Abstract
The thermal parameters of Mg–xZn cast alloys with 0·5–9 wt% Zn were evaluated by using computer aided cooling curve thermal analysis (CA–CCTA), whereas the corrosion behaviour was investigated by potentiodynamic polarization and immersion tests. Thermal analysis results revealed that the dendrite coherency temperature (T DCP ) decreased from 642·2 to 600 °C with the addition of Zn from 0·5 to 9 wt%. The liquid fraction at coherency point (\({f}_{ L}^{ DCP}\)) increased by 72% when Zn was increased up to 9 wt%. MgZn intermetallic phase was observed in samples with <3 wt% Zn. At higher percentages of Zn, the Mg 51Zn 20 intermetallic phase was also detected in addition to α-Mg and MgZn by first derivative cooling curves under non-equilibrium solidification. All these phases were observed along the grain boundary when Zn was rejected from the solid/liquid interface and enriched in the triple conjunction of grain boundary. The grain size decreased from 185·2 to 71·5 μm when Zn content was increased. The addition of Zn content had a significant effect on the corrosion rate and the corresponding mechanisms. The corrosion rate decreased from 2·1 to 1·81 mmpy as Zn content increased from 0·5 to 3 wt%; afterwards, however, this value increased with further increase of Zn. Mg–3Zn also had the lowest degradation rate and highest corrosion resistance which can be fully utilized for biodegradable orthopedic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Research and development of biodegradable materials has attracted a great deal of attention in recent years because of their importance in supporting tissue revival and healing which takes place by degradation of the biodegradable implant material and its replacement by the tissue (Witte et al 2008). In contrast to the current biodegradable materials based on ceramics, polymers or bioactive glasses, biodegradable metallic biomaterials exhibit superior behaviour in load-bearing applications due to their higher tensile strength and Young’s modulus (Wen et al 2004; Witte et al 2006, 2007). In recent years, investigation on corrosion behaviour and mechanical properties of magnesium alloys has assumed great importance (Ren et al 2005; Witte et al 2005; Song 2007; Somekawa et al 2009). Understanding the interfacial interaction between magnesium alloys and biological environment of the implanted area has been one of the major advances in research of biodegradable magnesium biomaterials. In contrast to earlier alloy development aimed at improving the mechanical properties, corrosion resistance and reducing cost of production of Mg alloys, the effect of alloying elements on the evolution of corrosion protective interface has been the main focus recently.
Recently, research work has reported an investigation on the possibility of using magnesium alloys in biomedical applications such as cardiovascular magnesium stents in animals (Waksman et al 2005, 2006). Di-Mario et al (2004), Erbel et al (2007) and Waksman et al (2009) reported that the first clinical human trials have been conducted. Use of Mg alloys as bone implants in various designs such as screws, plates and other types of fixture devices have also been investigated (Xu et al 2007; Zhang et al 2010). In addition, Mg alloys were also investigated as load bearing biomaterials for tissue engineering (Witte et al 2005, 2006, 2007), while Mg alloys shaped in the form of chips were also investigated for vertebral fusion in spinal surgery (Kaya et al 2007). It has also been reported that a high extracellular magnesium concentration may be helpful for cartilage tissue engineering (Feyerabend et al 2006).
Magnesium alloys exhibit lower elastic modulus and higher yield strength compared to metallic implants that are currently in use (Rosalbino et al 2010). However, their low corrosion resistance have prevented these alloys to be used as permanent medical implants. Thus, in order to maximize their potential use in biomedical applications, it is essential to improve their corrosion resistance and control the degradation rate (Song and Song 2006; Harandi et al 2011).
Indeed, previous research (Heublein et al 2003; Lévesque et al 2003; Alvarez-Lopez et al 2010; Stulikova and Smola 2010; Zhang et al 2010; Yu et al 2012) have shown that some of these alloys possess improved corrosion behaviour and can thus be considered as biocompatible materials. Most of the reported biodegradable magnesium alloys contain aluminum and rare-earth (RE) elements that are harmful to the human body. For example, Al3 + ions cause phosphate leakage bodies because it combines with inorganic phosphates. Elevated concentrations of Al3 + in the brain are related with Alzheimer’s disease (Ku et al 2002; El-Rahman 2003). Additionally, presence of RE elements (Pr, Ce, Y, etc.) could lead to hepatotoxicity. Unwarranted yttrium ions (Y3 + ) caused changes in the appearance of some rat genes and are believed to negatively affect DNA transcription factors (Yumiko et al 1997; Yang et al 2006). Zinc, on the other hand, is an essential nutritional element in the body and shows appropriate non-toxicity for biomedical applications (Zhang et al 2010). In addition to increasing the corrosion resistance of magnesium alloys, zinc also has a significant effect on the mechanical properties (Haferkamp et al 2003; Song 2007; Xu et al 2007; Somekawa et al 2009).
