Abstract
Pre-existing immune response against adenovirus could diminish transgene expression efficiency when Ad is employed in humans as gene therapy vector. We previously used Ad-hΔuPA (Recombinant adenovirus expressing human urokinase-type plasminogen activator) as antifibrotic gene therapy in cirrhosis models and demonstrated its effectiveness. As a further clinical approach, transient Cyclosporine A (CsA) immunosuppression was induced in cirrhotic animals to determine whether Ad-hΔuPA administration retained efficacy. Adenovirus sensitization was achieved by systemic administration of non-therapeutic Ad-βGal (Recombinant adenovirus expressing beta-galactosidase) after 4 weeks of intraperitoneal carbon tetrachloride (CCl4) regimen. Cirrhosis induction continued up to 8 weeks. At the end of CCl4 intoxication, immunosuppression was achieved with three CsA doses (40 mg/kg) as follows: 24 h before administration of Ad-hΔuPA, at the moment of Ad-hΔuPA injection and finally, 24 h after Ad-hΔuPA inoculation. At 2 and 72 h after Ad-hΔuPA injection, animals were sacrificed. Liver, spleen, lung, kidney, heart, brain, and testis were analyzed for Ad-biodistribution and transgene expression. In naïve animals, Ad-hΔuPA genomes prevailed in liver and spleen, while Ad-sensitized rats showed Ad genomes also in their kidney and heart. Cirrhosis and Ad preimmunization status notably diminished transgene liver expression compared to healthy livers. CsA immunosuppression in cirrhotic animals has no effect on Ad-hΔuPA biodistribution, but increments survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adenovirus (Ad) infections are common in humans and as much as 80–90% of the population has developed an adenoviral preimmunization status [1]. Gene therapy using adenovirus in patients is limited due to pre-existing cellular and humoral immune responses [2] that reduce expression efficiency and re-administration options [3]. In order to circumvent these inconveniences, immunosuppression with drugs has been tested to reduce pre-existing immune response [1, 4,5,6,7]. In several animal models, immunosuppressive drugs were shown to increase gene expression after adenovirus transgene delivery using different administration routes, doses, and numerous transgenes [8,9,10,11,12,13]. However, these studies had only been made in non-cirrhotic animals, considering that adenoviruses are naturally hepatotropic when administered systemically [14]. Biodistribution and measurements of transgene expression in cirrhotic livers of Ad-sensitized animals subjected to drug immunosuppression are needed to refine and optimize gene therapy strategies. In addition, our previous data demonstrated that gene therapy using a single injection of Ad-hΔuPA (Recombinant adenovirus expressing human urokinase-type plasminogen activator) in cirrhotic rats resulted in fibrosis improvement, rendering improved liver function [15, 16]. Therefore, we evaluated biodistribution and expression of Ad-hΔuPA in cirrhotic animals bearing a preimmunization status induced by Ad-βGal (Recombinant adenovirus expressing beta-galactosidase) exposition and evaluated whether transient immunosuppression near Ad-hΔuPA administration is able to enhance efficacy in gene transfer. Additionally, we also looked for therapeutic cognate protein expression, improvement in inflammation grading, and adenovector biodistribution alteration.
Materials and Methods
Animal Model and Adenovector Administration
The experimental model of cirrhosis consists of chronic I.P. administrations of carbon tetrachloride (CCl4) (Sigma Aldrich; St. Louis, Mo) mixed with mineral oil (Sigma Aldrich; St. Louis, MO) 3 times/week during 8 weeks [17]. Healthy rats were pair-fed to be employed as controls. Since the rat is not a natural host for adenovirus, cirrhotic and healthy animals (n = 5/group) were sensitized to adenovirus with a systemic administration of Ad-βGal (3 × 1011 vp/rat) at the 4th week of CCl4 intoxication protocol. Ad-hΔuPA was administered to Ad-sensitized and Ad-naïve rats and at the end of the eighth week CCl4 intoxication was done through the iliac vein (3 × 1011 vp/rat) using anesthesia (80 μL ZOLETIL 50 via ip). Ad-βGal and Ad-hΔuPA are both serotype 5. Wound was closed using 3.0 silk suture and local sepsis was applied. Figure 1a illustrates the intoxication regime with CCl4 and adenovirus administration. In order to analyze the Ad-hΔuPA biodistribution after two hours of administration, a subgroup of healthy and cirrhotic animals —-naïve to adenovectors—- was employed. The Ad-hΔuPA batch production was monitored for any endotoxin and/or mycoplasm contaminants and viral particles were tittered as previously described [18]. Animals received care according to the Mexican Official Norm NOM-062-ZOO-1999 and guidelines of the CUCS, Universidad de Guadalajara Animal Facility. The health of the animals was monitored daily in the morning for the survival study and 3 times per week for the rest of the studies. Animals were euthanized when identified in pain during the CCl4 intoxication, when they had ear infection, showed slow or not movement, had brittle hair or eye dehydration. The protocol was approved by the Research and Ethical Committee of the CUCS, Universidad de Guadalajara (Approval Number C.I. 67-2012) which reviewed and approved the mortality aspects of the protocol.
Experimental design and immunosuppression validation. a Scheme shows experimental design for CCl4 intoxication regimen for 8 weeks and Cyclosporine A administration. b Immunosuppression using 40 mg/kg of CsA was validated using lymphocyte proliferation assay. Statistically significant diminution in mitogen induction lymphocyte proliferation was achieved in group treated with PHA compared to control group administrated with vehicle only (p < 0.001). CsA cyclosporine A, CCl4 carbon tetrachloride, Ad-hΔuPA adenovirus with human-truncated uPA, PHA phytohemagglutinin
Validation of Immunosuppression Status
Animals subjected to transient immunosuppression were intragastrically administrated with 40 mg/kg of CsA at 24 h before, simultaneously with Ad-hΔuPA administration, and at 24 h after adenovector administration. Control groups (non-immunosuppressed) were administrated only with the vehicle.
