Abstract
In this work, we have synthesized MoS2 nanosheets (NSs) through hydrothermal as well as liquid-phase sonication methods and studied their ability to degrade organic dyes, methyl red (MR) and methylene blue (MB) under visible light irradiation. The as-synthesized photocatalysts have been characterized by powder X-ray diffraction, Raman Spectroscopy and transmission electron microscopy to confirm their structure and surface morphology. XRD patterns show that crystalline nature of MoS2 NSs prepared by hydrothermal method appears at 160°C for 15 h. In contrast, sharp and intense diffraction peaks appear for liquid exfoliation method even at room temperature, exhibiting better crystallinity of the as-prepared NSs. The difference (∆) of ~25 cm−1 between E12g and A1g modes of Raman spectra indicates formation of 5–6 layers of MoS2 from both the synthesis routes. UV–Vis spectroscopy has been utilized to study the degradation of dyes by MoS2 NSs as catalyst. Hydrothermally assisted synthesis of MoS2 NSs exhibit lesser degradation efficiency with 58.92% for MR and 49.28% for MB, while exfoliation-assisted MoS2 NSs act as better photocatalyst with 63.40 and 62.40% degradation of MR and MB, respectively. Faster degradation rate for MR compared to MB has been attributed to the presence of lesser chromophoric sites in MR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Photocatalysis proves to be an efficient technique for the degradation of variety of pollutants present in effluent water released from industries [1,2]. Semiconductor nano catalysts, such as CdS, TiO2, SnO2, etc. have been widely used as photocatalyst due to their wide band gap, high sensitivity and antibacterial activity [3,4,5,6,7]. However, restricted responsivity towards excitation by UV light and fast rate of electron–hole recombination limit their use [8,9]. Thus, an alternative candidate is always searched for, which will overcome the limitations of semiconductor photocatalysts and show improved degradation efficacy.
In this context, transition metal dichalcogenides (TMDC), particularly MoS2, proves to be a promising candidate for photocatalysis of dyes [10,11]. Its tuneable band gap compresses the electron–hole recombination rates, thereby giving MoS2 a unique property of getting self-excited under visible and UV light irradiation [5,12]. Compact degree of MoS2 structure increases the surface area for charge carrier mobility, leading to strong adsorption of dye molecules on the MoS2 catalyst surface [13]. Since the catalyst's surface structure greatly influences the charge transfer processes responsible for the catalytic activity and affects the photocatalysis kinetics and its mechanism, hence, preparation of MoS2 with specific morphology has attracted lot of interest [14,15]. Till now, various routes for synthesizing MoS2 have been reported, such as mechanical exfoliation, liquid exfoliation, hydrothermal, chemical vapour deposition (CVD) and sulphurization method, etc. [16,17,18]. However, hydrothermal synthesis proves to be quite an effective technique for synthesizing nanostructures of 2D TMDC. Ntakadzeni et al [19] have fabricated PEGylated MoS2 nanosheets (NSs) hydrothermally for photodegradation of organic dyes. Recently, Luo et al [20] have studied the hydrothermal synthesis of MoS2 with controllable morphologies and its adsorption properties for bisphenol A. Ho et al [21] have fabricated MoS2 nanoparticles using in situ photoreduction and deposition method to evaluate photocatalysis activity. The foremost advantage of the hydrothermal method is its ability to synthesize MoS2 with different morphologies [20,22], having many reactive edge states exposed for enhanced chemical activities in different environments for practical applications [23,24,25]. Therefore, this synthetic route is more favoured due to its advantage of forming functional nanocomposites of TMDC with other semiconducting materials like TiO2, carbon nanotubes (CNTs), graphene, etc. [26,27,28]. Hydrothermal method shows flexibility of controlling the morphological features, which depend upon various parameters like precursor, additives, temperature, reaction time, solvents, substrates, etc., on the growth mechanism of 2H-MoS2 [14]. However, besides all the advantages of the hydrothermal synthesis route, its major disadvantage is forming an uncontrolled number of layers and non-uniform morphology with poor crystallinity that affects the fabrication of quickly modulated optical devices for practical applications [29]. Hence, an alternative synthesis route needs to be considered for improving the crystallinity of MoS2 NSs at low reaction temperature, while maintaining uniformity over number of layers of these 2D materials, thereby, making it suitable for high-performance applications. Liquid-phase exfoliation overcomes the disadvantages of the hydrothermal synthesis route. It proves to be an efficient synthesis route for controlling the number of layers and maintaining uniformity avoiding aggregation of layers [17]. Sonication-assisted exfoliation with suitable solvent or in aqueous solutions, followed by centrifugation, helps in getting few layered MoS2 NSs at room temperature with better crystallinity [30,31]. Since, the solvent chosen in sonication-assisted exfoliation helps to stabilize the exfoliated NSs against re-aggregation, providing more reactive edge sites for longer time, thus MoS2 NSs exfoliated by this synthesis approach can be predicted to show enhanced photocatalytic response. Various researchers have reported hydrothermal synthesis routes to prepare MoS2 nanostructures for photocatalytic applications [19,20,21, 28,32,33,34,35]. But, not much work has been done to investigate the photocatalytic activity of MoS2 NSs exfoliated by liquid-phase sonication method.
