1× washing buffer five times, and the plate was dried by patting. Next, 50 μl each of chromogenic agents A and B was added into each well, and the plate was gently agitated to mix evenly before 10-min incubation in the dark at 37 °C to allow color development. Finally, 50 μl stop buffer was added to each well to quench the reaction. The absorbance value [optical density (OD)] of each well was measured at 450 nm, and the blank control well was set as zero. The linear regression equation of the standard curve was calculated using the concentrations of standards and corresponding OD values, and the sample concentrations were calculated using the regression equation.
Single-Nucleotide Polymorphism Detection
DNA samples were extracted using QIAamp DNA Mini and QIAmp Blood Mini kits (Qiagen, Shanghai, China). Three pairs of primers (Table 2) were designed to amplify DNA segments in gdnf promoter regions I and II, and the products were purified and sent to Sangon Biotech (Shanghai, China) for sequencing. Through alignment analysis of the acquired sequences, we could determine whether there were abnormal SNP sites in gdnf promoter regions I and II of GDNF in glioma tissues (−1991 to +151 and +3952 to +4904).
BSP
DNA was extracted as described in “Enzyme-Linked Immunosorbent Assay” and treated with bisulfite in strict accordance with the EZ DNA Methylation-Gold™ kit instructions (Zymo, Irvine, CA, USA). Five pairs of BSP primers (Table 3) covering promoter regions I and II were designed to amplify the treated DNA templates, and the PCR reaction protocol was as follows: denaturation at 98 °C for 4 min; 98 °C for 15 s, 60 °C for 15 s, 72 °C for 30 s, repeat 30 cycles; 72 °C for 10 min.
PCR products were separated by agarose gel electrophoresis, and the target bands were retrieved using a gel extraction kit (Axygen, Union City, CA, USA) and ligated with pMD19-T (Takara Bio, Otsu, Japan), and then, they were transformed into DH5α competent cells. The positive clones determined by bacterium medium PCR were sent to Sangon Biotech for sequencing. The methylation status of each sample was analyzed based on the sequencing result using the QUMA web server at http://quma.cdb.riken.jp/.
ChIP-PCR
ChIP was performed according to the manufacturer’s instructions of the EZ-ChIP chromatin immunoprecipitation kit (Millipore, Billerica, MA, USA). After the ChIP experiment, DNA binding to the target antibody was detected by real-time PCR. The PCR reaction system contained 12.5 μl SYBR Premix Ex Taq™ II (Takara Bio, Otsu, Japan), 1 μl each of upstream and downstream primers (10 μmol/l), and 3 μl DNA template. The total volume of 25 μl was reached by adding sterilized double-distilled water. Amplification was performed following a two-step PCR reaction protocol (predenaturation, 95 °C, 2 min; PCR reaction, 95 °C, 5 s; 60 °C, 4 s, 40 cycles), and relative quantitative analysis was carried out using the 2−ΔΔCt method [4]. The primers (Table 4) were synthesized by Kangcheng Biotech Co., Ltd. (Shanghai, China).
Statistical Analysis
Data were analyzed using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA) and expressed as mean ± standard deviation. The means of two samples were compared using independent samples t tests, and the means of multiple samples were compared using single factor analysis of variance (ANOVA). A value of P < 0.05 was considered as significantly different.
Results
Gdnf Transcription is Significantly Increased in Glioma Tissue
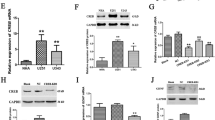
The relative expression level of gdnf mRNA in normal brain and low- and high-grade glioma tissues was determined with real-time PCR. Gdnf mRNA was detectable in all three groups (Fig. 1a), and the difference between glioma and normal brain tissues was statistically significant (P < 0.05). Gdnf mRNA levels also increased with pathological grade of glioma tissue (P < 0.05) (Fig. 1b).