As alternative biodegradable biomaterials, various tertiary Mg–Zn–X alloys have been investigated recently but very little work has been conducted on binary Mg–Zn alloys. Zhang et al (2010) studied in vitro and in vivo Mg–Zn alloys and from their results they argued that an amount of 6·2 wt% Zn is considered an appropriate content based on Mg–Zn binary phase diagram (Okamoto 1994). With regard to non-equilibrium solidification, different intermetallic phases are formed in Mg–Zn alloy, which significantly affect the corrosion behaviour. The formation of these intermetallic phases depends mostly on the amount of Zn added to the alloy. Research on the relationship between the formation of these intermetallic phases and Zn content has been lacking. Also little is known on the effect of Zn content on the microstructure and corrosion behaviour of Mg–xZn binary alloys. Thus, the aim of the present paper is to report results of an investigation of in vitro degradation, corrosion properties and solidification behaviour of Mg–xZn alloys solidified under non-equilibrium conditions.
2 Materials and methods
Mg–Zn (x = 0·5, 1·5, 3, 6 and 9 by weight percentage) were prepared by melting pure magnesium ingots (99·99%) and pure zinc chips (99·99%). The materials were melted in an electrical resistance furnace under argon gas atmosphere in a mild steel crucible at 750 °C. The molten metal was maintained at this temperature for about 45 min for stabilization. The molten alloys with different percentages of Zn were stirred for 30 s before being poured into mild steel moulds which had been preheated at 400 °C.
Specimens of 15 × 15 × 10 mm size were then cut from the cast alloys ingots for metallographic examination. The specimens were prepared according to standard metallographic procedures of grinding, polishing and etching. Microstructure analysis to examine phase formation and morphologies of the alloys were performed using optical microscopy and scanning electron microscopy equipped with energy-dispersive X-ray spectroscopy (EDS) analysis. X-ray diffraction (XRD) was used to identify the phases that were formed. In order to evaluate the effect of Zn additions on the hardness alloys, Vickers microhardness test was conducted with applying a 5 kg force.
Characteristics of temperature and time for each phase reaction as well as the solidification behaviour of Mg–xZn alloy were determined using thermal analysis. Thermal analysis was carried out by inserting two thermocouples based on the work of Backerud et al (1990). The first K-type thermocouple was inserted in the centre and the second one adjusted to the wall of the preheated steel mould with dimensions of 50 × 40 mm (height × diameter). The top and bottom parts of the mould were isolated by Fiberfax plates of 25 mm thickness to ensure thermal isolation during solidification. EPAD-Th8-K high-speed data acquisition system linked to DEWESoft.7 was used to obtain temperature–time data. The cooling and first derivative curves were plotted by applying FlexPro.8 data analysis software. Subsequently, the temperature between the central and wall thermocouples (ΔT = T C − T W) was established.
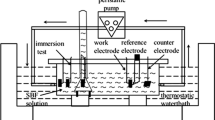
To investigate the corrosion behaviour in in vitro conditions, samples were cut and mechanically ground using silicon carbide papers with grit size ranging from 320 to 2000. The ground samples were then washed with distilled water, rinsed and degreased ultrasonically with ethanol, then dried at room temperature for immersion test. The prepared samples were immersed into beakers containing 200 ml Kokubo simulated body fluid (SBF) (Na 142·0, K 5·0, Ca 2·5, Mg 1·5, HCO3 4·2, Cl 147·8, HPO4 1·0 and SO4 0·5 mmol/L). The pH value of the solution for each sample was measured at 12 h intervals during the immersion test. The procedure of the immersion test was performed according to ASTM-G31-72 standard. The immersion test was conducted at the temperature of 37 °C for 144 h. After immersion for 144 h, the samples were taken out of SBF solution and corrosion products were removed using 20% CrO3 + 1% AgNO3 boiling solution. Samples were then rinsed in ethanol, dried in air and finally weighed (Yin et al 2008). Electrochemical potentiodynamic polarization test in Kokubo solution was conducted at pH 7·66 and temperature of 37 °C under air atmosphere. A three-electrode cell, consisting of graphite as the counter electrode, specimen as the working electrode and saturated calomel electrode (SCE) as the reference electrode, was used for the test. The potential scanning rate was fixed at 0·5 mV/s for all specimens with initial potential at − 250 mV below the open circuit potential.