In this study, we have attempted to exploit both the hydrothermal and the liquid-sonication method for exploring the effect of synthesis routes on the photocatalytic application of MoS2 NSs for the degradation of organic dyes methyl red (MR) and methylene blue (MB). The results will help us to choose an appropriate synthesis route for large-scale industrial application of MoS2 NSs to treat organic pollutants present in wastewater by photocatalysis.
2 Experimental
Materials: Analytical grade chemical reagents were used without further purification. Ammonium molybdate tetrahydrate [(NH4)6Mo7O24·4H2O], thiourea [CH4N2S], molybdenum disulphide (MoS2) powder (2 µm), ethanol, N-methyl 2-pyrrolidone (NMP) and isopropyl alcohol (IPA) were purchased from Sigma Aldrich. MR and MB were obtained from Molychem, India. Solutions were prepared in deionized water.
2.1 Synthesis of MoS2 NSs by hydrothermal method
MoS2 NSs were synthesized via conventional hydrothermal route using starting precursors: ammonium molybdate tetrahydrate as the source of molybdenum and thiourea as the source of sulphide ions. Briefly, 0.1 M solution of ammonium molybdate tetrahydrate and 0.5 M solution of thiourea were separately prepared in 40 ml of deionized water followed by mixing and sonication for 30 min. The sonicated solution was then transferred into a 100 ml Teflon-lined stainless-steel autoclave and the reaction was carried out in a muffle furnace for 15 h at 120, 140 and 160°C. The reaction was also carried out at 160°C for 7, 11 and 15h. After natural cooling of the furnace, the resulting dark brown precipitates were obtained through filtration. The precipitates were further washed with deionized water and absolute ethanol to remove any traces of residual ions. The final product was then dried at 60°C for 7 h.
2.2 Exfoliation of MoS2 NSs by liquid-exfoliation method
MoS2 NSs have been exfoliated via liquid-phase exfoliation method. In this process, 0.05 g of bulk MoS2 powder was dispersed in 20 ml of NMP and ethanol:distilled water (volume ratio 1:1) in two different sets. The solution was ultrasonicated at room temperature with samples collected at 6, 12, 18, 24 and 30 h. The resultant dispersion was centrifuged at 3000 rpm for about 10 min to remove the residual large size MoS2 powders. Further, the remaining solution was centrifuged at 10,000 rpm for 5 min. The supernatant was collected for further characterization to separate the black products. After 24 h of sonication, the colour changes from dark greyish black to greenish.
2.3 Photocatalytic test for dye degradation
Organic dyes MR and MB are poorly biodegradable and chemically stable contaminants present in effluent wastewater. We have used these organic dyes (MR and MB) model reactions to investigate the photocatalytic performance of MoS2 NSs. The dye concentration of 50 ml of MR and MB in 100 ppm aqueous solution was treated with 0.01 g of MoS2 NSs dispersed in 1 ml of distilled water for the MoS2 NSs obtained from hydrothermal synthesis route. However, for liquid-sonication method, 1 ml of exfoliated MoS2 NSs in NMP and ethanol:water was treated with the above concentration of dyes. Both the dye-catalyst solutions have been treated in dark as well as under white light (85-watt compact fluorescent lamp) for different exposure times (30, 60, 90 and 120 min). After treatment, the solutions were centrifuged for 10 min at 4000 rpm to remove the residual photocatalysts. Thus, obtained dye solutions were used to investigate the photocatalytic performance of the MoS2 NSs prepared by hydrothermal and liquid exfoliation methods for different exposure time using absorption spectroscopy.