Gdnf mRNA levels in glioma tissue and normal brain tissue. a hGAPDH and gdnf PCR results showing band lengths similar to the predicted values; M DL2000 marker; lanes 1–5 hGAPDH amplification using sample cDNA as a template, 6–10 gdnf using sample cDNA as a template. b Gdnf mRNA levels. One-way ANOVA showed that gdnf mRNA expression was significantly different among normal brain tissue and low- and high-grade glioma tissues (P < 0.05)
GDNF Protein is Significantly Increased in Glioma Tissue
The relative protein level of GDNF was assessed with ELISA. Consistent with the mRNA results, the difference among the three types of tissue was statistically significant (P < 0.05). GDNF protein was notably higher in glioma tissue compared to normal brain tissue and increased along with higher pathological grade (P < 0.05) (Fig. 2).
The gdnf Promoter Region Base Sequence is Unchanged in Glioma
To clarify the underlying mechanism of gdnf overexpression in glioma tissue, we cloned, amplified, and sequenced the promoter region of the gdnf gene in normal brain and low- and high-grade gliomas. Contrastive analysis revealed that the base sequence in low- and high-grade glioma tissues was unchanged versus normal brain tissue (Fig. 3).
Methylation is Altered in Promoter Regions I and II of the gdnf Gene in Glioma Tissue
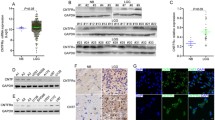
Next, we investigated the methylation status of promoter regions I and II of the gdnf gene using BSP (Fig. 4a). We found that methylation was significantly enhanced in promoter region I of the gdnf gene in glioma tissue compared to normal brain tissue (P < 0.05), but there was no significant difference between low- and high-grade glioma tissues (Fig. 4b). Conversely, methylation of promoter region II was significantly decreased in low-grade glioma (P < 0.05) and notably increased in high-grade glioma (P < 0.05) (Fig. 4c). Through further analysis of the CpG islands in promoter region II, we found that sites with significantly decreased methylation in low-grade glioma were mainly located in the enhancer region (P < 0.05), whereas those sites with significantly increased methylation in high-grade glioma were primarily localized in the silencer region (P < 0.05) (Fig. 4d).
Promoter region methylation in the gdnf gene. a Methylation status of each site in the two promoter regions detected by BSP; black circles indicate a methylated site, and white circles indicate a nonmethylated site. b Methylation status of promoter region I in normal brain tissue and low- and high-grade glioma tissues. c Methylation status of promoter region II in normal brain tissue and low- and high-grade glioma tissue. d Methylation status of cis-acting elements in promoter region II in normal brain tissue and low- and high-grade glioma tissues. One-way ANOVA showed that gdnf mRNA expression was significantly different among normal brain tissue and low- and high-grade glioma tissues (P < 0.05)
Changes of Methylation Level in Promoter Region I of the gdnf Gene Influenced its Expression in U251 Glioma Cell Line
In order to investigate the effect on methylation level of gdnf gene in promoter region I and its mRNA expression, the cell model was established by using 5-aza-CR to alter DNA methylation in U251 human glioma cell line. The results showed that the methylation level of gdnf gene in promoter region I began to decline after application with 5-aza-CR, the methylation level dropped significantly when adding 5 μmol/l 5-aza-CR (P < 0.05) (Fig. 5a). In contrary, the expression of gdnf mRNA researched the highest (Fig. 5b). The methylation level of gdnf gene in promoter region I was no longer changed when higher concentrations of 5-aza-CR (10 or 20 μmol/l) were added, but the expression of gdnf mRNA began to decline (P < 0.05) (Fig. 5b).
Effect of methylation state in gdnf’s promoter region I on its mRNA expression after treating with different doses of 5-aza-CR in U251 glioma cells. a Effect of different doses of 5-aza-CR on methylation state in gdnf’s promoter region I. b Effect of different doses of 5-aza-CR on the mRNA expression level of gdnf’ gene
Transcription Factor Binding to the gdnf Gene
We predicted transcription factors that might bind to gdnf’s promoter region using web-based tools (http://www.gene-regulation.com; Table 1 in Supplementary Material). We found that binding sites for AP-2, CREB, EGR-2, and SP1 were located in the region of the promoter where we had observed abnormal methylation. ChIP-PCR was employed to detect the binding of these four transcription factors to promoter regions I and II of the gdnf gene. The results showed that the binding capacity of the GDNF for these four transcription factors was altered to different degrees in glioma tissue (Tables 2 and 3 in Supplementary Material). The statistical results of these experiments are summarized in Fig. 6.
