Abstract
Usable pollination control systems have proven to be effective system for the development of hybrid crop varieties, which are important for optimal performance over varied environments and years. They also act as a biocontainment to check horizontal transgene flow. In the last two decades, many genetic manipulations involving genes controlling the production of cytotoxic products, conditional male sterility, altering metabolic processes, post-transcriptional gene silencing, RNA editing and chloroplast engineering methods have been used to develop a proper pollination control system. In this review article, we outline the approaches used for generating male sterile plants using an effective pollination control system to highlight the recent progress that occurred in this area. Furthermore, we propose possible future directions for biotechnological improvements that will allow the farmers to buy hybrid seed once for many generations in a cost-effective manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food security has become one of the challenging issues as the global population, particularly in the underdeveloped countries, is growing day by day. According to a report, the world population by 2050 is predicted to increase by 34% [1]. A significant proportion of the population lives in developing and underdeveloped countries and suffers from the deficiency of micronutrients of zinc, iron and vitamin A [2]. Severe public health problem arises due to food security and malnutrition [3].
During 1950–1980, there was a significantly increment in total food grain production as a result of green revolution [4]. This had made a balance between the food supply and the population growth. In the last few decades, the area of land for agriculture practices is decreasing consistently over the years because of urbanization and land degradation. Continuous population growth has raised the global alarm for the demand for food, feed, fiber and fuel along with economic advancement for keeping pace with global food supply. Therefore, it is required to increase the food production per unit area as cultivated lands are limited [5].
To increase productivity, genetic improvement in crop plants emerges as a key input. This again opens up the door (after the green revolution) to address food insecurity concerns. Development of hybrids has existed for a very long period as an effective method to increase agricultural productivity. Hybrid vigor has been observed in most of the crops, but its uses are limited to a few crops in which effective pollination mechanism is well developed.
Pollination control system described as a procedure in plant breeding adapted by a plant breeder in order to obtain desired hybrid cultivars [6]. In these systems, male floral parts of one parent line are removed (termed as the process of emasculation) making them male sterile to avoid self-pollination and to ensure pollination by the desired parent line. The objective is to develop a system for pollination control to facilitate hybrid crop varieties that exhibit heterosis in specific combinations resulting in wider adaptation to the environment over the years with the stability of performance. Removal of male flower or floral parts through manual emasculation is the most widely used method to avoid self-pollination. However, it is a tedious and costly procedure as the most crops have small, bisexual flowers [7].
Other pollination control systems include the use of male sterility in plants. Male sterility is defined as the failure of a plant to generate viable pollen grains retaining female fertility unaltered. The generation of male sterile plants is important for the production of hybrid seed. In nature, male sterility is more prevalent than female infertility, probably because the male gametophyte is less protected from the environment than the female ovule and the embryo sac. The male gametophyte development is a very well orchestrated and highly regulated process, in which anther performs a significant role in fertility of the plant and crop production through the generation of male gametes. Any disturbance in this process leads to male sterility.
Manifestation of Male Sterility
In nature, male sterility appears in plants in many different forms. It was broadly classified on the basis of phenotype and genotype [8]. Phenotypic male sterility can be structural, sporogenous and functional male sterility (Fig. 1). Structural male sterility arises because of anomalies in male sex organs [8]. Sporogenous male sterility displays the abnormal process of microsporogenesis, and functional male sterility results in the generation of non-viable pollen with the manifestation of abnormal pollen maturation and the failure of pollen grains to germinate on compatible stigma or it can be in the form of a barrier other than incompatibility, which prevents the pollen tube from reaching the ovule. Based on genotype, male sterility can be genic or genetic, cytoplasmic and cytoplasmic genetic (Fig. 1). Cytoplasmic and cytoplasmic genetic male sterility are the most commonly observed systems in nature [9].
Genetic Male Sterility (GMS)
GMS occurs as a result of spontaneous mutations in one or more (dominant or recessive) nuclear genes. The gene inheritance follows a Mendelian inheritance pattern. In nature, GMS has been identified in about 175 species. Most of them were due to spontaneous mutations in recessive nuclear genes and a few due to mutations in dominant genes [10, 11].
Cytoplasmic Male Sterility (CMS)
It arises due to spontaneous mutation in cytoplasmic genes, especially in mitochondrial genes. During microsporogenesis, anther cells are metabolically very active and are rich in mitochondria, which fulfill their energy demands. Any abnormalities in mitochondrial function can lead to cytoplasmic male sterility. Sterile cytoplasm is designated as S, while the normal cytoplasm as N. It follows maternal inheritance [12].