2.4 Characterization techniques
X-ray diffractometer (XRD) with CuKα radiation (λ = 0.154 nm) was used to determine the crystalline structure of as-prepared samples in the 2θ range from 10 to 70°. Transmission electron microscope (TEM) JEOL 2100F operated at 200 kV was used to analyse the morphologies of MoS2. A confocal Raman spectrometer having an excitation source of 532 nm laser was used to investigate the vibrational modes of MoS2. The UV–Vis absorption spectrophotometer (Labtronics, LT-2700, India) was used to record the absorption spectra of MoS2 and even for the photocatalytic study.
3 Results and discussion
3.1 Structural investigations of MoS2 NSs prepared by hydrothermal method
The MoS2 NSs were synthesized at different temperatures with variation in hydrothermal reaction time. The XRD patterns of hydrothermally synthesized MoS2 are presented in figure 1. In figure 1a, b and c, XRD patterns of MoS2 NSs are observed for samples prepared at temperatures 120, 140 and 160°C with hydrothermal reaction time maintained for 15 h. It is observed that at low hydrothermal temperatures of 120 and 140°C, diffraction peaks appear at 14.85 and 28.21 (2θ degrees), corresponding to (002) and (004) crystal planes with many extra peaks. Though this exhibits the formation of MoS2 NSs, concentration of synthesized MoS2 is quite low at low reaction temperature. However, with increase in hydrothermal temperature to 160°C, all diffraction peaks of MoS2 are distinctly observed at 14.85, 28.13, 32.89, 33.78, 36.19, 39.82, 44.33, 49.97, 56.19, 58.44, 60.70, 62.96 (2θ degrees) which arise from (002), (004), (100), (101), (102), (103), (006), (105), (106), (110), (008) and (107) crystal planes and matches well with JCPDS no. 37-1492 [8]. Higher intensity of (002) diffraction peak, as temperature increases from 120 to 160°C, attributes to the presence of random stacking of single layers of MoS2. No other extra diffraction peaks are observed for MoS2 NSs at 160°C-15 h that reveals presence of no impurities in the sample at this temperature. XRD patterns are also observed for MoS2 NSs with variation in hydrothermal reaction time from 7 to 15 h maintained at 160°C in figure 1d, e and f. Here also, proper diffraction peaks do not appear exhibiting exact structure of MoS2 NSs for lower reaction time of 7 and 11 h, but as the hydrothermal reaction time increases to 15 h maintained at 160°C, peaks of MoS2 NSs appear. Hence, it can be concluded that the minimum condition required for synthesis of pure 2H-MoS2 NSs with better crystallinity is 160°C-15 h. TEM results also provides direct evidence about the structural attributes of the sample. Figure 2a exhibits the TEM image of MoS2 NSs prepared hydrothermally at 160°C for 11 h. It is observed that MoS2 NSs are not properly formed within 11 h of hydrothermal reaction time at 160°C. In contrast, figure 2b exhibits the appearance of curled nanosheets of MoS2 indicating crystallization of MoS2 NSs at 160°C-15 h accompanied without any impurities. TEM results are in good agreement with the XRD results.
3.2 Optical investigations of MoS2 NSs prepared by hydrothermal method
Raman spectroscopy is an important technique for illustrating the vibrational modes and the number of layers of MoS2 NSs. In figure 3a, two typical Raman peaks for E12g and A1g modes appear at 381.04 and 406.09 cm−1 with sharp intensity indicating material’s crystallinity 160°C-15 h. The difference (∆) of ~25 cm−1 appears between the E12g and A1g modes, indicating the presence of 5–6 layers of MoS2 [35].
UV–Vis spectroscopy was used to analyse the absorption spectra of MoS2 NSs. Figure 3b shows the occurrence of two peaks of MoS2 around 608 and 670 nm due to spin-orbit splitting corresponding to the excitonic transitions related to band gap of the material [35]. This indicates the absorbance ability of MoS2 NSs in both UV and visible spectrum range that can be further exploited to use these MoS2 NSs as photocatalysts for the pollutant’s degradation.