Transcription factors binding capacity on promoter regions I and II of the gdnf gene. a AP-2 binding to promoter region I decreased along with greater pathological degree of glioma; the differences among the three groups were statistically significant (P < 0.05). AP-2 binding to promoter region II also decreased along with higher pathological degree of glioma, and the differences among the three groups were statistically significant (P < 0.05). b CREB binding to promoter region I varied among the three groups (P > 0.05). A significant difference in CREB binding capacity was only observed in high-grade glioma tissue, which was decreased versus normal brain tissue (P < 0.05). c ERG-2 binding to promoter region I was not significantly different between normal brain tissue and low-grade glioma tissue, but it significantly decreased in high-grade glioma tissue versus normal brain tissue and low-grade glioma tissue (P < 0.05). There was no significant difference in ERG-2 binding capacity on promoter region II between normal brain tissue and low-grade glioma tissue, but it was significantly decreased in high-grade glioma tissue (P < 0.05). d SP1 binding to promoter region I showed no significant difference between normal brain tissue and low-grade glioma tissue, whereas it significantly decreased in high-grade glioma tissue versus normal brain tissue (P < 0.05); the binding capacity of SP1 on promoter region II significantly decreased in glioma tissue and was the lowest in low-grade glioma tissue; the differences among them were statistically significant (P < 0.05)
Discussion
GDNF is an important neurotrophic factor that has been intensely studied since its discovery in the late twentieth century. Due to its effect on dopaminergic neurons, GDNF has attracted considerable attention as a potential therapy for Parkinson’s disease [10]. Recent studies have demonstrated a relationship between GDNF and glioma; gdnf expression level is significantly higher in glioma compared to normal glial cells [11]. To clarify the underlying mechanism of aberrant gdnf expression in glioma tissue, we investigated the methylation status of the promoter region of the gdnf gene and assessed the binding of specific transcription factors to the promoter region.
The gdnf gene has two promoter regions I which is upstream of exon 4 and II which is found to be an upstream region of exon 1. Baecker et al. [12] found that the transcriptional activity of promoter region II was stronger and thus concluded that region II played an important role in transcription initiation. To determine if mutations in the promoter region were involved, we assessed gdnf promoter region sequences in different grade gliomas; however, we did not identify any SNP sites that could discriminate glioma tissue from normal brain tissue. Considering other work in our lab that revealed that there was no mutation in the gdnf coding region sequence in glioma (data not shown), we surmised that the observed abnormal gdnf expression might be caused by epigenetic modifications.
A 2010 study showed that methylation on the 5′-side region of the gdnf gene promoted tissue development, neurogenesis, and neural proliferation; furthermore, this modification was closely related to upregulated transcription [13]. This led us to hypothesize that the increase in gdnf expression in glioma cells might be due to changes in the methylation of the promoter region of the target gene. We assessed the methylation status of both gdnf promoter regions in glioma tissue with BSP and found that promoter region I methylation was increased in glioma tissue. After the promoter of cancer suppressor gene Rb was found to have altered CpG island methylation, several genes were subsequently found, including p16, DAPK, and EBER1. Due to high methylation in the promoter region of these genes, they were silenced in specific tumors [14, 15].
Hypermethylation of gdnf promoter region I does not result in GDNF gene silencing; rather, gdnf transcription is highly active in glioma cells. Thus, we speculated that the “deactivation” of promoter region I was compensated by activation in promoter region II. In assessing the methylation status of promoter region II, we found that it was significantly decreased in low-grade glioma tissue and significantly elevated in high-grade glioma. Further sequence analysis revealed that the hypermethylated sites were located in the enhancer element of promoter region II in low-grade glioma tissue and in the silencer element of promoter region II in high-grade glioma tissue. Based on available methylation information, we speculated that promoter region II hypomethylation resulted in enhancer activation, whereas promoter region II hypermethylation caused silencer inhibition. Both of the modifications would ultimately induce gdnf gene transcription; however, how these modifications could do so was still unclear.