A lymphocyte proliferation assay was performed to validate immunosuppression [19]. Briefly, animal sacrifices were carried out under sterile conditions and spleen disgregation was performed. Two hundred thousand lymphocytes per well were cultured in 10% FBS DMEM (Invitrogen, Carlsbad, CA). Mitogen agent phytohemagglutinin (PHA) was used to stimulate spleen lymphocyte proliferation, measured by the incorporation of 3H-Thymidine. Cells were incubated 48 h after adding PHA (4 μg/mL) and then 1 μCi of 3H-thymidine was added and allowed to remain another 24 h. After washing the excess of tritium, radioactivity was measured with a BeckmanCoulter LS 6500 Liquid Scintillation Counter.
Animal Sacrifice and Sample Collection
Animal sacrifice was performed using an excess of anesthesia (150 μL of ZOLETIL 50 via ip) 2 and 72 h after Ad-hΔuPA administration. Blood was collected in anticoagulant-free tubes, serum was separated, and put to freeze (− 70 °C). Sections from all liver lobules, kidney, spleen, heart, lung, testis, and brain tissue were collected and kept frozen (− 70 °C) until further analysis to monitor Ad-hΔuPA biodistribution. Liver tissue was fixed in 4% paraformaldehyde and embedded in paraffin to evaluate fibrosis.
Fibrosis and Inflammation Assessment
Five-µm-thick paraffin-embedded liver section was stained with Masson’s trichromic, Sirius Red, and hematoxylin–eosin to determine the percentage of fibrosis, collagen, and inflammation, respectively. Computer-assisted automated image analyzer (Image Pro-Plus 6.3) was used to determine the percentage of fibrosis in liver tissue (20 microphotograph fields at random per slide). Fibrosis percentage was calculated according to the stained connective tissue in relation to the total tissue area. Two independent board-certified pathologists blinded to the study performed analysis of inflammatory cell infiltration and necroinflammation (Knodell) [20]. In addition, IL-6 and TNF-α mRNA levels were measured in liver homogenates by using qRT-PCR. Liver RNA was isolated with Trizol reagent (Invitrogen, Carlsbad, CA). Retrotranscription was prepared using 2 µg of total RNA and 200U of Superscrip III reverse transcriptase (Invitrogen, Carlsbad, CA). Two µL of cDNA were subjected to amplification in a Rotor Gene 3000 Thermocycler (Corbett Research, Cambridgeshire, UK) using manufacturer-recommended conditions. Gene amplification was normalized against GAPDH expression. Analysis was made using the 2−ΔCT method.
Functional Hepatic Test
ALT and AST serum levels were assessed in an automated Sincron-Cx7 analyzer. Results are reported as mean ± standard deviation (SD).
Adenovirus Biodistribution
Total DNA was extracted from liver, kidney, spleen, heart, lung, testis, and brain using proteinase K digestion followed by phenol extraction. Ad genomes were detected by RT-PCR, using 1 µg of total DNA along with specific TaqMan primers/probe for the Ad5-3´ITR/Psi signal region. A standard curve from 102 to 107 vp was used to interpolate sample values. Results are expressed as adenoviral vp/µg of total DNA.
Transgen Expression
A buffer (Tris–HCl 0.05 M, NaCl 0.15 M, HEPES 0.01 M, CaCl2 2 mM, Tween-80 0.01%, PMSF 1 mM pH 8.5, and complete protease inhibitors cocktail (Roche Diagnostics GmbH Mannheim, Germany)) along with 150 mg of liver, kidney, spleen, heart, lung, testis, or brain was used for extracting cellular proteins. Concentration of human protein uPA was measured by ELISA (Oncogene Science, Cambridge, MA).
Survival Rate in Transient Immunosuppressed Rats
Survival rate was monitored in 26 Ad-sensitized cirrhotic animals. Rats were systemically administrated doses of 3 × 1011 vp/rat of Ad-βGal at week 4 of CCl4 intoxication regimen. At week 8, 13 animals were treated with 40 mg/kg CsA 24 hours before, concomitantly, and 24 h after the Ad-hΔuPA dosage. Control animals received vehicle only. All animals continued with CCl4 intoxication 3 times a week until death.
Statistics
The statistic analysis was made using the Mann–Whitney t-test or one-way ANOVA with Bonferroni as a post hoc test. The data are expressed as mean ± SE or mean ± SD. Survival was analyzed using Kaplan–Meier.
Results
Cyclosporine A Induces a Transient Immunosuppression in Cirrhotic Animals
Transient immunosuppression was achieved with 40 mg/kg of CsA (Medifarma; Lima, Peru) administrated 24 h before, at the time of Ad-hΔuPA administration, and 24 h later (Fig. 1a). Figure 1b shows a significant decrease in mitogen-induced lymphocyte proliferation in animals administered with CsA and compared to controls when stimulated with phytohemagglutinin (PHA) (Sigma Aldrich; St. Louis, MO) (p < 0.001), which demonstrated an immunosuppressive status.