3.3 Structural investigations of MoS2 NSs exfoliated by liquid-phase sonication method
A few layered MoS2 NSs were exfoliated from bulk in two different solvents NMP and ethanol:water sonicated for 24 h. The XRD patterns of these exfoliated MoS2 NSs are presented in figure 4a and b. From figure 4a, diffraction peaks of exfoliated MoS2 in NMP are observed at 29.80, 32.71, 33.54, 35.91, 39.59, 44.17, 49.82, 56.01, 58.37, 60.44, 62.81 for 2θ (degree). However, from figure 4b diffraction peaks of exfoliated MoS2 are slightly displaced to 29.20, 32.89, 33.68, 36.08, 39.75, 44.33, 49.98, 56.17, 58.50, 60.61, 62.96 for ethanol:water as solvent. These diffraction peaks for both the solvents arising from crystal planes of (004), (100), (101), (102), (103), (006), (105), (106), (110), (008) and (107) and matches well with the JCPDS no. 37-1492, respectively [32]. These sharp and intense diffraction peaks present in both the solvents infer to better crystalline nature of the exfoliated MoS2 NSs from the sonication method than the hydrothermal method. The shape and size of MoS2 NSs were investigated using TEM. Figure 5a and b displays the TEM images of exfoliated MoS2 NSs in ethanol:water and NMP, respectively. Figure 5a and b reveals the successful formation of sheet-like structure of MoS2 NSs in ethanol:water and NMP, respectively. Figure 5b shows that the lattice spacing of MoS2 NSs exfoliated in NMP comes out to be of the order of 0.27 nm corresponding to (100) plane.
3.4 Optical investigations of MoS2 NSs exfoliated by liquid-phase sonication method
Figure 4c shows the Raman spectra of exfoliated MoS2 NSs in two different solvents, NMP and ethanol:water. For ethanol:water, the in-planer E12g mode appears at 382.27 cm−1, while the out-planer A1g mode appears at 407.54 cm−1. In solvent NMP, the E12g mode of the exfoliated NSs of MoS2 slightly blue shifts to 382.91 cm−1. However, the A1g mode appears at the same position at 407.54 cm−1. The Raman modes show high sensitivity towards thickness of layer. Generally, with decrease in number of layers, the difference between the E12g and A1g peaks decreases [35]. The Raman shift appears to be 25.27 cm−1 for MoS2 NSs exfoliated in ethanol:water. This shift slightly decreases to 24.63 cm−1 in case of exfoliated MoS2 NSs in NMP, which indicates decrease in the number of exfoliated layers for NMP as compared to ethanol:water system. However, from difference between the two modes, it can be inferred from previously reported works that bulk MoS2 is exfoliated to few-layer NSs with number of layers <5 in both the solvents [30]. The E12g/A1g peak intensity ratio for MoS2 NSs is 0.94 for both the solvents; therefore, by using Tuinstra Koenig relation, crystallite size measured is 17.82 nm. Sonication time is a key parameter for achieving high exfoliation ratio. To verify the exfoliation effect caused by sonication time, we have tailored the time of sonication from 6 to 30 h with same concentration of MoS2. The prepared samples were analysed for their optical properties by UV–Vis spectra in 400–800 nm. Figure 6a and b shows the absorption spectra of exfoliated MoS2 NSs with increasing sonication time from 6 to 30 h in ethanol:water and NMP, respectively. All the spectra exhibit three absorption bands. One of the broad band centred around 460 nm in both the solvents is attributed to direct electronic transition between conduction and valence band energy level. Two typical MoS2 peaks appear around 624–626 and 685 nm, for ethanol:water. However, in NMP as solvent, slight blue shift is exhibited in these peaks, which appears around 606–616 and 670–683 nm. Appearance of these peaks indicates the formation of MoS2 NSs and is attributed to the transitions that occur due to spin-orbit splitting [34]. By increasing the sonication time to 24 h, the optical absorbance increases, but at 30 h, some decrease in absorbance spectra is observed. Therefore, it can be concluded that increasing the time of sonication leads to an increase in NSs scission, reducing both the lateral dimension and thickness of the exfoliated sheets to an extent. However, further increase of sonication time leads to decrease in absorbance. Hence, analysing the absorbance spectra, we have decided to use exfoliated MoS2 NSs obtained at 24 h of sonication for measuring the degradation efficiency of organic dyes MR and MB.