In addition, we investigated the effect on methylation level of gdnf gene in promoter region and its mRNA expression in the cell model mentioned in the “Materials and Methods.” The results showed that 5-aza-CR can change the methylation level of gdnf gene in promoter region. The change of methylation level of gdnf gene in promoter region I influenced the expression of gdnf mRNA in glioma cell. Interestingly, we found the methylation level of gdnf gene in promoter region I was no longer changed when higher concentrations of 5-aza-CR (10 or 20 μmol/l) were added, but the expression of gdnf mRNA began to decline. It was suggested that the demethylation of the silencers in promoter region II may be the reason for the downregulation of the gdnf mRNA after treating with the high concentrations of 5-aza-CR.
Several studies have shown that DNA methylation is related to the binding affinity of transcription factors, thus providing new insights for exploring the abnormal gene expression in tumor cells [16]. Through bioinformatic analysis, we identified four transcription factors AP-2, CREB, EGR-2, and SP1 that could bind to the methylated promoter region and detected alterations in their binding using ChIP-PCR. The binding capacities of AP-2, CREB, EGR-2, and SP1 declined with increasing pathological degree of glioma, and the difference between high-grade glioma and normal brain tissue was significant (P < 0.05). These results indicate that transcription factor binding to the gdnf promoter is inhibited by methylation to some extent. AP-2, a DNA-binding protein that binds to a special sequence, regulates the transcription of many genes and participates in cell proliferation, differentiation, apoptosis, and carcinogenesis [17]. Here, we found that the binding capacity of AP-2 on promoter regions I and II of the gdnf gene decreased along with the increase in the pathological degree of glioma. However, it is difficult to identify the critical site involved, as there are many AP-2 binding sites in the promoter region. CREB is a nuclear transcription that binds to target DNA via its C-terminal basic amino acids [18]. Studies have shown that overexpression of CREB can induce cell apoptosis, and it is known to participate in tumor pathogenesis through its regulation of cAMP response elements on the promoters of many tumor-related genes [19]. There are two specific CREB binding sites on the enhancer and silencer elements of gdnf’s promoter region, respectively, making it an ideal candidate for studying transcription factor binding and cis element methylation. We found that CREB binding to the promoter region II of gdnf gene was decreased in high-grade but not low-grade glioma. Considering the methylation alterations in the cis elements in the promoter region, we hypothesized that abundant CREB binding in low-grade glioma was due to enhancer hypomethylation, while the decrease in high-grade glioma was due to silencer hypermethylation. Biological analysis of the promoter gdnf gene showed that EGR-2 merely bound to the enhancers in the core promoter region of gdnf gene, which provided us an ideal opportunity to investigate enhancer methylation and transcription factor binding activity. Analysis of enhancer element methylation in the promoter region of the gdnf gene showed that the promoter was hypo- and hypermethylated in low- and high-grade glioma tissues, respectively. EGR-2 binding to the promoter was only significantly decreased in high-grade glioma tissue, suggesting that the decrease was due to methylation in the enhancer region. SP1 plays important roles in various tumor cells during growth and metastasis through modulation of oncogenes and tumor suppressor genes [20, 21]. Our findings show that the binding capacity of SP1 on the promoter region of gdnf gene is decreased in glioma. Interestingly, the binding capacity of SP1 on the promoter region of gdnf gene increased with glioma progression; the specific mechanism underlying this phenomenon remains to be clarified.
Collectively, we investigated the methylation status of the promoter region of gdnf gene in glioma tissue and assessed transcription factor binding to the promoter region. We found that the methylation status of the promoter region of gdnf gene varied in different grades of glioma, and transcription factor binding to the methylated promoter region also changed with disease stage. We posit that transcription factor binding to the promoter region of gdnf gene, especially the cis elements, was affected by altered methylation of the promoter region of the gdnf gene, which resulted in aberrant regulation of gdnf gene transcription and overexpression. Our results provide a novel avenue to explore the molecular mechanism governing gdnf overexpression in glioma.