Ad-hΔuPA Induces Fibrosis Reversion in Ad-Sensitized and Ad-Naïve Cirrhotic Rats
Liver tissue was stained with trichromic staining. Cirrhotic nodules surrounded by thick fibrotic bands forming septums are seen in cirrhotic controls (Fig. 2a). Ad-sensitization with Ad-βGal did not affect the fibrosis occurrence significantly since the groups have similar fibrotic area values (10.9 ± 5.4 in Ad-naïve vs. 13.7 ± 5.5 in Ad-sensitized group). Treatment with Ad-hΔuPA significantly reduces fibrosis in all cirrhotic groups leading to 57% less (p < 0.001) fibrotic area in Ad-naïve cirrhotic animals, 46% less (p < 0.01) in the Ad-sensitized cirrhotic group, and 35% less (p < 0.05) in the Ad-sensitized immunosuppressed cirrhotic group compared to the untreated cirrhotic group (Fig. 2b). Then, fibrosis reversion was statistically noticeable in just 72 h-expression of the cognate therapeutic protein. Functionality of hΔuPA as therapeutic gene for fibrosis does not seem to be affected by the Ad-sensitization status or transient immunosuppression since all treated groups had similar fibrotic area values with no statistical significance between them (Fig. 2b). In Ad-hΔuPA-treated animals, thin fibrous bands extending from portal areas were observed as well as lower tissue deformation and mild central vein sclerosis compared to the untreated controls (Fig. 2a). It was also noticeable that adenovirus administration produces a negligible hepatic injury, even though two Ad doses were administrated, as observed in healthy animals. Sirius red staining showed a clear reduction in collagen observed in Ad-hΔuPA-treated groups, which is reflected in thinner fiber bands compared to untreated animals (Fig. 2c). Ad-hΔuPA transduction reduces collagen-staining area significantly in all cirrhotic groups (p < 0.05). The control group showed 9% of stained area, while treated Ad-naïve animals only presented 7.8%, Ad-sensitized group 5%, and Ad-sensitized immunosuppressed animals 6.4% (p < 0.05). The collagen staining values in healthy or cirrhotic animals subjected to Ad-sensitization did not reflect any difference compared to their Ad-naïve counterparts, which indicates that there is no harmful effect attributable to pre-immunization status (Fig. 2d).
Liver fibrosis is attenuated while CsA administration decreases inflammation in Ad-sensitized cirrhotic animals treated with Ad-hΔuPA. a Representative liver sections stained with Masson’s trichrome (× 40) showed in histological analysis blue-stained areas for ECM components and reticular fibers. Parenquima is stained in pink. Non-treated cirrhotic animals presented characteristic cirrhotic nodules surrounded by thick fibrotic bands stained in blue. Ad-hΔuPA groups showed reduced thickness in fibrotic septums and uncompleted regenerative nodules compared to non-treated cirrhotic controls. b Quantitative analysis performed with Image ProPlus software in at least 20 histological fields demonstrated a significant 22%, 50%, and 75% reduction (***p < 0.001) of fibrotic area after Ad-hΔuPA administration in ad-sensitized immunosuppressed, ad-sensitized, and ad-naïve cirrhotic groups, respectively, compared to cirrhotic control group. Tissue of healthy and healthy Ad-sensitized animals showed no important damage. Values were determined 72 h post-treatment and are presented as the mean ± SD. c Photographs of collagen staining by Sirius red in histological samples of animal groups (× 40). Healthy and healthy Ad-sensitized tissue showed no collagen staining. Cirrhotic untreated group presents thick collagen bands stained in red. Liver from cirrhotic animals treated with Ad-hΔuPA are shown in the second and third line where evidence is found for thinner collagen bands extending from portal areas, as well as lower tissue deformation compared to untreated controls. Also, there is an evident minor collagen staining even in Ad-sensitized cirrhotic animals compared to untreated cirrhotic controls. Besides, CsA administration in CCl4-cirrhotic groups seems to improve collagen diminution. d There is statistical significance in collagen reduction after hΔuPA treatment in immunosuppressed cirrhotic-CCl4 animals (*p < 0.05) Ad-sensitized or not, compared to untreated CCl4-cirrhotic animals. Collagen reduction was 55% in Ad-sensitized cirrhotic animals and 72% in Ad-sensitized immunosuppressed cirrhotic animals. Values were determined 72 h post-treatment and are presented as the media ± SD. e Inflammation and tissue damage were evaluated in hematoxylin–eosin-stained slides by two different pathologists in at least 10 fields (× 40). Adenoviral–preimmunizated animals presented increase in hepatic tissue injury and notable inflammatory cells infiltrate, while naïve groups showed tissue distortion, though a lower inflammation process. Transient immunosuppression with CsA in Ad-sensitized animals reduces inflammation compared to its non-immunosuppressed control group. Ad-pre-immunized healthy rats did not present significant inflammation or tissue distortion compared to Ad-naïve healthy animals. f Histological samples stained with Hematoxylin–Eosin were evaluated using Knodell grading. A significative reduction (3 point media) in inflammation Knodell score was observed (p < 0.05) in immunosuppressed Ad-sensitized animals comparing with non-immunosuppressed Ad-sensitized cirrhotic rats. Ad-sensitized cirrhotic groups present an increase in Knodell score compared to Ad-naïve animals (***p < 0.001), but healthy group had no difference in inflammation compared to healthy Ad-sensitized group. CsA immunosuppression in Ad-sensitized cirrhotic animals reduces inflammation Knodell grading compared to Ad-sensitized (**p < 0.01) and cirrhotic untreated (*p < 0.05) controls. Knodell grading was determined by 2 pathologists blinded to the study and data are presented as the media ± SD
Cyclosporine A Administration Decreases Inflammation in Ad-Sensitized Cirrhotic Animals
In Fig. 2c, untreated cirrhotic animals showed characteristics of liver damage as well as inflammation and hepatocyte-necrotic bodies. Adenovector administration by itself in healthy animals did not demonstrate a harmful effect in the liver due to lack of cellular alterations in naïve or Ad-sensitized rats. On the other hand, a severe inflammatory reaction and abundant inflammatory cell infiltration was developed in Ad-sensitized cirrhotic rats after Ad-hΔuPA administration. Moreover, hepatic cells seemed distorted, disintegrated, and to some extent lost their tissue structure when compared to the cirrhotic Ad-naïve animals. Transient administration of CsA in Ad-sensitized cirrhotic rats notably diminished cellular inflammatory infiltrate compared to the non-CsA-administrated group (Fig. 2e). The use of Knodell grading (Hematoxylin–Eosin stain) showed a meaningful reduction (3-point average) in the inflammation, Knodell score (p < 0.01), when the immunosuppressed Ad-sensitized cirrhotic animals were compared with non-immunosuppressed Ad-sensitized cirrhotic rats (Fig. 2f).