3.5 Photocatalytic activity of MoS2 NSs under visible light irradiation
The photocatalytic activity of MoS2 NSs prepared by two different synthesis routes, hydrothermal and exfoliation in ethanol:water and NMP were investigated under visible light irradiation (85-watt compact fluorescent lamp) for organic dyes MR and MB using UV–Vis spectrophotometer. The intensity of the characteristic absorption peak of MR at 522 and 665 nm for MB is used to track their degradation efficiency, respectively. A blank test has been undertaken in the absence of catalyst showing negligible self-degradation of the dyes. No noticeable change in colour is observed between untreated and treated dye solutions of MR and MB when placed in dark as compared to visible light irradiation for 4 h. Figure 7a and b shows the absorption spectra of MoS2 NSs prepared hydrothermally as catalyst for the degradation of MR and MB carried out for 4 h. By adding hydrothermally prepared MoS2 NSs, the absorption edge shifts towards lower wavelength (495 nm) for MR and correspondingly, intensity of the absorption peak decreases rapidly as the irradiation time increases, as shown in figure 7a. However, in case of MB as shown in figure 7b, the absorption edge shows no noticeable change. In contrast, decrease in intensity of the absorption peak is observed gradually with an increase in irradiation time up to 4 h.
Figure 8a and b displays absorption spectra of degradation of MR and MB dye by MoS2 NSs exfoliated in ethanol:water, respectively. Similarly, figure 9a and b exhibits a decline in MR and MB dye absorption spectra, indicating respective degradation of dyes by exfoliated MoS2 NSs in NMP. Irradiating with white light, intensity of the absorbance peak for MR shows a declining trend along with blue shifting of the absorption edge towards lower wavelength of 504 nm for MoS2 NSs exfoliated in ethanol:water as shown in figure 8a. Similarly, from figure 9a, MoS2 NSs exfoliated in NMP, the absorption edge blue shifts to 502 nm, exhibiting a decrease in the absorption peak. In case of MB (figures 8b and 9b), there is no noticeable change in edge of the absorption peak of dye solution treated with MoS2 NSs exfoliated in ethanol:water as well as in NMP under white light irradiation. However, similar decrease in absorption peak is observed with an increase in irradiation time (4 h) for MB.
The degradation efficiency of dyes MR and MB treated with MoS2 NSs prepared hydrothermally and exfoliated in solvents ethanol:water and NMP are measured using Beer–Lambert's law. According to this law, absorbance is proportional to concentration of dye. Hence, percentage degradation efficiency can be calculated by using equation (1) [33].
where Co, C, Ao, A are concentration and absorbance of dye at initial time 0 and after t minutes, respectively.
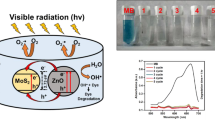
Figure 10a exhibits percentage degradation of organic dyes MR and MB by hydrothermally synthesized MoS2 NSs irradiated under white light (85-watt CFL) for 4 h. Correspondingly, degradation of both the dyes is clearly visible in the pictorial representation presented in figure 10b. Initially, degradation of dye MB is quite low 18.40% for 1 h, but with increase in irradiation time, degradation of MB increases from 18.40 to 49.28%. Similarly, degradation of MR increases from 30.35 to 58.92% in 4 h. This reveals that though MR degrades better than MB by hydrothermally synthesized MoS2 NSs as a catalyst, but efficiency is quite low and longer time may be needed for complete degradation of both the dyes.
For investigating better degradation of dyes, exfoliated MoS2 NSs by liquid-exfoliation method has been utilized. The percentage degradation of MR and MB by MoS2 NSs exfoliated in ethanol:water and NMP under white light irradiation are shown in figure 11a and b. Degradation of both the dyes in 4 h is quite visible from continuous fading of dye colour represented in the pictorial description in figure 12a and b, respectively. It is observed that MR degrades from 32 upto 61.04% in 4 h, whereas degradation of MB enhances upto 56.30% for same time duration, starting initially from 20.30% in 1 h for ethanol:water. A similar degradation trend has been observed for MoS2 NSs exfoliated in NMP for both the dyes MR and MB. In NMP, degradation of MR increases from 36 to 63.40%, and MB degrades from 34.40 to 62.40% in 4 h of irradiation. It has been explored that though degradation efficiency of dyes increases with exfoliated MoS2 NSs, however, the degradation efficiency of MR still shows better degradation trend than MB, similar to hydrothermally synthesized MoS2 NSs.
