Abstract
The aim of this study was to investigate biological, physicochemical and mechanical characteristics of a series of novel dental restorative nanocomposites that comprise dendritic methacrylate end-caped monomers, triethylene glycol dimethacrylate (TEGDMA; as diluting monomer) and modified silica nanoparticles (\(\hbox {M-SiO}_{2}\); as inorganic filler). The cytotoxicity effects of the monomers and fabricated nanocomposites were examined against NIH3T3 cells (the standard fibroblast cell line) through MTT and trypan blue cell viability tests, respectively. The antibacterial activities of the monomers were evaluated against Lactobacillus plantarum by standard agar disk diffusion approach. The mechanical properties (flexural strength (FS) and compressive strength (CS)) as well as some physicochemical characteristics such as water sorption (WS), sol fraction (SF) and double bond conversion (DC) were also investigated, and compared with corresponding characteristics of 3M Filtek Z250 as a reference. Thus, the fabricated nanocomposites have potential as dental restorative materials mainly due to their suitable biological, physicochemical and mechanical properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The last decade has witnessed significant progress on design and development of resin-based dental restorative materials. The most important part of this progress is related to synthesis of novel and more efficient monomers [1,2,3,4]. In this context, a new strategy is the use of multi-functional or dendritic monomers, mainly due to some advantages including low shrinkage stress and strain during photo-polymerization, high degree of conversion and high mechanical characteristics in comparison with bi-functional conventional dental monomers (e.g., 2,2-bis[4-(2-hydroxy-3-methacryloxypropoxy)phenyl]propane (Bis-GMA), TEGDMA and 1,6-bis(2-methacryloxyethoxycarbonylamino)-2,4,4-trimethylhexane (UDMA)) [1, 5, 6]. In addition, these monomers have high cross-link density (originated from their large number of reactive functional end groups) that resulted in a three-dimensional network as well as decrease in solubility and water sorption when compared with other similar molecular weight molecules [1, 5].
It is well established that the proliferation of cariogenic bacteria (e.g., Streptococcus mutans) into the microleakage between the resin-based composites (RBCs) and tooth structure is the main reason for secondary caries [7, 8]. In this context, antibacterial (especially fluoride-releasing) dental restorative materials can be considered as an efficient anticariogenic compounds for solving the mentioned thematic issue [9, 10]. It should be pointed out that the anticariogenic effect of fluoride-releasing dental restorative materials is resulted from their abilities to enhance re-mineralization and the formation of acid-resistant fluorapatite [11, 12]. The synthesis of quaternary ammonium fluoride monomers (QAFM) containing long-chains can be considered as an efficient approach towards fluoride-releasing dental restorative materials [13, 14].
Besides above-mentioned point, the most important problems of RBCs are low mechanical characteristics in comparison with dental amalgams and dental porcelain [15], shrinkage stress and strain during photo-polymerization [16] and low degree of monomer(s) conversion that caused to undesirable outcomes (e.g., allergic reactions) in patients due to releasing of residual monomer(s) [17]. These thematic issues could be solved through the use of multi-functional or dendritic monomers that discussed extensively in our pervious works [1, 18].
To the best of our knowledge, research on design and development of dendritic and antibacterial dental monomers is relatively new and no commercial products are available. We have previously synthesized and characterized two dendritic and antibacterial dental monomers. The chemical structures of these monomers are shown in schemes 1 and 2. Following this work; we report here the physicochemical (e.g., double bond conversion, water sorption and sol fraction), mechanical (e.g., flexural strength and compressive strength) and some biological (e.g., biocompatibility and antibacterial activity) aspects of novel dental restorative nanocomposites that fabricated from dendritic methacrylate end-capped dental monomers, TEGDMA as diluting monomer and modified silica nanoparticles as inorganic filler. The results obtained were compared with corresponding characteristics of 3M Filtek Z250 as a universal reference.
