Abstract
This paper describes the preparation and characterization of nanocrystalline CdxPb1−xS thin films by chemical bath deposition method with different Pb and Cd solute ratios. XRD, HRTEM and SAED patterns show the nanocrystalline nature of films with the co-existence of cubic, hexagonal and tetragonal phases, while micro-Raman spectra reveals the presence of cubic and hexagonal phases in the films. The average crystallite size of CdxPb1−xS estimated from TEM and XRD methods were nearly same. The chemical bonding present in the films was studied by FTIR spectral analysis. SEM images show uniformly distributed grains of different sizes over the substrates. Optical studies show the prominent blue shift in band gap energy (Eg) with the increase in Cd content in PbS. The increment of Cd addition to CdxPb1−xS has led to enhance the transmittance as well as band gap energy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The metal chalcogenide-based binary and ternary compound semiconductors are of great interest because of their relatively easy fabrication process and having their potential applications in electronic and optoelectronic devices [1]. By alloying appropriate binary constituents with changes in their relative concentrations, ternary alloys provide a class of semiconductors in which lattice parameters, band gap energy and other parameters could be continuously varied within specific limits [2]. The II–IV–VI group ternary semiconductor inorganic compounds, in thin-film form, have attracted great attention due to their unique physical properties and wide range of applications [3,4]. Lead sulphide (PbS) is a IV–VI group semiconductor with a narrow band gap of ~0.41 eV [5]. The narrow band gap of PbS could be used for certain photovoltaic devices with high sensitivity to the infrared (IR) spectral domain [6]. PbS has large Bohr radius of ~18 nm [7]. These properties of PbS provide strong quantum confinement of holes and electrons in nano-sized structure, so that the value of optical band gap can be controlled by modifying the crystallite size, according to the effective mass model [8,9]. By altering the material’s dimensionality, it is also possible to control electrical as well as optical properties [10]. PbS has been used in various devices viz. diode lasers, IR detectors, sensors, transistors, contact rectifiers and solar cells [11].
Cadmium sulphide (CdS) with direct band gap of ~2.46 eV and Bohr radius of ~2.5 nm, is a kind of II–VI group semiconductor material which has attracted much attention because of its application in various optoelectronic devices viz. solar cells, photo detectors, thin film transistors and light emitting diodes [12,13,14]. Owing to high electrochemical stability, high carrier concentration and high absorption coefficient, CdS is used as an optimize window layer for CdTe and other chalcopyrite-based solar cells [15,16,17]. Therefore, mixed nanostructures based on PbS and CdS, i.e., CdxPb1−xS has dealt with immense interest of research. Because, it offers the advantage of tuning the optical and optoelectronic properties of both CdS and PbS, viz. band gap, electrical conductivity and thermoelectric power in a controlled manner [18]. One of the most characteristic advantages of CdxPb1−xS semiconductor compound is high irradiation stability, which makes it useful for radiation hazardous facilities [19]. Tuneable composition of CdxPb1−xS provides a wide range of variations in optical band gap, and it is possible to widen the spectral window [20]. CdxPb1−xS ternary thin films have been chosen to enhance the solar energy conversion from ultraviolet (UV) to near infrared (NIR) spectral region [21]. There are various deposition techniques for the preparation of CdS and PbS thin films. Currently, the most used deposition methods to prepare ternary films are chemical bath deposition (CBD) [22,23,24,25], electro deposition [26,27,28,29], flash evaporation [30,31,32], spray pyrolysis [33,34], successive ionic layer adsorption and reaction (SILAR) [35,36], magnetron sputtering [37,38]. Among them, CBD is one of the simplest and highly efficient method. It has simple routine, low-cost, homogeneity and reproducibility [39,40].