In addition, Ad-sensitized cirrhotic groups presented an increase in the Knodell score compared to their Ad-naïve control group (p < 0.001).
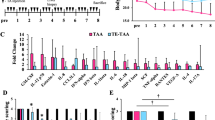
In CsA-administrated groups (Fig. 3a, b), liver homogenates contained a significant reduction in TNF-α and IL-6 mRNA levels, indicating a reduction of inflammation in naïve and Ad-sensitized animals. TNF-α and IL-6 cytokines are over expressed in cirrhotic livers compared to non-cirrhotic livers. Moreover, α-SMA mRNA levels (Fig. 3c) indicate that this profibrotic marker decreased in Ad-naïve cirrhotic immunosuppressed animals with CsA. Since fibrosis and collagen levels did not change in the CsA groups, this data suggest that the reduction in inflammation could be due to the administration of CsA and could diminish liver pro-inflammatory microenvironment known to induce HSC activation, which alters α-SMA expression.
Proinflammatory cytokines profile and α-SMA gene expression. a and b demonstrate a significant diminution in TNF-α and IL-6 mRNA levels in liver homogenate of CsA-administrated groups indicating a significant reduction in inflammation in naïve and ad-sensitized animals (*p < 0.05). Both pro-inflammatory cytokines were overexpressed in cirrhotic livers compared to non-cirrhotic livers. c α-SMA mRNA levels indicate that this profibrotic marker decreased in cirrhotic Ad-naïve animals immunosuppressed with CsA. (*p < 0.05). CsA Cyclosporine A, Ad-hΔuPA Adenovirus with human-truncated uPA, GAPDH Glyceraldehyde 3-phosphate dehydrogenase, TNF-α Tumor necrosis factor alpha, IL6 Interleukin 6, α-SMA Alpha-smooth muscle Actin
Adenovirus Biodistribution is Different in Cirrhotic and Healthy Animals
To evaluate the possible effect of cirrhosis-related vascular damage in adenovector biodistribution, we analyzed the liver, spleen, lung, kidney, heart, brain, and testis of healthy and CCl4-cirrhotic rats for Ad-hΔuPA genomes 2 h after systemic administration (3 × 1011 vp/rat). The biodistribution is different in cirrhotic animals from that of healthy rats (Fig. 4a). The number of Ad-hΔuPA in healthy animals is greater in the spleen > liver > lung; while in cirrhotic rats, decreasing numbers were shown in the liver > spleen > lung, respectively. Cirrhotic rat livers had twofold more genomes than undamaged livers, the same pattern as in lung tissue. In contrast, the spleen of healthy animals had threefold more adenovector genomes versus the cirrhotic-derived animals (p < 0.05). In the rest of the tissues, a negligible quantity of adenovirus genomes was present.
Effect of cirrhosis, Ad-sensitization, and CsA transient inmunosuppression in Ad-biodistribution and transgene expression. A dose of 3 × 1011 Ad-hΔuPA vp/rat was injected via iliac vein and adenovirus genomes were searched by PCR. a Adenovector genomes were examined in main organs two hours after Ad-hΔuPA administration in healthy and cirrhotic Ad-naïve animals. In healthy group, adenovirus were detected in spleen, liver, and lung in decreasing quantities. In CCl4-cirrhotic animals, viral particles detected in liver were the most, followed by those detected in spleen and lung. Spleen showed a significant difference in Ad genome quantity in healthy animals compared to cirrhotic rats (*p < 0.05). b 72 h after Ad-hΔuPA injection a large amount of adenovirus genomes was detected in Ad-naïve groups mainly in spleen and liver. Ad preimmunization status expands the quantity of organs positive for Ad genome detection in cirrhotic and healthy animals. Transient immunosuppression with CsA 24 h around Ad administration in non-sensitized and ad-sensitized animals did not affect Ad-biodistribution pattern or levels of genomes detected. c Clearly, cirrhotic animals express lower quantities of huPA than healthy rats and huPA was exclusively detected in liver, spleen, and kidney in all groups. Ad-naïve groups showed major levels of huPA than their Ad-sensitized counterparts with predominant detection of the therapeutic protein in liver and spleen. Specially, non-cirrhotic livers reached a peak quantity of 43 ng huPA protein per miligram of total protein (***p < 0.001); since Ad pre-immunized healthy livers did not reach such levels, an evident block of transgene expression is being caused by Ad-sensitization status. This phenomenon was also observed in cirrhotic groups and could not be reversed by CsA transient immunosuppression. CsA Cyclosporine A, Ad-hΔuPA Adenovirus with human-truncated uPA, vp viral particles, μg micrograms, mg milligrams
Influence of Ad-Sensitization in Adenovirus Biodistribution in Cirrhotic Animals
Adenovirus biodistribution was analyzed in cirrhotic Ad-naïve and Ad-sensitized rats after 72 h of Ad-ΔhuPA administration. Figure 4b showed a predominant localization of adenoviral genomes in the spleen and liver of healthy and cirrhotic naïve animals, but the spleen showed significantly higher adenoviral levels compared to liver tissue (p < 0.05). The quantity of vp in the liver of non-cirrhotic and cirrhotic naïve animals was around 5 × 103 vp/μg DNA. In naïve animals, immunosuppression did not influence the adenovector’s biodistribution. Biodistribution showed ten fold reduction in vp at 2 h (2 × 105 to 3 × 105 vp/µg DNA) compared to the quantity detected at 72 h (2 × 104 to 3 × 104 vp/µg DNA). This data agrees with other studies where 90% of virus is internalized by cells within the first 2 h after systemic administration.