2 Experimental
2.1 Materials
Monomers 1 and 2 and modified silica nanoparticles (\(\hbox {M-SiO}_{2}\) NPs) [using 3-(trimethoxysilyl)propylmethacrylate (MPS) and (3-mercaptopropyl)trimethoxysilane (MPT) (50:50 by mol)] were synthesized in our laboratory. Camphorquinone (CQ), 2-(dimethylamino)ethyl methacrylate (2-DMAEMA) and TEGDMA were purchased from Sigma-Aldrich (USA) and were used as received. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphe-nyltetrazolium bromide (MTT), foetal bovine serum (FBS) and other biological reagents were purchased from Invitrogen (Carlsbad, CA, USA) and were used as received. All other chemical reagents as well as solvents were purchased from Sigma-Aldrich (USA) or Merck (Darmstadt, Germany) and purified according to the standard methods.
2.2 Nanocomposite preparation
The synthesized QAFM, TEGDMA and M-\(\hbox {SiO}_{2} \) (see table 1) were added to 40 ml ethanol, stirred for about 1 h in the dark, and then, the solvent was distilled by using a rotary evaporator under reduced pressure. The composite was further mixed manually for about 20 min, and then, CQ (0.5 wt%) and 2-DMAEMA (0.5 wt%) (as visible-light initiator and reducing agent, respectively) were added to the mixture. The mixture was then mixed manually for another 20 min, and photopolymerized through the irradiation visible-light (800 mW \(\hbox {cm}^{-2}\)) for 40 s using a dental curing unit (Optilux 501, Kerr, USA).
2.3 Degree of double bond conversion
The degree of double bond conversion of formulated nanocomposites (see table 1) were measured using Fourier transform infrared (FTIR) spectroscopy (Shimadzu, Kyoto, Japan) through FTIR spectra of the sample before and after curing (40 s, 800 mW \(\hbox {cm}^{-2}\)). Degree of conversion (DC) was calculated from the ratio between the absorbance peaks of aliphatic C=C (peak at \(\sim 1630\,\hbox {cm}^{-1}\)) against the carbonyl group (C=O, peak at \(\sim 1720\,\hbox {cm}^{-1}\)) before and after curing of the specimen using the following equation [19].
2.4 Water sorption and sol fraction measurements
Sol fraction (SF) and water sorption (WS) of the cured samples were measured as follows. After photopolymerization, the specimen was weighed for every 24 h, until a constant mass (labelled as M1; variation was < 0.1 mg in any 24 h period). Then, the sample was immersed in double-distilled water. At fixed time intervals, the sample was removed, dried under vacuum to remove excess water, weighed and returned to the double-distilled water. Equilibrium mass (M2) was obtained until there was no significant change in the mass. Then, the samples were dried at \(65^{\circ }\hbox {C}\) until their mass was constant (M3). Water sorption and sol fraction of the samples were then calculated using the following equations [20].
2.5 Antibacterial activity test
The antibacterial activities of the synthesized monomers (M1 and M2) were investigated through standard agar disk diffusion approach against Lactobacillus plantarum (ATCC 8014) using sterile nutrient agar medium (MRS) during 72 h. The culture stocks were revived by inoculating them into freshly prepared sterile nutrient broth (MRS medium), and then incubating at \(37^{\circ }\hbox {C}\) and 100 rpm for about 24 h. Once the media got solidified by the addition of agar in respective sterile Petri plates, and then the bacterial cultures were smeared. It is worth to note that the monomers were dissolved in chloroform (\(\hbox {CHCl}_{3}\); 100 mg \(\hbox {ml}^{-1}\)), and 20 \(\upmu \hbox {l}\) was loaded on sterile paper disk with a diameter of 5 mm and a thickness of 1.5 mm. The disks were dried in laminar flow hood, and then inserted into the bacterial cultured Petri plate.