Comparative degradation efficiency of hydrothermally synthesized and exfoliated MoS2 NSs in both the solvents ethanol:water and NMP are presented in tables 1 and 2. From both the tables, it is clear that solvent-assisted liquid exfoliation of MoS2 NSs proves to be better photocatalyst than hydrothermally synthesized MoS2 NSs. From XRD data of the hydrothermal synthesis route, it has been observed that MoS2 NSs with better crystallinity are prepared at minimum 160°C for 15 h and higher temperature. At this temperature, there might be expansion of the interlayer distance of MoS2 NSs, indicating expansion of lattice. Due to high surface energy and attracting Van der Waals interaction, there will be aggregation of MoS2 layers during fabrication. As a result, hydrothermally synthesized MoS2 NSs might lose many of its active sites essentially needed for the photocatalytic activity. Whereas, the surface area of the NSs exfoliated by sonication method is quite increased and the solvent used to sonicate NSs prevent aggregation of sheets, thereby providing more active sites for reaction with dye molecules. Thus, availability of more active sites due to lesser non-aggregation of sheets in liquid exfoliation sonication method might be the reason for improved photocatalytic activity of this method compared to hydrothermally synthesized MoS2 NSs. However, in liquid exfoliation sonication, MoS2 NSs exfoliated in NMP exhibits better degradation efficiency than the MoS2 NSs exfoliated in ethanol:water system. Coulombic repulsion is created due to charged sites between neighbouring sheets, disrupting the bonding between the interlayer basal planes. Therefore, high-dipole solvents like NMP offers better electrostatic stabilization of NSs. Also, the O2 molecule present in NMP leads to an increase in moisture content of the solution, which improves the quality of the exfoliated layers by sonication in NMP. Thus, the exfoliated layers are smoother and more even than those obtained by ethanol:water promoting formation of redox-active species in situ. At the same time, there is an auto-oxidation pathway that converts NMP into N-methyl succinimide with intermediate hydroperoxide, leading to appearance of highly reactive sites that aids the exfoliation process as the edge sites due to oxidation.
The degradation efficiency can be better understood from the kinetic studies of MR and MB adsorption by MoS2 NSs obtained from both the methods. The constant rate k of the degradation was calculated using equation (2),
Co is the initial concentration and C the concentration at time t, k the rate constant and T the irradiation time. A graph is plotted between normalized absorption C/Co and time of irradiation given by equation (2) that explains the type of order of reaction followed by the degradation of dyes with photocatalysts.
Figure 13a and b exhibits the kinetic graph of MR and MB with photocatalysts MoS2 NSs obtained by hydrothermal and sonication methods. The figures reveal that normalized absorption declines exponentially with time, which exhibits that degradation reaction obeys pseudo-first-order reaction [8]. The degradation behaviour of both the dyes follows the same trend. Values of rate constant calculated from kinetic graphs are compiled in table 3. The rate constant values show that time taken for degradation of MR is 0.9333 h−1 faster than MB, which is 0.6723 h−1 for hydrothermally prepared MoS2 photocatalyst. Similarly, the reaction rate of MoS2 (ethanol:water) photocatalyst for MR degradation is 1.2595 h−1, while for MB, it is 0.9974 h−1. In the same way, MoS2 (NMP) has rate of reaction of 1.4881 h−1, however, for MB, it is 1.4022 h−1. Hence, these rate constant values confirm that the reaction rate is faster for MR than MB.
Degradation of dyes depend upon the presence of choromophoric sites. MR has only one chromophoric site, while there are four chromophoric sites present in MB. During the photocatalysis process, the ·OH or O2- radicals generated, attack these chromophoric sites present in dyes to destabilize or break the dyes into reducible components. Hence, lesser number of chromophoric sites present in MR leads to faster destabilization of the dye for photocatalytic degradation under white light irradiation.