3 Results and discussion
3.1 Microstructure observation and results
Figure 1 shows optical micrographs of as cast Mg–xZn (x = 0·5, 1·5, 3, 9 in weight percentage) alloys, respectively. It can be seen that the microstructure is composed of α-Mg and secondary phases which precipitated along the grain boundaries. In addition, it can also be observed that the grain size of Mg decreases with increasing Zn content. For instance, by increasing the Zn content from 0·5 to 3 wt%, the grain size decreased from 185·2 to 135·5 μm. With further increase of Zn content up to 9%, a reduction of 53% in grain size was obtained compared to the Mg–3Zn alloy.
The optical microstructures are confirmed by SEM micrographs shown in figure 2 (1·5% (a), 3% (b)). The corresponding EDS analysis also confirms that the dark area that is composed of Mg and Zn is related to the evolution of MgZn phase. The fraction of this MgZn phase is increased with increasing Zn in the alloy and became non-uniformly distributed as shown in figure 2(a and b). This is evident from the fact that Zn is concentrated in the secondary phase formed along the grain boundaries which also results in Mg matrix being depleted of Zn. This is confirmed by EDS analysis of point A in figure 2(c), where the Zn concentration is only 2·04 wt% compared with that of point B in figure 2(d) where Zn is 10·26 wt%.
Figure 3(a and b) shows a distinct phase distributed at the grain boundaries (bright phase) in addition to previous phases (dark phase MgZn) in 6 and 9 wt%, respectively. EDS analysis of the bright phase in Mg–6Zn alloy at point A in figure 3(c) indicates the presence of Zn with a value of 46·40 wt%, which suggests the formation of Mg51Zn20 intermetallic phase. This phase emerged in the form of eutectic phase with lamellar structure for more than 6 wt% Zn at grain boundaries (figure 3b). Thickness of the lamellar structure appears to have increased significantly with increasing Zn content at the grain boundaries. EDS analysis of Mg–6Zn alloy at point B in figure 3(d) also indicates the presence of Zn with a value of 10·34%, confirmed the formation of MgZn phase similar to that observed in alloys of Mg–1·5Zn and Mg–3Zn.
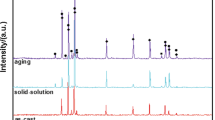
The microstructural observations discussed above were confirmed by XRD results as shown in figure 4, for the as-cast samples of Mg–Zn alloy. In addition to the defined Mg reflections, two more peaks were observed. It can be seen that the reflection of MgZn intermetallic phase appeared in Mg–1·5Zn and Mg–3Zn. However, by further addition of Zn content, a new phase was detected in addition to the previous MgZn phase. Mg51Zn20 phase was enriched with Zn which confirmed SEM and EDS observation of the same samples.
The effect of the addition of different Zn contents on the microhardness of Mg–xZn alloy is shown in figure 5. It was obvious that the hardness values increase with increasing Zn content for all the samples. This can be attributed to the refinement role of Zn on Mg alloys. However, for less than 3 wt% Zn addition to Mg–Zn alloy, hardness improves due to the solid solution strengthening mechanism of Zn dissolved in Mg matrix. In cases of more than 3 wt% Zn addition, the evolution of secondary phase at the grain boundary in the form of Mg51Zn20 in addition to MgZn intermetallic phase can be related to hardness enhancement (Yin et al 2008).