2.6 Biocompatibility tests
2.6.1 Cell culture
The biocompatibility of the synthesized monomers and fabricated nanocomposites were examined against NIH3T3 cell line (Iranian National Cell Bank, Pasteur Institute, Tehran, Iran). The cells were cultured into flasks and kept in a humidified incubator with \(\hbox {CO}_{2}\) (5%) at \(37^{\circ }\hbox {C}\). The cells were grown (up to 65–70% confluency) in RPMI1640 (Gibco, Invitrogen, CA, USA) with 10% (v/v) foetal bovine serum (FBS) and antibiotics (100 IU \(\hbox {cm}^{-3}\) penicillin G, 0.1 mg \(\hbox {cm}^{-3}\) streptomycin; Invitrogen, CA, USA) [21].
2.6.2 Cell viability assay
The cytotoxic effects of the synthesized monomers were evaluated using MTT assay vs. TEGDMA as the reference. For this purpose, the NIH3T3 cells was trypsinized, harvested and seeded in 96-well plates. After overnight incubation, the cells were treated with different concentrations (12.5, 25, 50 and 100 \(\upmu \hbox {g ml}^{-1}\)) of synthesized monomers (M1 and M2) as well as TEGDMA for time periods of 24, 48 and 72 h. After desired time, the medium containing monomers was replaced with cultivation medium and 50 \(\upmu \hbox {l}\) of phosphate-buffered saline (PBS) containing MTT reagent (2 mg \(\hbox {ml}^{-1}\)) was added to each well, then, the plates were placed in incubator for another 4 h. Then, the remaining MTT solution was aspirated, the formed formazan crystals were dissolved in dimethyl sulfoxide (DMSO; 200 \(\upmu \hbox {l}\)) containing sorenson buffer (25 \(\upmu \hbox {l}\)), and absorbance was measured at 570 nm using a spectrophotometric plate reader, ELx 800 (Biotek, San Francisco, CA, USA) [22].
The biocompatibilities of the fabricated nanocomposites were examined using trypan blue cell viability tests and compared with 3M Filtek Z250 as the reference. For this purpose, the nanocomposites (see table 1) and 3M Filtek Z250 were cured through the irradiation visible-light (800 mW \(\hbox {cm}^{-2}\)) for 40 s using a dental curing unit, and then, the samples were inserted into the 24-well plate. The cells were poured at a seeding density of \(1 \times 10^{5}\) per well, and incubated for about 24 h in a humidified incubator with \(\hbox {CO}_{2}\) (5%) at \(37^{\circ }\hbox {C}\). At the end of this period, the cells were trypsinized, and 10 \(\upmu \hbox {l}\) of cell suspension was removed and mixed with 10 \(\upmu \hbox {l}\) of trypan blue solution (0.4%; Sigma-Aldrich, USA) for 5 min. Brilliant blue and unstained cells representative of dead and live cells, respectively, were counted using a haemocytometer microchamber under an inverted microscope (Olympus CKX414, Minneapolis, MN, USA). The percent of viability was quantified using the following equation [23].
2.7 Mechanical tests
Compressive strength tests were carried out according to ISO 9917 using the stainless steel cylindrical moulds with a diameter of 4 mm and a height of 6 mm. The moulds were filled with the prepared nanocomposites in two step (layer by layer) and cured through the irradiation visible-light (800 mW \(\hbox {cm}^{-2}\)) for 40 s using a dental curing unit before the insertion of second layer. The samples were detached from the moulds and stored in distilled water at room temperature for 24 h prior to test. The compressive strength was then determined with the Hounsfield H5KS universal testing machine (Hounsfield, Redhill, UK) at a cross-head speed of 1 mm \(\hbox {min}^{-1 }\). The specimens (\(n = 5\)) were placed with their flat ends between the plates of the testing machine, so that the progressively increasing compressive load was applied along the long axis of the specimens [24].