The most recognized manifestation of nanocrystalline thin films is tunability of band gap with increase and decrease in crystal size. The narrow band gap of PbS (0.41 eV) semiconductor can be modified by adding different solute ratios of a wide band gap of CdS (2.4 eV). Nair et al [1] have investigated the effect of Cd/Pb atomic% on the structural, optical and electrical properties of CdPbS films by changing the molarity of the precursors used for the preparation of the films by CBD technique. Tan et al [2] have synthesized ternary CdxPb1−xS nanocrystal with clean surface and homogeneous composition by mechanical alloying process [2]. Deo et al [3] have prepared Cd0.5Pb0.5S thin films by CBD method to investigate the structural, morphological and optical characteristics as a function of temperature. Barote et al [4] have fabricated Cd1−xPbxS thin films by CBD method at various optimum parameters to study the growth mechanics and reduction kinetics. Many of the previous research articles of Cd1−xPbxS composition and their thin films were not exposed to the discussion of the influence of cadmium concentration on the structural and composition of CdxPb1−xS thin films. Therefore, the motivation of the present work is to study the influence of cadmium content on the structural, morphological and optical properties of CdxPb1−xS thin films. In the present work, CdxPb1−xS thin films are grown by cost-effective CBD technique at different solute ratios of (Pb:Cd) and also, to study the applicability of the prepared CdxPb1−xS thin films for photovoltaic device applications.
2 Experimental
CdxPb1−xS thin films were deposited onto chemically cleaned glass substrates at deposition temperature of 60°C for 120 min by using CBD technique. First, 3 ml of 0.1 M lead acetate (Pb(CH3COO)2·2H2O) was taken as Pb2+ ion source. To this, 0.1 M cadmium acetate (Cd(CH3COO)2·2H2O) solution with different volume (7, 12, 17 and 22 ml) was used as Cd2+ ion source. A 10 ml of 0.5 M thiourea (CH4N2S) was used as S2+ ion source for all the samples in the bath solution for the deposition of CdxPb1−xS thin films. The pH of the solution was maintained at 12.4 by adding liquid NH3 and triethanolamine (TEA) was used as a complexing agent. Deionized water was used as a solvent throughout the process. The prepared films are golden yellow in colour. In this work, four samples were prepared by changing the solute ratio of Pb and Cd (Pb:Cd) as 3:7, 3:12, 3:17 and 3:22. The samples are coded as S1, S2, S3 and S4, respectively. The sample details of the CdxPb1−xS thin films prepared by CBD method are listed in table 1.
X-ray diffractometer (XRD), model: X’Pert Pro, Phillips, using CuKα radiation (λ = 1.5406 Å) as a source is used to determine the crystal structure of the deposited powder sample. Fourier transform of infrared (FTIR), model: IMPACT410, spectra were taken on spectrometer with software OMNIC E.S.P.5.0, to investigate the chemical bonding structure of the CdxPb1−xS thin films. Raman spectroscopy (Renishaw basis series with 514 Laser) is used to perform micro-Raman studies. Surface morphology and the chemical composition of the prepared films were obtained by scanning electron microscope (SEM), model: JEOL JSM IT-300, equipped with energy-dispersive X-ray (EDX) spectroscopy attachment. High resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED) pattern were done by transmission electron microscope (TEM), model: JEM-2100, JEOL, operated with an acceleration voltage of electron beam ~200 kV. The thickness of the prepared films was estimated by Tolansky method using green line (λ = 5461 Å) of mercury. For optical studies, absorption and transmission spectra were recorded by UV–Vis spectrophotometer, model: Shimadzu UV–Vis 1800, in the wavelength range of 200–1100 nm.
3 Results and discussion
3.1 XRD analysis of Cd x Pb 1−x S thin films
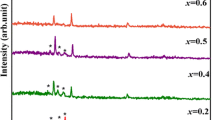
Figure 1 shows the XRD patterns of samples S1, S2, S3 and S4. A comparison of the peak position (2θ) values of XRD spectra with JCPDS data shows that the films have mixed phase structures of cubic, hexagonal and tetragonal, exhibiting the nanocrystalline nature. The peaks at 2θ of 20.65, 34.77 and 41.61° can be indexed to the planes (110), (211) and (113), respectively, of tetragonal PbS (JCPDS card no. 20-0596). The peaks at 24.80, 26.50, 43.68, 47.83, 51.82 and 58.27° match with the planes (100), (002), (110), (103), (112) and (202), respectively, which can be indexed to hexagonal CdS (JCPDS card no. 41-1049). The diffraction peaks at 53.45 and 71.03°, which match the planes (222) and (420), respectively, are indexed to cubic PbS (JCPDS card no. 05-0592). The intensities and the number of the peaks decrease considerably with the increase in Cd contents in CdxPb1−xS films. This is attributed to the increase in disorder with the incorporation of Cd at Pb sites in PbS [41]. A decrease in lattice spacing d for all the CdxPb1−xS films has been occurred. Due to the smaller ionic radii of Cd2+ (0.91 Å) [42] than Pb2+ (1.21 Å) [43], the lattice would undergo contraction as Cd2+ occupies the Pb2+ sites in the host lattice. Similar works have been reported for CdPbS thin films and other ternary systems by other researchers [44,45,46,47]. There is an increase in heterogeneity of the films for the occupation of Cd into the host lattice PbS. As a result, the peak broadening occurs in the films and also, the particle size decreases [48,49]. The stable crystal structures of PbS and CdS are different. Therefore, the crystal structure of CdxPb1−xS becomes unstable when Cd2+ occupies more sites of Pb2+ in the host lattice [50]. To stabilize the CdxPb1−xS crystal structure, the crystallite size is reduced to release the strain [51].