3.2 Solidification and phase transformation
Analysis of the obtained cooling curves was conducted to identify the phase evolution of α-Mg and MgZn intermetallics during solidification of Mg–xZn systems. Both cooling curves and the corresponding first derivative curves were plotted so that the dendrite coherency point, liquid fraction percentage, characteristic temperature and time are determined. Figure 6(a) shows cooling behaviour of Mg–0·5Zn during solidification and it is clear that the formation of α-Mg and MgZn phases occurred at two different temperatures according to the following equation:
Figure 6(a) shows that α-Mg and MgZn were formed at 649·6 and 509·1 °C, respectively. Similar solidification behaviour was observed for alloys of Mg–1·5Zn and Mg–3Zn with the formation of α-Mg and MgZn at temperatures of 640·1 and 507·8 °C, respectively for Mg–3Zn alloy as shown in figure 6(b). According to the binary Mg–Zn equilibrium phase diagram (Okamoto 1994), only α-Mg is expected to form under equilibrium solidification. However, the detection of MgZn is likely due to non-equilibrium solidification and rejection of Zn solute into the solidification front. This observation suggests that MgZn is formed during the last stage of solidification in the residual melt at the interdendritic space.
Figure 6(c) indicates that during solidification of Mg–6Zn alloy, an additional new phase believed to be Mg51Zn20 was formed and compared with alloys containing <6 wt% Zn. As shown in the plot, α-Mg, MgZn and Mg51Zn20 were detected at 636·2 , 597·3 and 337·5 °C, respectively. Similar results were also observed in alloy Mg–9Zn with Mg51Zn20 intermetallic phase being formed at 335·6 °C.
Figure 7 shows profile of temperature difference between the two thermocouples located at the wall and centre of the mould. This method was used in the current study to evaluate dendrite coherency point (DCP), corresponding coherent temperatures (T DCP) and times (t DCP) as well as liquid fraction at DCP with different Zn contents. Based on this approach, DCP occurred at the first detected maximum temperature difference between the centre and the wall thermocouples (ΔT = T W − T C) during the formation of α-Mg. Figure 7 also demonstrates a drop in ΔT, which indicates dendrite coherency point in all Mg–xZn specimens as a result of the enhanced thermal contact through the solid dendrite network.
It is shown that DCP occurred at 642·2 °C in 94·5 s and 640·5 °C in 94 s for Mg–0·5Zn and Mg–1·5Zn alloys, respectively. With addition of 3 wt% Zn, however, T DCP decreased to 632 °C and t DCP slightly increased to 97·1 s for Mg–3Zn alloy. T DCP and t DCP for Mg–6Zn were measured at 630 °C and 46·9 s. These characteristic parameters decreased to 600 °C and 70·2 s, respectively with the further addition of Zn up to 9 wt%. The results clearly show that t DCP did not change remarkably for 0·5–3 wt%, while it decreased for 6–9 wt% representing the role of Zn in refining of Mg grain size which confirms the results of microstructure observation.
Mg–Zn binary phase diagram shows that slope of liquidus is around 6·04 °C/wt indicating the ability of Zn to hinder the growth of α-Mg dendrite size. Therefore, the grain refinement induced by addition of Zn can be explained by the growth restriction theory of solute in the Mg melt (Lee et al 2000). Additionally, the solidification temperature range (ΔT = T s − T f) increases to 88% and total solidification time rises to 157%, when Zn content is increased from 0·5 up to 9 wt% Zn.
A base line was plotted for computing solid fraction at DCP for Mg–xZn alloys. The base line has been estimated by curve fitting; the data between the onset and end of solidification in the first derivative curve, using a cubic square function as per the following equation:
It should be noted that liquid fraction can be measured according to the following equation:
Based on (3), f L is found to be 43% for 0·5 wt% Zn content and this value increased to 49 and 58% for 1·5 and 3 wt% Zn additions, respectively. When Zn is increased to 9 wt%, f L increased to 74%. This indicates that the residual liquid volume fraction increased at DCP with increasing Zn addition due to the growth restriction effect of Zn on the growth of Mg dendrites.
3.3 Immersion test
Increase in pH values causes haemolysis of about 66% (Bakhsheshi-Rad et al 2012), which is not suitable for proliferation and cell growth. Thus, it is important that the pH values of samples immersed in SBF solution should be monitored throughout the immersion test. The pH variations of SBF with different Zn additions vs immersion time are shown in figure 8. It is evident from the graph that pH values of the samples during the immersion test initially increased from 7·66 to 8·70, which is likely caused by the release of OH ions into the solution. However, with increased immersion time, the pH value of all specimens exhibited a constant trend due to the formation of Mg(OH)2 and precipitation of calcium phosphate on Mg(OH)2 and the surface of Mg–Zn alloys as shown in figure 9.