References
Lin LF, Doherty DH, Lile JD et al (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260:1130–1132
Williams LR, Inouye G, Cummins V et al (1996) Glial cell line-derived neurotrophic factor sustains axotomized basal forebrain cholinergic neurons in vivo: dose–response comparison to nerve growth factor and brain-derived neurotrophic factor. J Pharmacol Exp Ther 277:1140–1151
He Z, Jiang J, Kokkinaki M et al (2008) Gdnf upregulates c-Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells 26(1):266–278
Reis RA, Cabral da Silva MC, Loureiro dos Santos NE et al (2002) Sympathetic neuronal survival induced by retinal trophic factors. J Neurobiol 50(1):13–23
Ng WH, Wan GQ, Peng ZN et al (2009) Glial cell-line derived neurotrophic factor (GDNF) family of ligands confer chemoresistance in a ligand-specific fashion in malignant gliomas. J Clin Neurosci 16(3):427–436
Golan M, Schreiber G, Avissar S (2011) Antidepressants elevate GDNF expression and release from C(6) glioma cells in a beta-arrestin1-dependent, CREB interactive pathway. Int J Neuropsychopharmacol 14(10):1289–1300
Nigro JM, Baker SJ, Preisinger AC et al (1989) Mutations in the p53 gene occur in diverse human tumour types. Nature 342(6250):705–708
Martinez R, Esteller M (2010) The DNA methylome of glioblastoma multiforme. Neurobiol Dis 39(1):40–46
Parrella P, la Torre A, Copetti M et al (2009) High specificity of quantitative methylation-specific PCR analysis for MGMT promoter hypermethylation detection in gliomas. J Biomed Biotechnol 2009:531692
Backman CM, Shan L, Zhang YJ et al (2006) Gene expression patterns for GDNF and its receptors in the human putamen affected by Parkinson’s disease: a real-time PCR study. Mol Cell Endocrinol 252(1–2):160–166
Wiesenhofer B, Stockhammer G, Kostron H et al (2000) Glial cell line-derived neurotrophic factor (GDNF) and its receptor (GFR-alpha 1) are strongly expressed in human gliomas. Acta Neuropathol 99(2):131–137
Baecker PA, Lee WH, Verity AN et al (1999) Characterization of a promoter for the human glial cell line-derived neurotrophic factor gene. Brain Res Mol Brain Res 69(2):209–222
Wu X, Rauch TA, Zhong X et al (2010) CpG island hypermethylation in human astrocytomas. Cancer Res 70(7):2718–2727
Banati F, Koroknai A, Salamon D et al (2008) CpG-methylation silences the activity of the RNA polymerase III transcribed EBER-1 promoter of Epstein-Barr virus. FEBS Lett 582(5):705–709
Jablonowski Z, Reszka E, Gromadzinska J et al (2011) Hypermethylation of p16 and DAPK promoter gene regions in patients with non-invasive urinary bladder cancer. Arch Med Sci 7(3):512–516
Cedar H, Bergman Y (2009) Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10(5):295–304
Jonckheere N, Fauquette V, Stechly L et al (2009) Tumour growth and resistance to gemcitabine of pancreatic cancer cells are decreased by AP-2alpha overexpression. Br J Cancer 101(4):637–644
Shaywitz AJ, Greenberg ME (1999) CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68:821–861
Giebler HA, Lemasson I, Nyborg JK (2000) p53 recruitment of CREB binding protein mediated through phosphorylated CREB: a novel pathway of tumor suppressor regulation. Mol Cell Biol 20(13):4849–4858
French SW, Malone CS, Shen RR et al (2003) Sp1 transactivation of the TCL1 oncogene. J Biol Chem 278(2):948–955
Safe S, Abdelrahim M (2005) Sp transcription factor family and its role in cancer. Eur J Cancer 41(16):2438–2448
Acknowledgments
This project was supported by the National Natural Science Foundation of China (grant number 31271358 and 81101899), the Natural Science Fund for Colleges and Universities of Jiangsu Province (08KJB180011), and the University Science Research Project of Jiangsu Province(No.11KJB180012), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Zheng-Quan Yu and Bao-Le Zhang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 54 kb)
Rights and permissions
About this article
Cite this article
Yu, ZQ., Zhang, BL., Ren, QX. et al. Changes in Transcriptional Factor Binding Capacity Resulting from Promoter Region Methylation Induce Aberrantly High GDNF Expression in Human Glioma. Mol Neurobiol 48, 571–580 (2013). https://doi.org/10.1007/s12035-013-8443-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8443-5