3.2 Crystallite size and strain
3.2.1 Scherrer’s method
The average crystallite size (D) of the films is estimated by using Scherrer’s equation (1) [52]:
where K is a constant equal to 0.99, β the full width at half maxima (FWHM) and θ the Bragg’s diffraction angle. The average crystallite sizes of samples S1, S2, S3 and S4 are found to be ~24, 20, 15 and 12 nm, respectively. The crystallite size decreases with the increase in Cd content in CdxPb1−xS thin films, and are shown in table 2.
3.2.2 Williamson–Hall method
Owing to the crystal imperfections and distortions, the strain (ε) induced-broadening of XRD peak arises and is related by ε ≈ βs/tan θ [53]. Williamson–Hall (W–H) method like Scherrer’s equation does not obey 1/cos θ dependency, but varies with tan θ. A simple integral breadth method is used in W–H method by considering the peak broadening as a function of 2θ, where both size- and strain-induced XRD peak broadenings are deconvoluted [54]. Addition of Scherrer’s equation and ε ≈ βs/tan θ, Williamson–Hall (W–H) suggested for broadened peaks as [53]:
Equation (2) represents the uniform deformation model (UDM), where the strain was assumed to be uniform in all the crystallographic directions. For a multiple-ordered diffraction pattern of a film, for each peak, found at different 2θ, β values are different. W–H plots for all samples S1, S2, S3 and S4 are shown in figure 2a–d. The average values of crystallite size (D) and strain (ε) are estimated from intercept and slope of the linear fitted W–H plot, respectively. The values of D and ε of the different films are summarized in table 2. The average value of crystallite size is found to decrease with the increase in Cd content in CdxPb1−xS, while strain is increased.
3.2.3 Size–strain plot method
W–H plots showed isotropic line broadening. However, a better evaluation of the size–strain parameters can be obtained in case of isotropic line broadening by considering an average size–strain plot (SSP) [53,55]. Accordingly, one can obtain [53]:
In SSP plot, (dhklβhkl cos θ)2 is plotted with respect to (d2hklβhkl cos θ) for all the orientated peaks of CdxPb1−xS thin films. SSP plot for samples S1, S2, S3 and S4 are shown in figure 3a–d. The values of the crystallite size and the strain are determined from the slope and the square root of the y-axis intercept of linearly fit data. The obtained values are listed in table 2. From these plots, it is observed that with the increase in Cd content, the average crystallite size decreases and average strain increases. The variation in the estimation of crystallite size by Scherrer’s, W–H and SSP methods is due to different average particle size distribution [56].
3.3 FTIR characteristics of Cd x Pb 1−x S thin films
To study the molecular arrangements present in the sample, FTIR spectra of samples S1, S2, S3 and S4 are recorded in the wavenumber range of 1100–400 cm−1, as shown in figure 4. The frequency bands at ~1032 and 1074 cm−1 correspond to the C–O stretching vibration due to acetate group present in the sample [57,58,59]. The peaks at ~850 and 900 cm−1 are assigned to C–S bond due to sulphide group present in thiourea for all the samples [60,61]. In addition, other strong characteristics for Cd–S stretching vibrations occurred at ~651 and 680 cm−1 [62,63,64]. The characteristic absorption peaks for weak Pb–S vibrational bond of all the samples are identified at ~419 cm−1 [7].
