Abstract
Nanocrystalline \(\hbox {ZnS:Pb}^{2+}\) phosphor was synthesized by microwave-assisted co-precipitation method. The phase purity and surface morphology of prepared material were investigated using an X-ray diffractometer (XRD) and a scanning electron microscope. The photoluminescence property was studied by near-UV (nUV) excitation. The XRD pattern of prepared phosphor matches well with that of an ICDD (International Center for Diffraction Data) file. Surface morphology of prepared phosphor was found to be in submicron range. As-prepared \(\hbox {ZnS:Pb}^{2+}\) phosphor shows a green emission under nUV excitation. As \(\hbox {Pb}^{2+}\) ions are located on a regular \(\hbox {Zn}^{2+}\) site they produce a green emission band in the range of 400–650 nm, centred at 500 nm, under excitation wavelength of 357 nm, exhibiting luminescence properties typically observed for \(\hbox {Pb}^{2+}\). Effect of concentration of \(\hbox {Pb}^{2+}\) ions on emission intensity was studied. The colour co-ordinates of prepared phosphor were calculated and found to lie in the green region of Commission Internationale de l’Eclairage diagram.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
ZnS exhibits direct bandgaps of 3.83 eV for the cubic zinc-blende (ZB) phase and 3.91 eV for the hexagonal wurtzite (WZ) phase [1]. Although much research has been devoted to the photoluminescence (PL) properties of 1D ZnS nanostructures, very few studies have reported possible UV bandgap emission at room temperature. This is mainly due to high sensitivity of the 1D ZnS nanostructure optical properties to the synthesis conditions, its crystal size and shape, and intrinsic defects such as vacancies and interstitials.
The emission wavelength strongly depends on the synthesis conditions, suggesting the presence of multiple optically active centres [2, 3]. In the middle of 1980s it became clear that rare-earth doping of ZnS would not lead to sufficiently bright PL materials. As a result, several new activator–host combinations were tested and found to yield bright emission, CaS:Ce (green) [4], SrS:Ce (blue-green) [5], CaS:Eu (red) [6] and SrS:Eu (orange) [7] being the most successful combination.
Nanometre-sized semiconductor particles are currently investigated in many laboratories over the world. Due to spatial confinement of electrons and holes, the chemical and physical properties of these particles strongly differ from those of the bulk solids (size quantization effect) [8,9,10,11]. They form a totally new group of materials with potentially valuable applications [9]. Nanocrystals doped with optically active luminescence centres create new opportunities for luminescence study and applications of nanometre scale materials [12, 13]. ZnS is the most typical and important crystalline phosphor both for application and basic science [14].
Doped ZnS nanocrystals have changed properties; especially, ZnS nanoparticles activated by the transition metallic ions form a new group of luminescent materials. Sulphide phosphors could be the phosphors in future for colour conversion in white LEDs. Here we present PL characteristics of ZnS nanoparticles doped with \(\hbox {Pb}^{2+}\). New luminescence characteristics like strong and stable broad band emission covering entire spectrum of visible light have been observed in \(\hbox {ZnS:Pb}^{2+}\).
2 Experimental
2.1 Sample preparation
\(\hbox {Pb}^{2+}\) (0.001, 0.003, 0.005, 0.01 and 0.02 mol)-doped zinc sulphide nanophosphors have been prepared by microwave-assisted co-precipitation method. All ingredients used were of AR grade. \(\hbox {ZnS:Pb}^{2+}\) was first synthesized through the co-precipitation method by the reaction of freshly prepared solution of \(\hbox {Zn}(\hbox {NO}_{3})_{2}\cdot 6\hbox {H}_{2}\hbox {O}\) and \(\hbox {Pb}(\hbox {NO}_{3})_{2}\) in stoichiometric amount with dropwise addition of freshly prepared and filtered greenish solution of sodium sulphide (\(\hbox {Na}_{2}\hbox {S}\cdot 9\hbox {H}_{2}\hbox {O}\)) [15].
The precipitate was washed repeatedly by double-distilled water and then dried slowly at \(40^{\circ }\hbox {C}\). The dried precipitate was then heated in a slightly reducing atmosphere in the presence of charcoal in a closed crucible (in order to avoid formation of sulphate) at \(700^{\circ }\hbox {C}\) for 4 h in a microwave furnace and then allowed to cool very slowly to room temperature. Thus, greenish yellow colour fine powder of \(\hbox {ZnS:Pb}^{2+}\) phosphor was obtained.