Cytoplasmic Genetic Male Sterility (CGMS)
CGMS involves the interaction of both cytoplasmic and nuclear genes. The terms CMS and CGMS are often used interchangeably. It has been reported in more than 200 species [8, 13,14,15]. CGMS has two types of cytoplasm, N (Normal) and S (Sterile) along with nuclear genes, restorers of fertility (Rf). The Rf genes are distinct from genetic male sterility genes and are required for restoration of male fertility in S cytoplasm-induced sterility in plants. The Rf genes display expression in the presence of sterile cytoplasm only. Therefore, male sterile plants possess S cytoplasm with rfrf genes, while fertile plants exhibit a combination of either N cytoplasm with rfrf genes or S cytoplasm with Rf genes. These Rf genes mostly encode pentatricopeptide repeat (PPR) proteins, which post-transcriptionally modify the expression of CMS gene in mitochondria, and silence the expression of ORFs causing male sterility [16]. It was one of the conventional methods for hybrid seed production. But, it has some limitations such as undesirable pleiotropic effects, imperfect fertility restoration among other features.
Genetic Engineering for Male Sterility
Conventional methods for commercial hybrid seed production have certain limitations, and it cannot fulfill global demands of the continuously growing population. Genetic Engineering provides a precise method for the generation of transgenic male sterile plants as an effective pollination control system. A suitable pollination control system is required for hybrid seed production. It also acts in transgene biocontainment, which can minimize unwanted transgene movement via pollen dispersal. Several approaches have been employed for generating transgenic male sterile plants.
Dominant Nuclear Male Sterility (barnase and barstar System)
In 1990, Mariani et al. developed the first genetically engineered male sterility system in crop plants. Two types of RNases were used to manipulate this trait. One is RNase T1 from Aspergillus oryzae, and the other is barnase from Bacillus amyloliquefaciens. The coding sequences of both were fused with tapetum-specific TA29 promoter separately and then transformed individually into oilseed rape and tobacco. About 10% of the TA29-RNaseT1 transformants and 92% of TA29-barnase transformed were completely male sterile [17]. Furthermore, studies revealed that the 5′ region of TA29 gene had driven the expression of RNaseT1 and barnase genes specifically in tapetal cell lineage that caused selective and early cell death of tapetal cells that surround the pollen sacs, presumably by hydrolyzing and ablating the tapetal cells. This manipulated phenomenon disrupted the pollen nourishment resulting in the male sterility of the plants expressing these constructs.
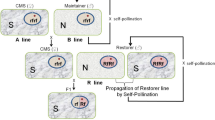
Later, Mariani et al. [18] used the approach of inhibiting the barnase action in the tapetal cells by targeting the expression of barstar also to the same cell layer for restoring fertility in the earlier described barnase-expressing transgenic plants. Barnase-expressing male sterile plants were crossed with barstar-expressing fertile plants, which resulted in the coexpression of barnase and barstar genes in the tapetal cell layers of the anthers of F1 plants leading to the inactivation of barnase by barstar by forming an inactive protein complex leading to the restoration of fertility in the hybrid plants (Fig. 2).
Schematic representation of male sterility–fertility restoration system in barnase/cysteine protease–barstar/cystatin method. Tapetal expression of barnase/cysteine protease resulted in male sterility and coexpression of barstar/cystatin with barnase/cysteine protease restored the male fertility [17, 18, 22, 23]
Huang et al. [19] observed that the expression of barnase gene as a fusion gene with SOLO DANCERS (SDS) gene under the 1.5 kb SDS promoter resulted in complete male and female sterility. Contrary to this, barnase gene expression driven by 1.5 kb SDS promoter did not display sterility phenotype. It was argued that the fusion of SDS gene with barnase (SDS:: SDS-barnase) is required for barnase gene expression through SDS promoter. SDS gene encodes a meiosis-specific cyclin, and when it was expressed as a fusion gene with barnase (SDS:: SDS-barnase), it interferes with the formation of male and female gametes, which resulted in complete male and female sterility [19].