3.4 Micro-Raman characteristics of Cd x Pb 1−x S thin films
In case of wurtzite CdS, there are six observable Raman active modes, E2 at 43 cm−1, A1 transverse optical, A1(TO) at 234 cm−1, E2 at 256 cm−1, E1(TO) at 243 cm−1, A1 longitudinal optical, A1(LO) at 305 cm−1 and E1(LO) at 307 cm−1 [65]. TO and LO modes of cubic modification coincide with E1(TO) and E1(LO) of the hexagonal modification. Figure 5a–d shows the Raman spectra of all samples S1, S2, S3 and S4, respectively. The prominent Raman peaks of samples S1, S2, S3 and S4 are at ~303, 302.3, 302.4 and 302 cm−1, respectively. These peaks are attributed to either cubic 1LO phonon or hexagonal A1(LO)/E1(LO) phonons, whereas 2 and 3 cm−1 are the respective shifts from 305 cm−1 of the single crystal CdS [66]. All the films have least shifts indicating that the films have better crystal structure [67]. Another characteristic peak, 2LO phonon is barely noticeable at about ~612.5–610.1 cm−1. Also, in the spectrum, some tiny peaks are observed. The peaks observed at ~266.7 (S1), 268.4 (S2), 267.4 (S3) and 266.7 cm−1 (S4) are the E2 peaks of hexagonal CdS [68,69,70]. However, the peaks observed at ~276–287 cm−1 are attributed to either the TO peak of cubic CdS or E1(TO) peak of hexagonal CdS [65,71,72]. Both the cubic and hexagonal phases of CdS are observed in the samples. All the observed Raman peaks are listed in table 3.
A Gaussian fit is applied to the 1LO peak observed at ~302–303 cm−1 for all samples S1, S2, S3 and S4 are shown in figure 6a–d, respectively. The observed peaks have FWHM of ~21.7, 22, 24.5 and 27.8 cm−1 for S1, S2, S3 and S4, respectively. It shows that FWHM of the prepared samples increases with the increasing Cd content in CdxPb1−xS films and indicates a diminution in film crystallinity [66,67]. This result is consistent with the XRD results.
3.5 Surface morphology and compositional analysis of Cd x Pb 1−x S thin films
The surface morphology of all the CdxPb1−xS thin films deposited at different solute ratios (Pb:Cd) with the magnification of ×5000 is shown in figure 7. The morphology of the samples represents layered type structure in samples S1 and S2, as shown in figure 7a, b, respectively. By increasing the Cd content in CdxPb1−xS, more cabbage like structures are observed in samples S3 and S4, which are shown in figure 7c, d, respectively. In each sample, the surface is composed of largely compact interconnected grains with different sizes and sharp edges. Grains are uniformly distributed over the glass substrates without any visible microscopic defects, like cracks or pinholes. The average grain sizes of samples S1, S2, S3 and S4 are determined by Image J software and found to be ~92, 86, 72 and 63 nm, respectively. It is observed that the grain size of the samples decreases with the increase in Cd content in the deposited films. This confirms that the disordering increases with the increase in Cd incorporation in host lattice and results in a small grain growth [44]. This result is corroborated by the XRD results. The grain size in SEM images is greater than those of calculated from XRD as SEM shows an aggregate image of composed small grains or crystals [73].
Quantitative analysis of the CdxPb1−xS thin films is carried out using EDX spectroscopy to study the stoichiometry of the films. The EDX analysis confirms the presence of Cd, Pb and S in all the prepared films along with Si, C and O. Figure 8 shows the EDX spectra of CdxPb1−xS thin films for all samples S1, S2, S3 and S4, respectively, along with the atomic% ratio S/(Cd+Pb) of the films listed at the upper right corner of the respective EDX images. The presence of carbon is due to the carbon adhesive tape used to mount the samples. The presence of oxygen in the prepared CdxPb1−xS thin film samples can be attributed to the glass substrates where the thin films are fabricated and also, due to the deionized water solution from which the samples are synthesized. Si is attributed to only glass substrates. It is observed that the element Pb shows a decreasing trend, whereas Cd content shows an increasing trend in the samples. Similar results have been reported for PbS thin films with Ni doping by Rajashree and Balu [74]. This confirms that there is more Cd in the overall sample as expected, since Cd:Pb ratio in the films was higher. The atomic% ratio of S/(Cd+Pb) for samples S1, S2, S3 and S4 are 0.83, 0.86, 0.95 and 0.98, respectively. The results show that all the films show a consistent stoichiometry as atomic% ratio of S/(Cd+Pb) increases from 0.83 to 0.98 and approaches to stoichiometric ratio 1 with the increasing Cd in the deposited films.