The flexural strength of specimens was investigated according to ISO 4049 through the insertion of the resin composites into a rectangular stainless-steel mould with 2 mm \(\times \) 2 mm \(\times \) 25 mm dimensions, which was placed on a glass slide. Another glass slide was placed on the top of the mould and the specimens were cured from both top and bottom sides through the irradiation visible-light (800 mW \(\hbox {cm}^{-2}\)) for 40 s using a dental curing unit. The specimens were removed from the mould and stored in distilled water at room temperature for about 24 h prior to test, and then, both the surfaces of specimens were polished using a silicon carbide paper in a moist environment. A three-point bending test was performed using a universal testing machine (Z 010, Zwick/Roell, Ulm, Germany) at a cross-head speed of 0.5 mm \(\hbox {min}^{-1}\) [25].
3 Results and discussion
The conventional RBCs have some disadvantages including accumulation of more biofilm and plaque, polymerization shrinkage and low double bond conversion. Thus, great deals of academic and industrial efforts were devoted to solve these thematic issues. In this context, the use of dendritic ammonium fluoride monomers can be considered as an efficient strategy [1, 18]. Nevertheless, this field is still growing and many pivotal issues remain to be addressed before translation into clinical therapies and achieving commercial success.
3.1 Physicochemical properties (DC, WS and SF)
Degree of monomer conversion is an important parameter in RBCs and many researches conducted for its improvement [1]. In this study, we have investigated the DC of the samples using FTIR spectroscopy and the results obtained were compared with DC of the 3M Filtek Z250 as a universal reference (table 2). As seen, all fabricated nanocomposites using the synthesized monomers (as shown in schemes 1 and 2) have higher DC in comparison with 3M Filtek Z250. This may be resulted from the multi-functionality of the synthesized monomers that provide higher DC. Another reason for this may be the formation of thiol–methacrylate systems by M-\(\hbox {SiO}_{2}\) NPs. In addition, the monomer 2, which possesses 12 methacrylate terminal groups has a slightly higher DC than those of the monomer 1, which possesses six methacrylate terminal groups.
Water sorption and sol fraction of the fabricated nanocomposites (see table 1) were investigated in this study and compared with corresponding characteristics of a commercial sample (3M Filtek Z250). As seen in table 2, in comparison with 3M Filtek Z250, the prepared nanocomposites did not show significant differences in water sorption and sol fraction values.
3.2 Antibacterial activities of monomers
Intense interest was focussed on the design and development of fluoride-releasing dental restorative materials mainly to prevent accumulation and proliferation of cariogenic bacteria or plaque that resulted to secondary caries [7,8,9,10].
It is well established that the QAMs have antibacterial activity characteristic through two antibacterial mechanisms as follows:
1. The bacteria attached to the surface of composite is killed by QAMs, which are immobilized in the polymer.
2. The QAM compound released from the composite into bacterial suspension can devitalize bacteria in the suspension [20, 26].
The antibacterial activities of the M1 and M2 were evaluated using agar disk diffusion test (ADT) method against Lactobacillus plantarum. As seen in figure 1, in comparison with gentamicin (a strong antibiotic as positive control; C), the synthesized M1 exhibited approximately the same antibacterial activities. In contrast, the M2 exhibited higher inhabitation zone due to higher number of fluoride ions in the structure of this monomer.
3.3 Biocompatibility of monomers and nanocomposites
The biocompatibility of any material is the first concern regarding its application for biomedical purpose. Thus, the cytotoxic effects of the synthesized monomers as well as biocompatibility of the cured nanocomposites were investigated through MTT and trypan blue cell viability tests against NIH3T3 cell line, respectively. Figure 2 shows the cytotoxic effects of the synthesized monomers (M1 and M2) after 24, 48 and 72 h using MTT assay.
As seen in this figure, the synthesized monomers approximately exhibited no toxicity in all the mentioned time periods. At the higher concentration (100 \(\upmu \hbox {g ml}^{-1}\)) and long-time incubation (after 72 h), the monomers show a little cytotoxic effects, however, the cells viabilities are over than 80 and 65% for monomers 1 and 2, respectively, even at this condition. It is worth to note that in comparison with TEGDMA, the synthesized monomers showed less toxicity as seen in figure 2. The potential in vitro cytotoxic, genotoxic, mutagenic, estrogenic effects of TEGDMA at higher concentrations were established [27].