Transgene Expression Does Not Correlate with Adenoviral Genome Biodistribution
Seventy-two hours after Ad-hΔuPA administration, levels of human uPA protein were measured in liver, spleen, lung, kidney, heart, brain and testis tissue homogenates. In all groups, therapeutic protein was exclusively detected in liver, spleen and kidney tissue. Ad-naïve animals showed a significantly (p < 0.05) higher hepatic expression than their Ad-sensitized counterparts. Figure 4c indicates that non-Ad-sensitized animals preferentially express therapeutic protein in liver and spleen. Alternatively, transient immunosuppression changes transgene expression pattern in naïve and ad-sensitized animals, making spleen expression predominant over liver expression. Healthy and cirrhotic Ad-sensitized animals expressed quantitatively lower amounts of therapeutic protein than naïve control groups (p < 0.05). In these Ad-sensitized groups, the mean value of human uPA protein in the liver was around 50 pg/mg total proteins independently of CsA administration. On the other hand, livers of naïve animals expressed a media of 43 ng huPA/mg total proteins for the healthy group; a value tenfold higher than cirrhotic tissue where hepatic expression barely reached 4.5 ng uPA/mg total proteins.
Hepatic Enzyme Serum Levels Improve After Ad-hΔuPA Gene Therapy
Ad-sensitized animals with Ad-βGal showed increased enzyme levels compared to Ad-naïve groups. Twofold increase for ALT and threefold for AST are observed in cirrhotic Ad-sensitized groups versus their non-sensitized counterparts, indicating that preexisting immunity had a deleterious effect on liver function after a second adenovirus administration. Figure 5a shows reduced serum levels of ALT in cirrhotic Ad-naïve groups (administrated or not with CsA) after Ad-hΔuPA gene therapy compared to cirrhotic untreated group. Besides, CsA immunosuppression diminished ALT in Ad-naïve animals from 191 ± 91 to 105 ± 97 U/L (p < 0.05), a diminution that reproduces in Ad-sensitized group, even when no statistical difference was achieved. This indicates that transient immunosuppression obliterated the acute liver damage caused by adenoviral administration.
Hepatic enzyme serum levels and survival test in Ad-sensitized cirrhotic animals. a Ad-hΔuPA treatment induces a decline in hepatic enzymes serum levels compared to cirrhotic untreated group, but Ad preimmunization status aggravates liver enzymes levels, effect that was reduced by CsA immunosuppression. In Ad-naïve groups CsA administration significatively (*p < 0.05) diminishes ALT and AST serum levels. Ad-sensitized deleterious effect is not present in healthy rats. b Transient immunosuppression with CsA significantly increases survival (**p < 0.01) in Ad-sensitized cirrhotic animals compared to non-immunosuppressed control group as apparent effect due to inflammation diminution. Both groups continued the 3 time/week CCl4 intoxication until the death of the animals. CsA cyclosporine A, Ad-hΔuPA adenovirus with human-truncated uPA, ALT Alanine aminotransferase, AST aspartate aminotransferase
On the other hand, Fig. 5b shows that the Ad-sensitized cirrhotic group presented a significant increment in AST levels compared to Ad-naïve cirrhotic groups, indicating that liver damage after a second adenovector dosing is increased due to adenoviral sensitization. Transient immunosuppression with CsA successfully decreased AST levels from 352 ± 265 to 228 ± 209 U/L in Ad-naïve rats (p < 0.01) and from 3208 ± 2455 to 913 ± 371 U/L in Ad-sensitized animals.
Transient Immunosuppression in Second Adenoviral Administration Increases Survival in Cirrhotic Ad-sensitized Animals
For this experiment, 9 out of 13 animals (70%) died in the first 3 days after a second adenovirus administration in the non-immunosuppressed group. Meanwhile, in the CsA immunosuppressed group, 4 out of 13 (25%) deaths were detected in the first 3 days. Figure 5c presents the accumulated survival test analyzed by Kaplan–Meier statistics. Transient immunosuppression with CsA significantly increases survival (p < 0.01) in adenovirus-sensitized animals, increasing survival from 22 to 41 days after the second adenovirus administration. This effect could be due in part to different biological effects of CsA besides immunosuppression, since CsA is known to have anti-inflammatory and anti-chemotactic properties and its ability to fight viral, fungal, and parasitic infections.
Discussion
Since the liver is a preferred target organ when adenovirus are administrated systemically [14], this study explores the potential of temporary immunosuppression in a cirrhotic rat model, to allow for repetitive gene transfer. Parameters related to liver inflammation indicate that healthy animals sensitized or not to adenovirus did not show a significant inflammatory reaction caused by Ad administration nor any damage attributable to CsA metabolism. In the cirrhosis-CCl4 model, cell necrosis and strong inflammation are induced in the liver [17], leading to activation of Kupffer cells with an increase of TNF-α and IL-6 levels. However, transient immunosuppressed cirrhotic animals showed a reduced inflammatory score (Knodell) and a decrease in TNFα and IL-6 mRNA levels. CCl4 is known to be converted into CCl3· + Cl− by the cytochrome P450 [21], the same enzyme system that metabolizes CsA. Consequently, these substances concurrently administrated could alter CsA blood levels or CCl4 metabolites; though our data suggest no harmful effect on the group combining CCl4 and CsA. Noticeably, pre-exposition to Adenovirus seems to have different effects on cirrhotic and non-cirrhotic animals, rendering a greater inflammation reaction in the damaged liver when re-exposed to adenovirus. Also, adenovirus sensitization produces a deleterious effect on hepatic enzyme serum levels in cirrhotic animals, but not in healthy animals. These results could be interpreted as a synergism between the hepatic alterations due to cirrhosis and those caused by the pre-existing immune response to adenovirus. This could be attributed to the presence of antibodies against Ad in the Ad-sensitized animals that could have produced antigen–antibody (Ag–Ab) complexes that could exacerbate the already present liver inflammation. At the same time, these antigen-neutralizing antibodies (nAb) could diminish adenovirus hepatic transduction as observed in the Ad-sensitized groups. For adenovirus, nAb are anti-hexon, anti-penton, or anti-fiber, and serotype-specific, and elevated circulating titers can entirely abolish the ability of adenovirus to infect or transfect cells [22, 23]. Cyclosporine A itself is not expected to have effects on neutralizing Ab titers. Another important complication of gene therapy is the formation of inhibitory antibodies directed against the therapeutic protein [24]. Several studies suggest that both genetic and environmental factors impact the risk of mounting an immune response against the recombinant protein. In our case, this possibility could be the explanation for the diminution of protein expression since healthy and cirrhotic-naïve animals expressed quantitatively bigger amounts of the uPA therapeutic protein than Ad-sensitized control groups. On the other hand, the adenoviral dose administrated (3 × 1011 vp/rat) is considered a high dose [25]; and it is well known that high doses administrated systemically induce activation of the complement; this could explain the increase in inflammation and cytotoxicity in Ad-sensitized animals [26].