2.2 Instruments
A microwave muffle furnace of CEM corporation, Phoenix Standard Unit (a high-capacity model, maximum temperature \(1000^{\circ }\hbox {C}\)), working at nominal line voltage of 220–240 \(\hbox { V}_{\mathrm{AC}}\), 50 Hz, 10 A and magnetron frequency 2455 MHz with power output of 1350 W (\(\pm 50\hbox { W}\)) was used for heating the samples during the synthesis process. The phase purity of the prepared phosphors was investigated using a Rikagu Meniflex II X-ray diffractometer (XRD) with Cu \(\hbox {K}\upalpha \) radiation (\(\lambda = 1.54060\,\, {\AA }\)) at 40 kV and 30 mA. The XRD pattern was recorded in the range of \(10^{\circ } \le 2\theta \le 70^{\circ }\). PL measurement at room temperature was performed on a Hitachi F-7000 spectrofluorimeter equipped with a 450-W Xenon lamp, in the range of 200–700 nm, with spectral width of 2.5 nm, and spectra were corrected using Rhodamine B standard following the procedure prescribed by Hitachi. Effect of dopant concentration on emission intensity of prepared phosphor was studied and chromaticity coordinates for optimum concentration were also calculated. Surface morphology and elemental composition of phosphors were studied by scanning electron microscopy (SEM) using a JEOL’s model JSM-6360A fitted with EDS facility.
3 Results and discussion
3.1 XRD analysis
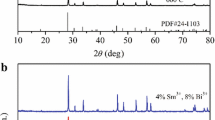
XRD pattern of the synthesized \(\hbox {ZnS:Pb}^{2+}\) phosphor is shown in figure 1. The peak positions in the diffraction pattern of the synthesized material when compared to the standard data available with International Center for Diffraction Data were found to match with those of ICDD file 01-072-4841, confirming the formation of the material. The crystallographic data are given in table 1. No additional peaks corresponding to any impurity phase were reported in the XRD pattern, showing that the dopant has a good solubility in the host matrix for this range of concentration. The average crystalline size of this prepared phosphor was found to be 45.36 nm when estimated from XRD information using the Debye–Scherrer formula [16]
where \(\theta \) is the Bragg angle of diffraction lines, K is a shape factor taken as 0.90, \(\lambda \) is wavelength of incident X-rays (\(\lambda = 0.154~\hbox {nm}\)) and \(\beta \) is full-width at half-maximum (FWHM in radians).
3.2 Surface morphology study of as-synthesized phosphor
The morphological structures of the prepared \(\hbox {ZnS:Pb}^{2+}\) phosphor characterized by SEM are shown in figure 2. Though exact dimensions are difficult to measure, the particles can be approximately considered, from the resolution scale, as micro–nanostructures (submicron), i.e., mix-up of nanoparticles 200–400 nm (0.2–0.4\(\,\,\upmu \hbox {m}\)) in diameter (figure 2a) and nanorods of diameter 300–500 nm (0.3–0.5\(\,\, \upmu \hbox {m}\)) and 0.5–1\(\,\, \upmu \hbox {m}\) length (figure 2b). The SEM images also reveal that some of the crystals of prepared phosphor are almost similar to nanoawls, i.e., nanorods with needle-shaped tips, having tips of length 1–5\(\,\,\upmu \hbox {m}\), as shown in figure 2c.
The EDS pattern of prepared phosphor shown in figure 3 contains prominent peaks of Zn and S along with small peaks of Pt, as platinum was used during sample preparation for SEM. From the EDS pattern, it was observed that Zn and S are not in appropriate proportion of elemental composition; instead, sulphur deficiency is indicated, as reported in other papers [17]. In the ZnS lattice, the \(\hbox {Pb}^{2+}\) cation has an oxidation state of +2 and on doping it is located on a tetrahedral site with replacement of \(\hbox {Zn}^{2+}\) (purple balls), where it is coordinated by four \(\hbox {S}^{2-}\) anions (yellow balls) as shown in figure 3.
3.3 PL study of synthesized phosphor \(ZnS{:}Pb^{2+}\)
A combined excitation and emission spectrum of \(\hbox {ZnS:Pb}^{2+}\) studied at room temperature is shown in figure 4a. The excitation monitored at 500 nm shows broad excitation band with the highest intensity peak at 280 nm along with a broad and flat region with a maximum at 357 nm. Some low-intensity peaks as compared with these were also observed on smaller wavelengths side, indicating direct transition from valence band to conduction band associated with the peculiar nanostructure of the host ZnS lattice (figure 4b).
The curve representing emission spectrum of the synthesized \(\hbox {ZnS:Pb}^{2+}\) phosphor monitored at 357 nm shows a broad emission band in the wavelength range 400–650 nm (covering almost the entire visible spectrum) centred at 500 nm exhibiting green emission with FWHM of around 100 nm having a slight shouldering at 475 nm (figure 4a). The stable and wide green emission band centred at 500 nm is attributed to a characteristic transition between \(\hbox {Pb}^{2+}\) levels and the slight shouldering at 475 nm, blue–green emission, could be ascribed to the transitions involving self-activated centres, vacancy states or interstitial states associated with the peculiar nanostructure (figure 4b).