The cytotoxicity assay (MTT) results of the synthesized monomers (M1 and M2) and TEGDMA at different concentrations (12.5, 25, 50 and 100 \(\upmu \hbox {g ml}^{-1}\)) after 24, 48 and 72 h against NIH3T3 cells (Neg; negative control: 0.05% DMSO and Pos; positive control: 5% DMSO) (* represents significant difference between groups and untreated NIH3T3 cell line (\(P < 0.05\))).
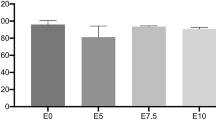
The biocompatibilities of the fabricated nanocomposites after curing (see table 1) were examined using trypan blue cell viability test and compared with the 3M Filtak Z250 as the reference. As seen in figure 3, in comparison with 3M Filtak Z250, the fabricated nanocomposites using the synthesized monomers have good biocompatibilities after incubation time of 24 h. Among the samples, the prepared nanocomposite (S4) using only TEGDMA as the monomer showed the highest cytotoxic effect in accordance with the MTT assay results.
The trypane blue cell viability assay results of fabricated nanocomposites (see table 1) and 3M Filtak Z250 (* represents significant difference between groups and untreated NIH3T3 cell line (\(P < 0.05\))).
3.4 Mechanical properties
As mentioned in the literature, the RBCs have lower mechanical characteristics in comparison with dental amalgam [1, 28]. Thus, each year, a great deal of research effort in the field of RBCs was focussed on the improvement of their mechanical properties. As mentioned in ‘Introduction’ section, the use of multi-functional or dendritic monomers can be considered as an efficient strategy for solving this issue in part, due to inherent characters of these compounds (e.g., high cross-link density that resulted to a three-dimensional network). Thus, the mechanical properties are amongst the most important factors that require consideration in RBCs.
Compressive strength (CS) of the samples (see table 1).
Flexural strength (FS) of the samples (see table 1).
The compressive strength (CS) and flexural strength (FS) of the fabricated nanocomposites were investigated and the results obtained are summarized in figures 4 and 5.
As shown in figure 4, the fabricated nanocomposites exhibited close values of CS to the 3M Filtek Z250. In detail, all fabricated nanocomposites showed slightly lower CS values in comparison with 3M Filtek Z250 sample that polymerized in the same condition.
The FS studies of the samples (figure 5) revealed that this property is relatively different for the samples. In detail, the 3M Filtek Z250 sample showed the highest FS, and S1 exhibited the lowest FS values. The results obtained from CS and FS studies are summarized in table 3. According to the results, it seems that the higher number of methacrylate end-capped groups resulted in the better mechanical characteristics.
4 Conclusion
Fabrication and investigation of some biological, physicochemical and mechanical characteristics of a series of novel dental restorative nanocomposites that comprise dendritic QAMs, TEGDMA and M-\(\hbox {SiO}_{2}\) NPs were successfully demonstrated. The cytotoxicity effects of the monomers and biocompatibilities of the fabricated nanocomposites were examined by MTT and trypan blue cell viability tests against NIH3T3 cells, respectively. The monomers exhibited no significant toxicity effects after 24, 48 and 72 h. In comparison with 3M Filtek Z250 (a universal resin-based dental composite), the fabricated nanocomposites exhibited better biocompatibilities. The antibacterial activities of the monomers were approved using agar disk diffusion test (ADT) method against Lactobacillus plantarum. The mechanical (flexural and compressive strengths) properties study revealed that the fabricated nanocomposites have acceptable mechanical characteristics in comparison with 3M Filtek Z250 sample. As a result, the fabricated nanocomposites have potential as dental restorative materials in part due to their acceptable biological, physicochemical and mechanical characteristics. In conclusion, further experiments are under progress to evaluate the effects of other inorganic fillers (e.g., zirconium nanoparticles) and diluting monomer on the mechanical, physicochemical and biological properties of the resultant nanocomposites.