3.6 TEM, HRTEM and SAED pattern analysis of Cd x Pb 1−x S thin films
TEM and HRTEM image analyses are carried out to investigate the size of the particle and crystallographic orientation of the prepared thin film. TEM image and the corresponding particle size distribution histogram of sample S3 are shown in figure 9a and b, respectively. The average size among 24 particles is ~13 nm. The result is in good agreement with the result obtained from XRD method for sample S3. Figure 9c shows the bright field image of the sample S3 corresponding to SAED pattern and the SAED pattern reveals the polycrystalline nature of the film. The lattice planes (002) and (202) are corresponding to hexagonal CdS phase (JCPDS: 41-1049), the lattice plane (211) is corresponding to tetragonal PbS phase (JCPDS: 20-0596), and the plane (420) is corresponding to cubic PbS phase (JCPDS: 05-0592). Highly ordered and parallel lattices are observed in respective HRTEM images, which are shown in figure 9d. This suggests that the film’s crystallization with lattice spacing ~0.334 nm, which is associated with (002) lattice plane of hexagonal CdS phase (JCPDS: 80-0006). This result is confirmed by XRD and SAED pattern analyses. For sample S3, the interplanar spacing along with crystal planes observed in HRTEM and SAED pattern are listed in table 4.
3.7 Estimation of optical band gap energy
The transmission spectra of the CdxPb1−xS thin films at different solute ratios of Pb:Cd is shown in figure 10 and the inset shows photographs of samples S1, S2, S3 and S4. All the films have good transmittance in the visible light range with an average transmittance of >70%, indicating good optical quality of the deposited films with low scattering or absorption losses. When Cd content increases in CdxPb1−xS, the films become more transparent in wavelength >500 nm. The blue shift of the absorption edges is observed as compared to the band gaps of bulk CdS ~2.4 eV [12] and bulk PbS ~0.41 eV [5].
From the transmission data, the absorption coefficient α is calculated by equation (4) [75]:
where t is the thickness and was estimated by Tolansky method using equation (5) [76]:
where L is the fringe width or spacing and ΔL/L the fractional discontinuity of the fringe system. The calculated values are listed in table 5. The film thickness decreases with the increase in Cd content due to the varying rates of Cd2+ ions generation with different Cd additions in CdxPb1−xS thin films, which affects the growth rate of the films [77]. In addition, the difference in solubility values between Cd(OH)2 and Pb(OH)2, also affects the film thickness [78].
The optical band gap (Eg) value of the CdxPb1−xS films is estimated by equation (6) [75]:
where A is the Tauc’s slope, hν the photon energy and m the transition probability. CdPbS semiconducting thin films show the direct band gap nature [1,66,67,68]. For direct band gap semiconductor, m = 1/2. Therefore, the (αhν)2 vs. hν plots for samples S1, S2, S3 and S4, are shown in figure 11a. The calculated values of band gap are estimated in table 5. The optical band gap values for samples S1, S2, S3 and S4 are ~2.8, 2.82, 3 and 3.42 eV, respectively. Alloying of bulk PbS (Eg ~ 0.41 eV) with bulk CdS (Eg ~ 2.46 eV) is expected to vary the optical band gaps between 0.41 and 2.46 eV. The values of band gap increase with the increase in Cd content in CdxPb1−xS thin films and which are greater than the value of bulk CdS and PbS. This can be explained with Burstein–Moss effect [79]. In this effect, the Fermi level and its position depend on the concentration of the free electrons present in the conduction band [80]. In n-type CdxPb1−xS semiconductor, according to Burstein–Moss effect, the proximity of the conduction band will form additional carrier electrons leaving the Fermi level of PbS in the conduction band. The transition process will occur above the Fermi level rather than in the bottom of the conduction band because the gap between the bottom of conduction band and Fermi level will be filled with electrons [81]. Therefore, the energy band gap is associated with the excitation of the electrons from the valence band to Fermi level. The Fermi level which lies on the conduction band causes the absorption side to increase in energy and hence, results in widening of the band gap energy. Zhao and Li [81] synthesized Eu-doped PbS quantum dots and confirmed that band gap of Eu-doped PbS is bigger than both EuS and PbS. Yilmaz et al [82] synthesized Al-doped ZnO thin films and observed band gap values were greater than pure ZnO. These changes are similar to that observed in CdxPb1−xS thin films. The variation in the optical band gap as a function of Pb:Cd ratios is shown in figure 11b. The large modification in Eg confirms the formation of ternary CdxPb1−xS thin films [83]. So, it is concluded that by changing the Cd content in CdxPb1−xS films, the band gap as well as transmittance of the films can be enhanced. Due to this property, the nanocrystalline CdxPb1−xS films are suitable for photovoltaic cells as a window layer.