Recently, our group has developed a plant gene-based male sterility–fertility restoration system using cysteine protease and cystatin genes, respectively. It was found that in a wild peanut Arachis diogoi, which displays absolute resistance against the late leaf spot pathogen Pheaoisariopsis personata, the cysteine protease gene was found to be expressed differentially [20, 21]. The role of these genes in programmed cell death was well documented. Therefore, tapetal expression of cysteine protease resulted in early degeneration of tapetal cell layer and induced male sterility in the high expression plants of tobacco [22]. Subsequently, the male fertility was restored in F1 hybrids of cysteine protease-induced male sterile plants through crosses using the pollen from high expression line of cystatin, which was also expressed in tapetal cell layer (Fig. 2) [23].
Male sterility by Altering a Metabolic Process
Carbohydrate supply to the developing microspores is essential, and they rely on the sucrose hydrolysis through invertase enzyme bound to the cell wall. Therefore, male sterility was achieved by impeding the carbohydrate supply through antisense suppression of the invertase activity or through expressing invertase inhibitor in anthers using an invertase promoter P-Nin88 [24, 25]. Later, male fertility was restored by expressing yeast invertase under the same promoter [24].
Similarly, glutamine is crucial for nitrogen metabolism in cells. Glutamine is synthesized by glutamine synthetase (GS) through the catalytic conversion of glutamate to glutamine in an ATP-dependent manner utilizing ammonia as a substrate. GS is an octameric enzyme, found in two isoenzymatic forms, cytoplasmic GS1 and chloroplastic GS2. The expression of both GS1 and GS2 is reported during male reproductive development [26,27,28]. Using genetic engineering approach, Ribarits et al. [29] developed male sterile transgenic plants of Arabidopsis having mutated tobacco GS1, and GS2 driven by microspore-specific promoter (NTM19) and tapetum-specific (TA29) separately. Three constructs were prepared. In the first construct, N-terminal chloroplast targeting signal and 45 amino acid component of C-terminal activity domain of GS2 were deleted, and the resultant sequence was fused with TA29 promoter (pTA29-∆GS2-L construct). In the second and third constructs, two point mutations at critical sites (N56A, R291L) were introduced in GS1 (GS1A56L291), and the mutants were fused with TA29 and NTM19 promoter. Mutated GS1 or GS2 expression in tapetum leads to the change in anther color from green to olive and subsequently drying up before dehiscence. Transgenic tobacco expressing the mutated GS1 in the microspore leads to 50% sterile pollen in T 0 transgenic, which has been conferred by the single copy insertion of the transgene, and transgenic plants were able to give rise to the seeds. Later, double haploids were generated from the microspores of the heterozygous plant. These doubled haploids were homozygous, and 100% male sterile were used as female parents for crossing with a male fertile inbred line [29].
Callose is a plant polysaccharide. It is composed of β-1-3 glucan. During meiosis, β-1-3 glucan gets accumulated around the tetrads of microspores. But, as the meiosis completed and microspore exine wall formation starts, the enzyme callase, a tapetally secreted β-1-3 glucanase, degrades the callose and releases the microspores from the tetrad in a precisely timed manner. Hence, tight developmental regulation and timing of callase activity are necessary for proper microspore development. Worrall et al. [30] used a modified pathogenesis-related glucanase (PR-β-1-3 glucanase), which mimics the tapetal β-1-3 glucanase and expressed it in the tapetal cell lineage under A3 and A9 promoters. Expression of modified PR-β-1-3 glucanase in transgenic tobacco plants resulted in the premature dissolution of microsporocyte callose wall leading to moderate to complete male sterility [30].
Chang et al. [31] developed a rice hybrid breeding system by using the rice nuclear gene Oryza sativa No Pollen 1(OsNP1). It encodes a putative glucose–methanol–choline–oxidoreductase, which has roles in tapetum degeneration and pollen exine formation. The ethyl methanesulfonate-induced rice mutant, osnp1-1, exhibited complete male sterility. Chang et al. [31] transformed osnp1-1 mutant using a binary vector pZhen18B with two T-DNAs. First T-DNA had NPTII gene as a selection marker under CaMV 35S promoter. The second T-DNA had three gene cassettes, OsNP1 gene driven by its native promoter, the maize α-amylase gene ZM-AA1 driven by pollen specific PG47 promoter to make pollen non-viable and red fluorescence protein gene from Discosoma sp. (DsRed) with aleurone-specific LTP2 promoter for identification of transgenic seeds. The T1 progenies from the self-pollinated seeds of T0 plants were screened for the plants having only single copy of second T-DNA but lacking first T-DNA. One of the selected T1 plant was named as Zhen18B, which has been analyzed further. This plant displayed normal vegetative and male organ development, 1:1 ratio of fertile and non-viable pollen grains, and 1:1 ratio of red fluorescent seeds and non-fluorescent seeds (no transgene). All plants from the red fluorescent seeds were genetically the same as Zhen18B parent, and plants from non-fluorescent seeds (named as Zhen18A) were male sterile. Zhen18B acts as a maintainer line, and it was maintained through self-pollination and selection of red fluorescent seeds via seed sorting. Zhen18A male sterile plants were utilized in the hybrid seed production through the crossing with the pollen of normal rice plants [31].