Figure 9 shows corrosion morphologies of Mg–xZn specimens after immersion in Kokubo solution for 144 h using SEM. Figure 9(a) reveals that cracks with uniform distribution formed on Mg–0·5Zn and the formation of cracks could be attributed to dehydration of the layer after drying in an ambient atmosphere. EDS analysis in figure 10 shows that the precipitates are composed of little amounts of Ca and P and a high amount of Mg (point A), whereas the crack layer consisted mainly of high amounts of Ca and P and a little amount of Mg (point B).
It is also clear from figure 9(e) that homogeneous corrosion product precipitates on the surface of Mg–3Zn alloy. Generally, when exposed to SBF solution, Mg alloys are reactive, generating Mg2 + rich interface at which these extra Mg ions between the sample and solution serve to prevent nucleation of calcium phosphate deposition (Song et al 2010). The formation of corrosion product follows the below reactions:
Mg transforms to stable Mg2 + ion; subsequently the cathodic reaction occurs at the same time by formation of hydroxide ion according to reactions (4) and (5).
Mg ion reacts with hydroxide ion and forms magnesium hydroxide. By evolution of Mg(OH)2 as a barrier layer, the degradation rate of the sample declines and corrosion products such as CO\(_{3}^{2-}\), PO\(_{4}^{3-}\) and Cl − ions are precipitated on the surface of the specimen. Based on (7), chlorine reacts with the deposited Mg(OH)2 on the specimens and forms MgCl2 with higher solubility compared to Mg(OH)2. Cl − ions can decrease the corrosion resistance of the specimens. Moreover, Cl − ion can easily penetrate the deposition layer due to its small radius which causes preferential adsorption and OH − replacement by Cl − (Wang et al 2010). As the reaction progresses, high amounts of Mg2 + are dissolved and as a result more HCO3 and HPO4 are consumed causing a decrease in the corrosion rate.
From figure 9(g and j), it can be seen that the thickness of the calcium phosphate film gradually increases with increasing the amount of Zn to 6 and 9 wt%. For Mg–6Zn alloy, corrosion attack takes place in the eutectic (Mg+Mg51Zn20) which relates to galvanic corrosion between the cathodic α-Mg matrix and the anodic eutectic. The corrosion attack can be developed firstly within the eutectic and then occurs in α-Mg grain (Amira et al 2008). Therefore, it can be assumed that a preferential dissolution may occur resulting in increased corrosion attack of Mg51Zn20 phase.
Mg–9Zn suffers from heavy corrosion by formation of heterogeneous laminar corrosion products, while the inner layer is composed of Mg(OH)2 phase. EDS analysis confirmed that the product of the inner layer contains O, C, Mg, Cl, Ca and Zn. However, the outer layer includes calcium phosphate with irregular morphology which precipitated loosely. The corresponding EDS analysis also revealed that the amount of calcium phosphate in the outer layer is significantly higher than that of the inner layer. This result is in agreement with previous finding by Song et al (2010).
The degradation rates (in units of mm year − 1) were obtained according to ASTM-G31-72. The corrosion rate is given by (8):
where the constant K = 8·76 × 104, W is weight loss (g), A is the sample area exposed to solution (cm2), T is the exposure time (h) and D is the density of the material (g/cm − 3). The weight loss corrosion rate of Mg–3Zn alloy was calculated and found to be 1·84 mm year − 1 for Mg–3Zn alloy compared to 5·19 and 2·91 mm year − 1 for Mg–0·5Zn and Mg–1·5Zn alloys, respectively. The results also showed that increasing Zn content in Mg alloy to 6 and 9 wt% increased the corrosion rates to 7·23 and 11·78 mm year − 1, respectively. These results are in good agreement with the results of Kirkland et al (2010).