In addition, memory T cells against Ad could increase the necrotic damage present in cirrhotic Ad-sensitized animals; these cytotoxic cells lysate hepatocytes, releasing hepatic enzymes into circulation. Transient immunosuppression around the second adenovector administration in Ad-sensitized cirrhotic animals diminished the histological necrotic signals, pro-inflammatory cytokines, and hepatic enzyme levels, probably due to the downregulation of the T cytotoxicity activation.
Now, the preexisting immune response decreases transgen expression. In contrast to the expected results, transient immunosuppression with CsA did not increase transgene expression in cirrhotic Ad-sensitized nor Ad-naïve animals. Despite reduction of transgen expression in Ad-sensitized animals, hΔuPA levels achieved seem to be enough for a therapeutic effect causing fibrosis reversion. In our experience, Ad-hΔuPA transduction has been shown to undoubtedly have antifibrotic properties, a fact confirmed in this study. Nonetheless, cirrhotic animals non-sensitized to adenovirus showed the greatest fibrosis reversion (57%). Even the other Ad-hΔuPA-treated cirrhotic groups also showed significant fibrosis reduction compared to the untreated cirrhotic group.
Normal and cirrhotic animals showed a greater quantity of adenovirus genomes at 2 h rather than 72 h after adenovirus administration. This data agree with other reports discussing adenovirus access into the cells within the first 2 h [27, 28]. Using the iliac vein as administration route, at 2 h, values reached 2 × 105 or 3 × 105 genomes/µg DNA in liver and spleen. This distribution could be explained because liver resident macrophages and dentritic cells, together with the spleen compartment (marginal zone) are the cells that clear out most of all circulating adenovirus [29]. In our study, after 72 h of Ad administration liver and organs from the reticulo-endothelial system are the remaining organs positive for adenovirus, probably due to its elevated amount of antigen-presenting cells (APC) that can be directly transduced by recombinant Ad [30, 31]. It seems that just after the reticulo-endothelial system is saturated with high doses of adenovirus, excess adenoviruses remain free with the chance to transduce hepatic cells [32].
Other studies have also detected adenovirus genomes in spleen and lung after systemic injections [32,33,34]; while most transgen expressions have been detected in the liver and spleen [14] and a few times in lung, heart, kidney [32], gonads, bone marrow and brain [35]. Healthy, cirrhotic, and transient immunosuppressed cirrhotic-naïve animals illustrated the same Ad-biodistribution pattern 72 h post-administration, mostly in spleen and liver with small amounts in the lung. Transient immunosuppression did not influence adenovirus biodistribution qualitatively or quantitatively. On the other hand, Ad-sensitization triggered a wider spread of the adenoviral genome’s distribution. In these groups, viral particles in the non-cirrhotic animals predominate in liver and spleen and in cirrhotic rats within the liver, spleen, heart, and lung. Immunosuppressive drug administration did not modify any patterns.
Only few papers have studied vector-sensitized animals [35,36,37] and to our knowledge, this is the first paper focused on cirrhotic Ad-sensitized rats. In other studies, regardless of the administration route, Ad-sensitization significantly diminishes transgene expression. Besides, a wider spread of the Ad-biodistribution caused by the adenovirus sensitization status has been reported in a dose-dependent manner [25].
In addition, we must take into account that immune memory is present 2–4 weeks after adenovirus exposure and it is route and dose dependent [38, 39]; administration by systemic route being the main inducer of immune response. Based on this, it is possible that a re-exposure to the adenovirus may trigger an immune response that produces broader transduction efficiency in immune-related organs compared to the first exposition. At 72 h post-administration, our results showed the expression of the protein exclusively in liver, spleen and kidney in all groups. The liver showed huPA levels of 43 ng/mg of total protein in healthy animals and only 4.5 ng/mg of total protein in cirrhotic animals; similar levels were found in the spleen. However, huPA liver expression in Ad-sensitized animals decreased to 50–150 pg/mg of total protein, which correlates with the lower percentage of fibrosis reversion achieved in this group compared to non Ad-sensitized animals.
Our study concurs with others that showed that at 72 h post adenovector administration, protein predominates in the liver of healthy and cirrhotic rats [14, 32, 34]. It is also noteworthy to mention that in other studies, the location of Ad genome not always correlated with transgene expression. Although in our study some organs were positive for viral particles such as the lung and heart, they did not express hΔuPA protein. Moreover, transient immunosuppression did not modify the quantity of therapeutic protein in the liver, but adenovirus sensitization status did. Remarkably, other studies using immunosuppressive drugs demonstrated an extended transgen expression, but did not mention any increment in protein levels [8, 9, 13, 40]. CsA seems to have some quantitative or qualitative effect on transgene expression though it did increase survival rate in cirrhotic ad-sensitized animals exposed to a second adenovirus administration. This could be due to the fact that CsA decreases IL-2 transcription and T cell activation, leading to less inflammatory reactions against the adenovirus. In addition, it has been reported in rats that 4 days of treatment with CsA increases hepatocyte proliferation [41]; then it may also improve liver function and animal survival [42]. Shen et al. [9] administrated adenovirus and measured the duration of transgene expression after CsA and Sirolimus immunosuppression, achieving a 4-week expression and reducing liver damage. We believe that these studies, along with the one described here, offer a possibility for immunosuppressive drugs to be used in different clinical scenarios using gene therapy involving adenovirus.