4 Photocatalytic degradation mechanism
Photocatalytic degradation behaviour of organic dyes under irradiation by visible light may proceed via two possible processes: photocatalytic oxidation and photosensitized oxidation. In the former mechanism, when MoS2 NSs are irradiated by visible light, the photoexcitation of the semiconductor leads to the generation of electron–hole pair (\(e_{{{\text{CB}}}}^{ - }\) from the conduction band and \(h_{{{\text{VB}}}}^{ + }\) from the valence band) on the surface of the catalyst given by equation (3):
Oxygen vacancies on catalyst surface act as a trap for electrons to inhibit electron–hole recombination. The photogenerated electrons oxidize with \({\text{O}}_{2}\) (sink for electron acceptors) adsorbed on the MoS2 surface leading to the formation of superoxide radical anion (\({\text{O}}_{2}^{ \bullet - }\)) given by equation (4):
The photoinduced hole can be easily oxidized by the organic molecule or react with \({\text{H}}_{2} {\text{O}}\) or \({\text{OH}}^{ - }\) ion to oxidize to hydroxyl radical (\({\text{OH}}^{ \bullet }\)) given by equations (5) and (6).
Finally, the hydroxyl radical (\({\text{OH}}^{ \bullet }\)) and the superoxide radical anions (\({\text{O}}_{2}^{ \bullet - }\)) are responsible for the overall photodecomposition reaction given by equations (7) and (8).
On the other hand, in the photosensitizing mechanism, instead of catalyst, the adsorbed dye itself gets excited to singlet or triplet states under visible light irradiation. It injects electrons to the conduction band of MoS2 nanostructures given by equations (9 and 10).
The cationic dye radical (\({\text{Dye}}^{ \cdot + }\)) undergoes degradation to hydroxyl radical (\({\text{OH}}^{ \bullet }\)). The hydroxyl radical (\({\text{OH}}^{ \bullet }\)) from both the mechanisms actively break the various bonds present in organic dyes and leads to mineralization. Both the mechanisms of photo-oxidation and photosensitization go side by side. It is not possible to explain which of the mechanism is superior. Still, it is established that the photosensitizing mechanism improves the efficiency of photo-oxidation degradation of dyes and makes the process more feasible.
5 Conclusion
In summary, MoS2 NSs have been synthesized by two routes: hydrothermal and liquid-phase sonication. Degradation of organic dyes MR and MB by MoS2 NSs synthesized by both the routes have been studied by illumination under UV–Vis light. The as-synthesized photocatalysts characterized by XRD reveal that the exfoliated NSs from the sonication method at room temperature exhibit sharp and intense diffraction peaks inferring better crystallinity than NSs prepared at 160°C-15 h by conventional hydrothermal method. For both the synthesis routes, the difference (∆) of ~25 cm−1 appears between the E12g and A1g modes of Raman spectra, indicating the presence of 5–6 layers of MoS2. Among these three routes, hydrothermally synthesized MoS2 NSs exhibit least degradation efficiency with 58.92% for MR and 49.28% for MB. Simultaneously, the exfoliated MoS2 NSs in NMP act as a better photocatalyst for the degradation with 63.40% for MR and 62.40% for MB. Lesser efficiency of the MoS2 NSs prepared by the hydrothermal method might be due to re-aggregation of sheets and availability of lesser number of active sites essentially needed for the photocatalytic activity.
On the other hand, in high-dipole solvent NMP, the O2 molecule present in NMP promotes the formation of redox-active species leading to appearance of highly reactive edge sites enhancing the photocatalytic degradation of both the dyes. It is also observed that MR shows faster degradation ability with rate constant 0.9333 and 1.4881 h−1 than MB with rate constant 0.6723 and 1.4022 h−1 for hydrothermal method and liquid sonication with NMP, respectively. Better degradation of MR might be attributed to the presence of lesser chromophoric sites than MB.