4 Conclusions
Nanocrystalline CdxPb1−xS thin films are fabricated on glass substrates by CBD method as a function of different Pb:Cd ratios. The XRD studies indicate the mixture of cubic, hexagonal and tetragonal phase structures of CdxPb1−xS thin films. The line broadening was analysed by Scherrer’s formula, W–H analysis and SSP method. From the result, it is observed that lattice strain increases and crystallite size decreases with increasing Cd content in CdxPb1−xS thin films. The film crystallinity is relatively good when the Cd content is low and deteriorates its crystallinity with increase in Cd content as confirmed by XRD spectra. Existence of different peaks of CdS and PbS nanoparticles in FTIR revealed the formation of CdxPb1−xS and the presence of different functional groups in the films. Micro-Raman spectra show the phase transition in all the prepared CdxPb1−xS thin films, due to variation in the Cd content in the films, where modes of cubic and hexagonal phases were observed. An increase in FWHM of cubic 1LO or hexagonal A1(LO)/E1(LO) peak with increase in Cd content in the films is observed, which implies an increase in lattice damage. SEM image analysis confirms that all the films have uniform surface morphology with layered type structure. EDX confirms the presence of Cd, Pb, S in the prepared CdxPb1−xS films. The TEM image analysis shows an average particle size of ~13 nm. The TEM result was in good agreement with the result of XRD method. HRTEM and SAED analyses reveal that CdxPb1−xS thin film exhibits polycrystalline nature with co-existence of hexagonal CdS, tetragonal and cubic PbS phases. The optical band gap energy values increase with the increase in the Cd content in the CdxPb1−xS films. The band gap energy values are greater than that of bulk value of both CdS and PbS.
References
Nair S B, Abraham A, Pradeep B, Shripathi T, Ganesan V and Philip R R 2014 AIP Conf. Proc. 1620 511
Tan G L, Liu L and Wu W 2014 AIP Adv. 4 067107
Deo S R, Singh A K, Desmukh L, Paliwai L J and Singh R S 2015 Optik 126 2311
Barote M A, Yadav A A and Masumdar E U 2011 Chalcogenide Lett. 8 129
Paulraj K, Ramaswamy S, Shkir M, Yahia J S, Hamdy M S and Al Faify S 2020 J. Mater. Sci.: Mater. Electron. 31 1817
Forostyanaya N A, Maskaeva L N, Smirnova Z I, Santra S, Zyryanov G V, Markov V F et al 2018 Thin Solid Films 657 101
Rajathi S, Kirubavathi K and Selvaraju K 2017 J. Taibah Univ. Medical Sci. 11 1296
Yıldırım A K and Altıokka B 2020 Emerg. Mater. Res. 9 1
Fouda A N, Marzook M, Abd EI-Khalek H M, Ahmed S, Eid E A and EI Basaty A B 2017 Silicon 9 809
Hone F G and Dejene F B 2020 Inorg. Chem. Commun. 111 107583
Ali S M, Al Garawi M S, Al Dawood S, Al Salman S A and Al Gamdi S S 2020 Radiat. Phys. Chem. 171 108732
Isik M, Gullu H H, Delice S, Parlak M and Gasanly N M 2019 Mater. Sci. Semicond. Process. 93 148
Laukaitis G, Lindroos S, Tamulevicius S, Leskela M and Rackaitis M 2000 Appl. Surf. Sci. 161 396
Ma L, Ali X and Wu X 2016 J. Alloys Compd. 691 399
Ahmad F R, Yakimov A, Davis R J, Her J H, Cournoyer J R and Ayensu N M 2013 Thin Solid Films 535 166