References
Yadvinder, S. A., Dinesh, S. B., & Suresh, K. M. (2011). Adenoviral vector immunity: Its implications and circumvention strategie. Current Gene Therapy,11(4), 307–320.
Tomita, K., Sakurai, F., Tachibana, M., & Mizuguchi, H. (2012). Correlation between adenovirus-neutralizing antibody titer and adenovirus vector-mediated transduction efficiency following intratumoral injection. Anticancer Research,32(4), 1145–1152.
Sack, B. K., & Herzog, R. W. (2009). Evading the immune response upon in vivo gene therapy with viral vectors. Current Opinion in Molecular Therapeutics,11(5), 493–503.
Cao, H., Yang, T., Li, X. F., Wu, J., Duan, C., Coates, A. L., et al. (2011). Readministration of helper-dependent adenoviral vectors to mouse airway mediated via transient immunosuppression. Gene Therapy,18, 173–181.
Chirmule, N., Raper, S. E., Burkly, L., Thomas, D., Tazelaar, J., Hughes, J. V., et al. (2000). Readministration of adenovirus vector in nonhuman primate lungs by blockade of CD40-CD40 ligand interactions. Journal of Virology,74, 3345–3352.
Unzu, C., Melero, I., Hervás-Stubbs, S., Sampedro, A., Mancheño, U., Morales-Kastresana, A., et al. (2015). Helper-dependent adenovirus achieve more efficient and persistent liver transgene expression in non-human primates under immunosuppression. Gene Therapy,22, 856–865.
Kay, M. A., Meuse, L., Gown, A. M., Linsley, P., Hollenbaugh, D., Aruffo, A., et al. (1997). Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proceedings of the National Academy of Sciences of the United States of America,94, 4686–4691.
Ilan, Y., Jona, V. K., Sengupta, K., Davidson, A., Horwitz, M. S., Roy-Chowdhury, N., et al. (1997). Transient immunosuppression with FK506 permits long-term expression of therapeutic genes introduced into the liver using recombinant adenoviruses in the rat. Hepatology,26, 949–956.
Shen, W. Y., Lai, M. C., Beilby, J., Barnett, N. L., Liu, J., Constable, I. J., et al. (2001). Combined effect of cyclosporine and sirolimus on improving the longevity of recombinant adenovirus-mediated transgene expression in the retina. Archives of Ophthalmology,119, 1033–1043.
Vilquin, J. T., Guerette, B., Kinoshita, I., Roy, B., Goulet, M., Gravel, C., et al. (1995). FK506 immunosuppression to control the immune reactions triggered by first-generation adenovirus-mediated gene transfer. Human Gene Therapy,6, 1391–1401.
Fontanellas, A., Hervás-Stubbs, S., Mauleón, I., Dubrot, J., Mancheño, U., Collantes, M., et al. (2010). Intensive pharmacological immunosuppression allows for repetitive liver gene transfer with recombinant adenovirus in nonhuman primates. Molecular Therapy,18(4), 754–765.
Fang, B., Eisensmith, R. C., Wang, H., Kay, M. A., Cross, R. E., Landen, C. N., et al. (1995). Gene therapy for hemophilia B: Host immunosuppression prolongs the therapeutic effect of adenovirus-mediated factor IX expression. Human Gene Therapy,6, 1039–1044.
Durham, H. D., Alonso-Vanegas, M. A., Sadikot, A. F., Zhu, L., Lochmuller, H., Massie, B., et al. (1997). The immunosuppressant FK506 prolongs transgene expression in brain following adenovirus-mediated gene transfer. NeuroReport,8, 2111–2115.
Garcia-Banuelos, J., Siller-Lopez, F., Miranda, A., Aguilar, L. K., Aguilar-Cordova, E., & Armendariz-Borunda, J. (2002). Cirrhotic rat livers with extensive fibrosis can be safely transduced with clinical-grade adenoviral vector. Evidence of cirrhosis reversion. Gene Therapy,9, 127–134.
Salgado, S., Garcia, J., Vera, J., Siller, F., Bueno, M., Miranda, A., et al. (2000). Liver cirrhosis is reverted by urokinase-type plasminogen activator gene therapy. Molecular Therapy,2, 545–551.
Miranda-Diaz, A., Rincon, A. R., Salgado, S., Vera-Cruz, J., Galvez, J., Islas, M. C., et al. (2004). Improved effects of viral gene delivery of human uPA plus biliodigestive anastomosis induce recovery from experimental biliary cirrhosis. Molecular Therapy,9, 30–37.
Perez, T. R. (1983). Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology,3, 112–120.
Armendáriz-Borunda, J., Bastidas-Ramírez, B. E., Sandoval-Rodríguez, A., González-Cuevas, J., Gómez-Meda, B., & García-Bañuelos, J. (2011). Production of first generation adenoviral vectors for preclinical protocols: amplification, purification and functional titration. Journal of Bioscience and Bioengineering,112, 415–421.
Sitz, K. V., & Birx, D. L. (1999). Lymphocyte proliferation assay. Methods in Molecular Medicine,17, 343–353.
Ishak, K., Baptista, A., Bianchi, L., Callea, F., De Groote, J., Gudat, F., et al. (1995). Histological grading and staging of chronic hepatitis. Journal of Hepatology,22, 696–699.