References
Jaymand M, Lotfi M and Lotfi R 2016 RSC Adv. 6 43127
Milia E, Cumbo E, Cardoso R J A and Gallina G 2012 Curr. Pharm. Des. 18 5542
Huyang G, Debertin A E and Sun J 2016 Mater. Des. 94 295
Zhang L, Gao Y, Chen Q, Tian M and Fong H 2010 J. Mater. Sci. 45 2521
Viljanen E K, Skrifvars M and Vallittu P K 2007 Dent. Mater. 23 1420
Viljanen E K, Langer S, Skrifvars M and Vallittu P K 2006 Dent. Mater. 22 845
Wang Z, Shen Y and Haapasalo M 2014 Dent. Mater. 30 e1
Featherstone J D and Doméjean S 2012 Adv. Dent. Res. 24 28
Flausino J S, Soares P B F, Carvalho V F, Magalhães D, Da Silva W M, Costa H L et al 2014 J. Mater. Sci. 49 6820
Dionysopoulos D, Eugenia K, Maria H and Nikolaos K 2013 Dent. Mater. J. 32 296
Xu X, Wang Y, Liao S, Wen Z T and Fan Y 2012 J. Biomed. Mater. Res. Part B 100 1151
Burke F M, Ray N J and McConnell R J 2006 Int. Dent. J. 56 33
Cheng L, Weir M D, Zhang K, Arola D D, Zhou X and Xu H H K 2013 J. Dent. 41 345
DePaola P F, Soparkar P, Foley S, Bookstein F and Bakhos Y 1977 Community Dent. Oral Epid. 5 7
Ferracane J L 2011 Dent. Mater. 27 29
Kaisarly D and Gezawi M E 2016 Odontology 104 257
Van Landuyt K L, Nawrot T, Geebelen B, De Munck J, Snauwaert J, Yoshihara K et al 2011 Dent. Mater. 27 723
Jaymand M, Lotfi M, Barar J and Kimyai S 2017 Res. Chem. Intermed. 43 5707
Goncalves L, Filho J D N, Guimaraes J A, Poskus L T and Silva E M 2008 J. Biomed. Mater. Res. B 85 320
Liang X, Huang Q, Liu F, He J and Lin Z 2013 J. Appl. Polym. Sci. 129 3373
Hatamzadeh M, Najafi-Moghadam P, Baradar-Khoshfetrat A, Jaymand M and Massoumi B 2016 Polymer 107 177
Jaymand M, Sarvari R, Abbaszadeh P, Massoumi B, Eskandani M and Beygi-Khosrowshahi Y 2016 J. Biomed. Mater. Res. A 104 2673
Miller L, Leor J and Rubinsky B 2005 Technol. Cancer Res. Treat. 4 699
Wang L, D’Alpino P H P, Lopes L G and Pereira J C 2003 J. Appl. Oral Sci. 11 162
Rodríguez H A, Giraldo L F and Casanova H 2015 Dent. Mater. 31 789
Rupf S, Balkenhol M, Sahrhage T O, Baum A, Chromik J N, Ruppert K et al 2012 Dent. Mater. 28 974
Emmler J, Seiss M, Kreppel H, Reichl F X, Hickel R and Kehe K 2008 Dent. Mater. 24 1670
Lohbauer U, Belli R and Ferracane J L 2013 J. Dent. Res. 92 584
Acknowledgements
We wish to express our gratitude to the Research Center for Pharmaceutical Nanotechnology (RCPN), Tabriz University of Medical Sciences, for financial support for this project (Grant Number: 93002, which was a part of PhD thesis Number: 93/002/131/4).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaymand, M., Lotfi, M., Barar, J. et al. Novel dental nanocomposites: fabrication and investigation of their physicochemical, mechanical and biological properties. Bull Mater Sci 41, 84 (2018). https://doi.org/10.1007/s12034-018-1589-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-018-1589-z