3.4 Potentiodynamic polarization test
Potentiodynamic polarization test in Kokubo’s solution was performed to assess the corrosion behaviour of Mg–xZn alloy as shown in figure 11. From the graph, it can be seen that the hydrogen evolution (cathodic polarization) in Mg–0·5Zn is higher than the other Mg alloys containing higher Zn, which indicates that the corrosion potential (E corr) is more negative (active) in 0·5 wt% Zn. The kinetic of cathodic reaction in Mg–0·5Zn is noticeably faster compared with other alloys with a percentage of Zn content. By addition of 3 wt% Zn to the alloy, the cathodic and anodic reactions were kinetically decreased compared to other concentrations of Zn. As shown in table 1, E corr of Mg–0·5Zn is more negative than 9, 6, 3 and 1·5 wt% Zn alloy. However, the Mg–3Zn alloy showed better corrosion properties, which can be attributed to the formation of a barrier film in this alloy which is more protective.
The corrosion current density decreased from 144 μA/cm2 in 0·5 wt% Zn to 103 μA/cm2 in 3 wt% Zn, whereas this value for 6 and 9 wt% Zn increased to 122 and 147 μA/cm2, respectively. This indicates higher corrosion resistance of Mg–3Zn. The presence of a higher amount of MgZn phases as confirmed by results of thermal analysis in Mg–3Zn suggests the enhanced corrosion resistance of the alloy, while formation of Mg51Zn20 phase in Mg–6Zn and Mg–9Zn specimens can decrease the corrosion resistance. Zhang et al (2009) reported that the addition of more Zn content results in an increase in the corrosion current density, I corr and reduced corrosion resistance.
The linear polarization resistance, R p, can be calculated using (9) (Liu et al 2010; Argade et al 2012), where β a is the anodic and β c is the cathodic Tafel slopes. The calculated results (table 1) showed that additions of Zn from 0·5 to 3 wt% into Mg–xZn alloy increased the R p value from 0·84 to 1·17 kΩcm2. However, further additions of Zn up to 9 wt% decreased the R p value to 0·82 kΩcm2. It is obvious that the corrosion behaviour of the alloy improved as R p values increased with the addition of Zn up to 3 wt%:
As a result, it can be suggested that in the galvanic couple of Mg–3Zn, the α-Mg phase is assumed as the anode and MgZn phase is the cathode. However, in Mg–6Zn and Mg– 9Zn, the Mg51Zn20 phase acts as the anode and α-Mg phase plays the cathode role. Therefore, the corrosion rate in Mg– xZn binary alloys follows this order: Mg–9Zn > Mg–0·5Zn > Mg–6Zn > Mg–1·5Zn > Mg–3Zn.
4 Conclusions
Mg alloys with various Zn content ranging from 0·5 to 9 wt% in as-cast condition were investigated in terms of microstructure, thermal analysis and corrosion behaviour. Microstructure analysis shows an evenly distributed MgZn phase for alloys with Zn content of 3 wt% or less. Another intermetallic phase Mg51Zn20 was identified with further additions of Zn up to 9 wt%. Corresponding reactions for these phases were detected via cooling curve thermal analysis. Liquid fraction (\(f_{\rm L}^{\rm DCP})\) at the coherency point increased with increasing Zn content leading to an increase in volume fraction of the secondary phases at the end of the solidification process. T DCP decreased with increasing Zn content for all the samples. Moreover, t DCP did not change remarkably for 0·5–3 wt%, while it decreased from 6 to 9 wt%. The Mg–3Zn alloy exhibited the lowest degradation rate and the highest corrosion resistance compared to other alloys, hence, it is the most suitable candidate for biomedical applications.