Recknagel, R. O., Glende, E. A., Dolak, J. A., & Waller, R. L. (1989). Mechanisms of carbon tetrachloride toxicity. Pharmacology & Therapeutics,43, 139–154.
Tomita, K., Sakurai, F., Iizuka, S., Hemmi, M., Wakabayashi, K., Machitani, M., et al. (2018). Antibodies against adenovirus fiber and penton base proteins inhibit adenovirus vector-mediated transduction in the liver following systemic administration. Science Reports,8, 12315–12327.
Klasse, P. J. (2018). Collusion between neutralizing antibodies and other immune factions in the destruction of adenoviral vectors. Proceedings of the National Academy of Sciences of the United States of America,115, 10201–10203.
Arruda, V. R., Favaro, P., & Finn, J. D. (2009). Strategies to modulate immune responses: A new frontier for gene therapy. Molecular Therapy,17(9), 1492–1503. https://doi.org/10.1038/mt.2009.150.
Harvey, B. G., Maroni, J., O'Donoghue, K. A., Chu, K. W., Muscat, J. C., Pippo, A. L., et al. (2002). Safety of local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of morbid conditions. Human Gene Therapy,13, 15–63.
Liu, Q., & Muruve, D. A. (2003). Molecular basis of the inflammatory response to adenovirus vectors. Gene Therapy,10, 935–940.
Meier, O., & Greber, U. F. (2004). Adenovirus endocytosis. Journal of Gene Medicine,6, S152–163.
Ganesan, L. P., Mohanty, S., Kim, J., Clark, K. R., Robinson, J. M., & Anderson, C. L. (2011). Rapid and efficient clearance of blood-borne virus by liver sinusoidal endothelium. PLoS Pathogens,7(9), e1002281.
Zhang, Y., Chirmule, N., Gao, G. P., Qian, R., Croyle, M., Joshi, B., et al. (2001). Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Molecular Therapy,3, 697–707.
Jooss, K., Yang, Y., Fisher, K. J., & Wilson, J. M. (1998). Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. Journal of Virology,72, 4212–4223.
Schiedner, G., Bloch, W., Hertel, S., Johnston, M., Molojavyi, A., Dries, V., et al. (2003). A hemodynamic response to intravenous adenovirus vector particles is caused by systemic Kupffer cell-mediated activation of endothelial cells. Human Gene Therapy,14, 1631–1641.
Smith, J. S., Tian, J., Muller, J., & Byrnes, A. P. (2004). Unexpected pulmonary uptake of adenovirus vectors in animals with chronic liver disease. Gene Therapy,11, 431–438.
Awasthi, V., Meinken, G., Springer, K., Srivastava, S., & Freimuth, P. (2004). Biodistribution of radioiodinated adenovirus fiber protein knob domain after intravenous injection in mice. Journal of Virology,78, 6431–6438.
Smith, J. S., Tian, J., Lozier, J. N., & Byrnes, A. P. (2004). Severe pulmonary pathology after intravenous administration of vectors in cirrhotic rats. Molecular Therapy,9, 932–941.
Varnavski, A. N., Zhang, Y., Schnell, M., Tazelaar, J., Louboutin, J. P., Yu, Q. C., et al. (2002). Preexisting immunity to adenovirus in rhesus monkeys fails to prevent vector-induced toxicity. Journal of Virology,76, 5711–5719.
Vlachaki, M. T., Hernandez-Garcia, A., Ittmann, M., Chhikara, M., Aguilar, L. K., Zhu, X., et al. (2002). Impact of preimmunization on adenoviral vector expression and toxicity in a subcutaneous mouse cancer model. Molecular Therapy,6, 342–348.
Thomas, C. E., Schiedner, G., Kochanek, S., Castro, M. G., & Lowenstein, P. R. (2001). Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Human Gene,12, 839–846.
Harvey, B. G., Hackett, N. R., El-Sawy, T., Rosengart, T. K., Hirschowitz, E. A., Lieberman, M. D., et al. (1999). Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. Journal of Virology,73, 6729–6742.
Seregin, S. S., & Amalfitano, A. (2010). Improving adenovirus based gene transfer: Strategies to accomplish immune evasion. Viruses,2(9), 2013–2036.
Lochmuller, H., Petrof, B. J., Pari, G., Larochelle, N., Dodelet, V., Wang, Q., et al. (1996). Transient immunosuppression by FK506 permits a sustained high-level dystrophin expression after adenovirus-mediated dystrophin minigene transfer to skeletal muscles of adult dystrophic (mdx) mice. Gene Therapy,3, 706–716.
Pedroso Baretta, G. A., Gama, F. O., Toderke, E. L., Dall'Oglio Tolazzi, A. R., & Fouto Matias, J. E. (2015). Effect of cyclosporine on liver regeneration in partial hepatectomized rats. Acta Cirurgica Brasileira,30(1), 54–59.
Smith, T. A., White, B. D., Gardner, J. M., Kaleko, M., & McClelland, A. (1996). Transient immunosuppression permits successful repetitive intravenous administration of an adenovirus vector. Gene Therapy,3(6), 496–502.
Acknowledgements
Authors want to thank Conacyt for Grant Number 103772 awarded to Ana Sandoval Rodriguez and Conacyt Grant Number 259096 awarded to Juan Armendariz-Borunda
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice where the studies were conducted. The protocol was approved by the Research and Ethical Committee of the CUCS, Universidad de Guadalajara (Approval Number C.I. 67-2012) which reviewed and approved the mortality aspects of the protocol.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sandoval-Rodríguez, A., Mena-Enriquez, M., García-Bañuelos, J. et al. Adenovirus Biodistribution is Modified in Sensitive Animals Compared to Naïve Animals. Mol Biotechnol 62, 260–272 (2020). https://doi.org/10.1007/s12033-020-00247-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-020-00247-x