3.4 Effect of \({Pb}^{2+}\) concentration on emission intensity of \({ZnS{:}Pb}^{2+}\) phosphor
Emission spectra for different concentrations of \(\hbox {Pb}^{2+}\) ions are shown in figure 5. When the molar concentration of \(\hbox {Pb}^{2+}\) ions was increased from 0.1 to 2% it was observed that 1 mol% concentration was the optimum for the prepared phosphor, showing the maximum emission intensity. Thereafter, emission intensity decreased due to the well-known phenomenon of concentration quenching [18].
3.5 Study of colour chromaticity
The chromaticity diagram established by the Commission Internationale de l’Eclairage (CIE) in 1931 is a two-dimensional graphical representation of any colour perceivable by the human eye on an x–y plot, in which colours are represented by means of colour coordinates. To confirm the chromaticity in the present case, the colour coordinates of phosphors \(\hbox {ZnS:Pb}^{2+}\) (Pb = 0.01 mol) have been calculated from their corresponding emission spectra. Calculated colour coordinates (\(x = 0.192\), \(y = 0.371\)) fall in the blue–green boundary region of CIE diagram and it is closer to green than blue colour as shown in figure 6.
4 Conclusions
\(\hbox {Pb}^{2+}\)-doped ZnS nanocrystalline phosphor was successfully synthesized by microwave-assisted co-precipitation method. The crystal structure and phase purity of prepared phosphor was confirmed with XRD analysis. Broadening of peaks in XRD pattern and particle size calculated by the Debye–Scherrer formula signify that the prepared phosphor is in nanometre range. Also from surface morphology it was observed that the prepared phosphor is in submicron range. The emission spectra of \(\hbox {Pb}^{2+}\)-doped ZnS demonstrate stable broad-band green emission centred at 500 nm for nUV excitation of 357 nm. From the afore-mentioned discussion, \(\hbox {ZnS:Pb}^{2+}\) phosphor can be used as a green component of nUV-excited RGB tricolour LED for production of white light in solid-state lighting application.
References
Shionoya S 1999 in: Shionoya S and Yen W M (eds) Phosphor handbook (Boca Raton, FL: CRC Press) p 239
Rennie J, Takeuchi K, Kaneko Y and Koda T 1991 J. Lumin. 48 787
Gruzintsev A N, Volkov V T and Pronin A N 1992 J. Cryst. Growth 117 975
Shankar V, Tanaka S, Shiiki M, Deguchi H, Kobayashi H and Sasakura H 1984 Appl. Phys. Lett. 45 960
Barrow W A, Coovert R E and King C N 1984 SID Symposium Digest of Technical Papers, San Francisco, CA, USA, p 249
Tanaka S, Shankar V, Shiiki M, Deguchi H and May K H 1985 SID Symposium Digest of Technical Papers, Orlando, FL, USA, p 255
Kane J, Harty W E, Ling M and Yocom P N 1985 Proceedings of the International Display Research Conference, San Diego, CA, USA, p 163
Alivisatos A P 1996 Science 271 933
Pool R 1990 Science 248 1186
Weller H 1993 Adv. Mater. 5 88
Hasselbarth A, Eychmuller A and Weller H 1993 Chem. Phys. Lett. 203 271
Bhargava R N, Gallagher D and Welker T 1994 J. Lumin. 60–61 275
Li Y, Ding Y, Zhang Y and Quian Y 1993 J. Phys. Chem. Solids 60 13
Marfunin A S 1979 Spectroscopy (New York: Springer)
Ingale N B, Omanwar S K, Muthal P L, Dhopte S M, Kondawar V K, Gundurao T K et al 2008 Radiat. Meas. 43 1191
Sawala N S, Palan C B, Chauhan A O and Omanwar S K 2016 Optik: Int. J. Light Electron Opt. 127 6419
Manzoor K, Vadera S R, Kumar N and Kutty T R N 2003 Mater. Chem. Phys. 82 718
Palan C B, Bajaj N S and Omanwar S K 2016 Mater. Res. Bull. 76 216
Acknowledgements
DNG is thankful to The Chairman, FIST-DST Project, Sant Gadge Baba Amravati University, Amravati, for providing all the necessary research facilities and also to the Head, Deccan Education Society’s Technical Institute, Fergusson College Campus, Pune, for constant encouragement and guidance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Game, D.N., Palan, C.B., Ingale, N.B. et al. Microwave-assisted synthesis and photoluminescence properties of \(\hbox {ZnS:Pb}^{2+}\) nanophosphor for solid-state lighting. Bull Mater Sci 40, 1441–1445 (2017). https://doi.org/10.1007/s12034-017-1489-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-017-1489-7