Mathew J and Anila E I 2018 Sol. Energy 172 165
Sinha T, Lilhare D and Khare A 2019 J. Mater. Sci. 54 12189
Arora L, Singh V N, Gupta P, Chhikara N, Jain K and Chand S 2014 J. Nanosci. Nanotechnol. 14 5324
Vaganova I V, Maskaeva L N, Markov V F, Voronin V I and Bamburov V G 2018 Nanosyst.: Phys. Chem. Math. 9 811
Nicholos P L, Liu Z, Yin L, Turkdogan S, Fan F and Ning C Z 2015 Nano Lett. 15 909
Gode F and Unlu S 2019 Mater. Sci. Semicond. Process. 90 92
Ahire R R and Sharma R P 2006 Indian J. Eng. Mater. Sci. 13 140
Agawane G L, Shin S W, Suryawanshi M P, Gurav K V, Moholkar A V, Lee J Y et al 2013 Mater. Lett. 106 186
Anuar K, Tan W T, Atan S, Kuang Z, Haron M J, Ho S M et al 2010 Chem. Asian J. 22 222
Ahmed H H, Khazeal A S and Atallah F S 2013 J. Univ. Anbar Pure Sci. 7 1
Bicer M and Sisman I 2011 Appl. Surf. Sci. 257 2944
Mahalingam T, Kathalingam A, Lee S, Moon S and Kim Y D 2007 J. New Mater. Sci. 10 15
Xiao F, Yoo B Y, Ryan M A, Lee K H and Myung N V 2006 Electrochim. Acta 52 1101
Ngo T T, Chavhan S, Kosta I, Miguel O, Grande H J and Ramon T Z 2014 ACS Appl. Mater. Interfaces 6 2836
Takashiri M, Tanaka S, Miyazaki K and Tsukamoto H 2007 J. Appl. Phys. 101 074301
Heras C and Sanchez C 1991 Thin Solid Films 199 259
Dizaji H R, Ghasemian M and Ehsani M H 2012 Surf. Rev. Lett. 19 1250012
Dedova T, Krunks M, Volobujeva O and Oja I 2005 Phys. Status Solidi C 5 1161
Isac L A, Duta A, Kriza A, Enesca I A and Nanu M 2007 J. Phys. Conf. 61 477
Sankapal B R, Mane R S and Lokhande C D 2000 Mater. Res. Bull. 35 2027
Guzeldir B, Saglam M and Ates A 2012 Acta Phys. Pol. A 121 33
Willeke G, Dasbach R, Sailer B and Bucher E 1992 Thin Solid Films 213 271
Hwang D H, Ahn J H, Hui K N, Hui K S and Son Y G 2012 Nano. Res. Lett. 7 1
Yao H, Shen H, Zhu X, Jiao J, Li J and Wang W 2016 Ceram. Int. 42 2466
Gogoi L, Chaliha S and Saikia P K 2018 AIP Conf. Proc. 1942 140020
Thangavel S, Ganesan S and Saravanan K 2012 Thin Solid Films 520 5206
Portillo-Moreno O, Lima-Lima H, Ramirez-Falcon V, Martinez-Juarez J, Juarez-Diaz G, Lozada-Morales R et al 2006 J. Electrochem. Soc. 153 926
Portillo M C, Moreno O P, Parez R G, Merino R P, Juarez H S, Tehuacanero Cuapa S et al 2017 Mater. Sci. Semicond. Process. 72 22
Yadav H K, Sreenivas K, Katiyar R S and Gupta V 2007 J. Phys. D: Appl. Phys. 40 6005
Badera N, Godbole B, Srivastava S B, Vishwarkarma P N, Sharath Chandra L S, Jain D et al 2008 Appl. Surf. Sci. 254 7042
Tsai C S, Chen S H and Chuu D S 1996 Phys. Rev. B 54 11555
Maskaeva L N, Markov V F and Guev A I 2003 Dokl. Phys. Chem. 390 147
Bhushan S, Mukherjee M and Bose P 2002 J. Mater. Sci.: Mater. Electron. 13 581
Kim D H, Lee D J, Kim N M, Lee S J, Kang T W, Woo Y D et al 2007 J. Appl. Phys. 101 094111
Prathap P, Revathi N, Venkata Subbaiash Y P and Reddy K T R 2008 J. Phys.: Condens. Matter 20 035205
Palomino Merino R, Portillo Moreno O, Chaltel Lima L A, Gutiérrez Pérez R, De Icaza Herrera M and Castaño V 2013 J. Nanomater. 507647 1
Kittel C 2004 Introduction to solid state physics 8th edn (Wiley) p 25
Zak A K, Majid W H A, Abrishami M E and Yousefi R 2011 Solid State Sci. 13 251