References
Alvarez-Lopez M L, Del-Valle M D, Fernandez-Lorenzo J A, Garcia-Alonso M, Ruano M C and Escudero O A 2010 Acta Biomater. 6 1763
Amira S, Dubé D, Tremblay R and Ghali E 2008 Mater. Charact. 59 1508
Argade G R, Kandasamy K, Panigrahi S K and Mishra R S 2012 Corros. Sci. 58 321
Bäckerud L, Chai G and Tamminen J 1990 Solidification characteristics of aluminum alloys, Vol 2: Foundry alloys, Des Plaines, Illinois, AFS/Skanaluminium
Bakhsheshi-Rad H R, Idris M H, Kadir M R A and Farahany S 2012 Mater. Des. 33 88
Di-Mario C, Griffiths H, Goktekin O, Peeters N and Verbist B M J 2004 J. Interv. Cardiol. 17 391
El-Rahman S S A 2003 Pharmacol. Research 47 189
Erbel R et al 2007 Lancet 369 1869
Feyerabend F, Witte F and Kammal M 2006 Tissue Eng. 12 3545
Haferkamp H et al 2003 Magnesium alloys and technology (ed.) K U Kainer (Weinheim: WILEY-VCH Verlag GmbH & Co. KG aA) 226
Harandi S E, Idris M H and Jafari H 2011 Mater. Des. 32 2596
Heublein B, Rohde R, Kaese V, Niemeyer M, Hartung W and Haverich A 2003 Heart 89 651
Kaya R A et al 2007 J. Neurosurg. Spine 6 141
Kirkland N T, Lespagnol J, Birbilis N and Staiger M P 2010 Corros. Sci. 52 287
Ku C H, Pioletti D P, Browne M and Gregson P J 2002 Biomaterials 23 1447
Lee Y C, Dahle A K and St John D H 2000 Metall. Mater. Trans. A31 2695
Lévesque J, Dubé D, Fiset M and Mantovani D 2003 Mater. Sci. Forum 426–432 521
Liu M, Schmutz P, Uggowitzer P J, Song G and Atrens A 2010 Corros. Sci. 52 3687
Okamoto H 1994 Phase Equilibria Diffusion 15 129
Ren Y, Huang J, Yang K, Zhang B, Yao Z and Wang H 2005 Acta Metallurgica Sinica 41 1228
Rosalbino F, De Negri S, Saccone A, Angelini E and Delfino S 2010 J. Mater. Sci.: Mater. Med. 21 1091
Somekawa H, Singh A and Mukai T 2009 Scr. Mater. 60 411
Song G 2007 Corros. Sci. 46 1696
Song G and Song S 2006 Adv. Eng. Mater. 9 298
Song Y, Zhang S, Li J, Zhao C and Zhang X 2010 Acta Biomater. 6 1736
Stulikova I and Smola B 2010 Mater. Charact. 61 952
Waksman R et al 2009 J. Am. Coll. Cardiol. Intv. 2 312
Waksman R, Pakala R, Hellinga D, Baffour R, Kuchulakanti P and Seabron R 2005 Eur. Heart. J. 26 417
Waksman R, Pakala R, Kuchulakanti P K, Baffour R, Hellinga D and Seabron R 2006 Catheter Cardiovasc. Interv. 68 607
Wang J, Wang L, Guan S, Zhu S, Ren C and Hou S 2010 J. Mater. Sci.: Mater. Med. 21 2001
Wen C E, Yamada Y, Shimojima K, Chino Y, Hosokawa H and Mabuchi M 2004 Mater. Lett. 58 357
Witte F, Vogt H N, Cohen C, Kainer S, Willumeit K U and Feyerabend R 2008 Curr. Opin. Solid State Mater. Sci. 12 63
Witte F, Kaese V, Haferkamp H et al 2005 Biomaterials 26 3557
Witte F et al 2006 Mater. Werkstoff. 37 504
Witte F, Ulrich H, Palm C and Willbold E 2007 J. Biomed. Mater. Res. A81 757
Xu L P, Yu G, Zhang E, Pan F and Yang K 2007 J. Biomed. Mater. Res. A83 0711
Yang W, Zhang P, Liu J and Xue Y 2006 J. Rare Earth 24 369
Yin D S, Zhang E and Zeng S 2008 Trans. Nonferrous Met. Soc. China 18 763
Yu S, Zhang B, Wang Y, Geng L and Jiao X 2012 Mater. Des. 34 58
Yumiko N, Yukari T, Yasuhide T, Tadashi S and Yoshio I 1997 Fundam. Appl. Toxicol. 37 106
Zhang C, Zeng R, Chen R, Liu C and Gao J 2010 Frontiers Mater. Sci. China 4 116
Zhang E, Yin D, Xu L, Yang L and Yang K 2009 Mater. Sci. Eng. C29 987
Zhang S et al 2010 Acta Biomater. 6 626
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
LOTFABADI, A.F., IDRIS, M.H., OURDJINI, A. et al. Thermal characteristics and corrosion behaviour of Mg–xZn alloys for biomedical applications. Bull Mater Sci 36, 1103–1113 (2013). https://doi.org/10.1007/s12034-013-0566-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-013-0566-9