References
Sharma M, Mohapatra P K and Bahadur D 2017 Front. Mater. Sci. 11 366
Zhu S and Wang D 2017 Adv. Energy Mater. 7 1700841
Xu C, Ravi Anusuyadevi P, Aymonier C, Luque R and Marre S 2019 Chem. Soc. Rev. 48 3868
Altintas Yildirim O, Arslan H and Sönmezoğlu S 2016 Appl. Surf. Sci. 390 111
Vattikuti S V P and Byon C 2016 Superlattices Microstruct. 100 514
Tab A, Bellal B, Belabed C, Dahmane M and Trari M 2020 Optik (Stuttg). 214 164858
Malligavathy M, Iyyapushpam S, Nishanthi S T and Padiyan D P 2018 Pramana–J. Phys. 90 1
Rani A, Singh K, Patel A S, Chakraborti A, Kumar S, Ghosh K et al 2020 Chem. Phys. Lett. 738 136874
Sumesh C K and Parekh K 2019 Pramana–J. Phys. 92 87
Mishra A K, Lakshmi K V and Huang L 2015 Sci. Rep. 5 15718
Quinn M D J, Ho N H and Notley S M 2013 ACS Appl. Mater. Interfaces 5 12751
Johari P and Shenoy V B 2012 ACS Nano 6 5449
Li Z, Meng X and Zhang Z 2018 J. Photochem. Photobiol. C: Photochem. Rev. 35 39
Li M, Wang D, Li J, Pan Z, Ma H, Jiang Y et al 2016 RSC Adv. 6 71534
Paul K K, Sreekanth N, Biroju R K, Narayanan T N and Giri P K 2018 Sol. Energy Mater. Sol. Cells 185 364
Chhowalla M, Shin H S, Eda G, Li L-J, Loh K P and Zhang H 2013 Nat. Chem. 5 263
Prabukumar C, Mohamed Jaffer Sadiq M, Krishna Bhat D and Udaya Bhat K 2019 Res. Express 6 085526
Ding Q, Czech K J, Zhao Y, Zhai J, Hamers R J, Wright J C et al 2017 ACS Appl. Mater. Interfaces 9 12734
Ntakadzeni M, Anku W W, Kumar N, Govender P P and Reddy L 2019 Bull. Chem. React. Eng. Catal. 14 142
Luo L, Shi M, Zhao S, Tan W, Lin X, Wang H et al 2019 J. Saudi Chem. Soc. 23 762
Ho W, Yu J C, Lin J, Yu J and Li P 2004 Langmuir 20 5865
Hou S X, Wu C and Huo Y J 2017 Ceram. - Silikaty 61 158
Huang Q, Liu X, Chen Z, Gong S and Huang H 2019 Chem. Phys. Lett. 730 608
Chaudhary N, Khanuja M, Abid and Islam S S 2018 Sens. Actuator. A: Phys. 277 190
Luo S, Dong S, Lu C, Yu C, Ou Y, Luo L et al 2018 J. Colloid Interface Sci. 513 389
Sabarinathan M, Harish S, Archana J, Navaneethan M, Ikeda H and Hayakawa Y 2017 RSC Adv. 7 24754
Chen M, Dai Y, Wang J, Wang Q, Wang Y, Cheng X et al 2017 J. Alloys Compd. 696 900
Kaur M, Umar A, Mehta S K and Kansal S K 2019 Appl. Catal. B: Environ. 245 143
Peng Y, Meng Z, Zhong C, Lu J, Yu W, Jia Y B et al 2001 Chem. Lett. 30 772
Nguyen T P, Sohn W, Oh J H, Jang H W and Kim S Y 2016 J. Phys. Chem. C 120 10078
Mukherjee S, Maiti R, Midya A, Das S and Ray S K 2015 ACS Photonics 2 760
Tian Y, He Y and Zhu Y 2004 Mater. Chem. Phys. 87 87
Singh R, Barman P B and Sharma D 2017 J. Mater. Sci. Mater. Electron. 28 5705
Eda G, Yamaguchi H, Voiry D, Fujita T, Chen M and Chhowalla M 2011 Nano Lett. 11 5111
Lee C, Yan H, Brus L E, Heinz T F, Hone J and Ryu S 2010 ACS Nano 4 2695
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rani, A., Singh, K. & Sharma, P. Investigation of visible light photocatalytic degradation of organic dyes by MoS2 nanosheets synthesized by different routes. Bull Mater Sci 45, 63 (2022). https://doi.org/10.1007/s12034-022-02655-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-022-02655-y