Borah D J and Mostako A T 2020 Appl. Phys. A 126:818 1
Tagliente M A and Massaro M 2008 Nucl. Instrum. Meth. Phys. Res. B 266 1055
Mote V D, Purushotham Y and Dole B N 2012 J. Theor. Appl. Phys. 6 6
Pawar S B, Shaikh J S, Devan R S, Ma Y R, Haranath D, Bhosale P N et al 2011 Appl. Surf. Sci. 258 1869
Aziz B S, Rasheed M A, Saeed S R and Abdullah O G 2017 Int. J. Electrochem. Sci. 12 3263
Heiba Z K, Mohamed M B, Abdellatief M and Albassam A A 2020 Appl. Phys. A 126:518 1
Sharma S, Reddy A V D, Jayarambabu N, Kumar N V M, Saineetha A, Kailsa S et al 2020 Mater. Today Proc. 26 162
Ranjan R and Sinha A S K 2019 Int. J. Hydrog. Energy 44 5955
Poornaprakash B, Chalapathi U, Kumar M, Subramanyam K, Vattikuti S V P, Reddy M S P et al 2020 Ceram. Int. 46 21728
Sabah A, Siddiqui S A and Ali S 2010 World Acad. Sci. Eng. Technol. 69 82
Gahramanli L, Muradov M, Kukovecz A, Balayeva O and Eyvazova G 2020 Inorg. Nano Met. Chem. 50 1
Zahn D R T, Maierhofer Ch, Winter A, Reckzugel M, Sarma R, Thomas A et al 1991 J. Vac. Sci. Technol. B 9 2206
Khallaf H, Chai G, Lopan O, Chow L, Park S and Schulte A 2008 J. Phys. D: Appl. Phys. 41 185304
Lee J 2004 Thin Solid Films 451–452 170
Arguello C A, Rousseau D L and Porto S P S 1969 Phys. Rev. 181 1351
Tell B, Damen T C and Porto S P S 1966 Phys. Rev. 144 771
Nusimovici M A and Birman J L 1967 Phys. Rev. 156 925
Enderlein T R and Leite J R 1996 J. Appl. Phys. 79 4137
Najmaei S, Liu Z, Ajayan P M and Lou L 2012 Appl. Phys. Lett. 100 013106
Munaga V V P, Krishnan T and Borra R K 2020 SN Appl. Sci. 2:552 1
Rajashree C and Balu A R 2016 Optik 127 8892
Borah D J, Mostako A T T, Saikia P K and Dutta P 2019 Mater. Sci. Semicond. Process. 93 111
Tolansky S 1970 Phys. Today 23 95
Kapriper A, Guneri E, Code F, Gamus C and Ozpozan T 2011 Mater. Chem. Phys. 129 183
Mahdi M A and Al-Ani S K J 2012 Int. J. Nanoelectron. Mater. 5 11
Yogamalar N R and Bose A C 2011 Appl. Phys. A 103 33
Kaur M, Kaur P, Kaur G, Dev K, Negi P and Sharma R 2018 Vacuum 155 689
Zhao Y and Li W 2019 Mater. Res. Express 6 115908
Yilmaz M, Tatar D, Sonmez E, Cirak C, Aydogan S and Gunturku R 2015 Synth. React. Inorg. Met. Org. Chem. 46 489
Thangavel S, Ganesan S, Chandramohan S, Sudhagar P, Kang Y S and Hong C H 2010 J. Alloys Compd. 495 234
Acknowledgements
We acknowledge the Department of Physics, Bahona College, Jorhat (India), for providing laboratory facilities. We acknowledge the CSIC, Dibrugarh University, Dibrugarh (India), for providing the SEM and EDX facilities. We acknowledge SAIF, Gauhati University, Guwahati (India), for providing PXRD facilities. We also acknowledge SAIF-NEHU, Shillong, Meghalaya (India), for TEM, HRTEM and SAED facilities. We acknowledge SAIC, Tezpur University, (India), for providing FTIR and micro-Raman facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gogoi, L., Chaliha, S., Borah, D.J. et al. Influence of Cd content on structural and optical properties of chemical bath deposited CdxPb1−xS thin films. Bull Mater Sci 44, 225 (2021). https://doi.org/10.1007/s12034-021-02506-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-021-02506-2