The maize Ms26 gene and its ortholog in rice (OsCYP704B2) encode a cytochrome P450 mono-oxygenase. This enzyme catalyzes the ω-hydroxylation of C16 and C18 fatty acid and specifically expresses in tapetum. Cigan et al. [32] generated male sterility through the targeted mutagenesis of Ms26 orthologs in rice and sorghum using Ems26+ endonuclease, which recognizes the 22nd sequences of 5th exon of Ms26. Ems26+ creates a double-strand break (DSB) and then NHEJ repair resulting in mutation in Ms26 gene, which leads to male sterility in rice and sorghum [32]. Further, fertility was restored in sorghum through crossing with the pollen of transgenic sorghum expressing maize ZmMs26 gene [32].
Conditional Male Sterility
Conditional male sterility refers to a situation in which plants are normally fertile, but when a particular condition is applied, it results in male sterility. Hawkes et al. [33] described the application of inactive d-glufosinate as a male sterility causing agent on the transgenic plant expressing a modified (F58 K, M213S) form of the d-amino acid oxidase (DAAO) from Rhodosporidium toruloides. DAAO converts oxidized d-glufosinate to its 2-oxo derivative (2-oxo-4-methyl phosphinyl-butanoic acid), which is phytotoxic to the plants. For inducing male sterility, modified DAAO encoding gene was fused with TAP1 promoter from Antirrhinum majus and transformed into tobacco plants for generating the transgenic plants. When d-glufosinate was sprayed on these transgenic plants, it caused complete male sterility which was endured for two or more weeks without affecting female fertility [33].
Singh et al. [34] developed a three-component tapetum-specific and steroid-inducible expression system for inducing conditional male sterility in the plant. For this, AtBECLIN1 gene from Arabidopsis thaliana, mutated TA29 promoter (TA29TGTA), TATA-binding protein mutant 3 (TBPm3) and ligand-binding domain (LBD) of the glucocorticoid receptor (GR-LBD) from rat were used. AtBECLIN1 gene is shown to be involved in a cross-talk between apoptosis and autophagy. Mutated TA29 promoter having TATA box with mutant TGTA does not recognize the native TATA-binding protein, but with the coexpression of a mutant TBPm3, the mutated TGTA promoter function is restored to a higher level. Three constructs were prepared. In the first construct, AtBECLIN1 gene was driven by mutated TA29 promoter (TA29TGTA) as component one (Fig. 3a). In the second construct, AtBECLIN1 gene was expressed from mutated TA29 promoter (TA29TGTA) along with the constitutive expression of TBPm3 as component two (Fig. 3b). In the third construct, the component one was the same, but the component-II, TBPm3, transcribed as a fusion protein with the ligand-binding domain of the glucocorticoid receptor (GR-LBD) as component-III (Fig. 3c). The GR-LBD gets associated with a chaperone of heat shock proteins (HSPs) in the cytoplasm and remains inactive without its cognate ligand or its agonist, and therefore, there would be no transcription from TBPm3 and no TBPm3 protein. However, GR-LBD gets dissociated from HSPs in the presence of its ligand (glucocorticoid) or agonist (dexamethasone) and then GR-LBD-TBPm3 fusion protein with functionally active TBPm3 restore the function of mutated TA29 promoter (TA29TGTA). There was no sterility in transgenic plants expressing construct-I. But in transgenic lines carrying the construct-II, non-viable pollen grains were observed and severe abnormality in microspore development, which resulted in male sterility. Dexamethasone treatment is required to induce male sterility in transgenic plants expressing the third construct [34].
Schematic explanation of conditional male sterility developed through utilization of AtBECLIN1 gene, mutated TA29 promoter (TA29TGTA), TATA-binding protein mutant 3 (TBPm3) and ligand-binding domain (LBD) of the glucocorticoid receptor (GR-LBD) from rat. In construct-I, BECLIN1 gene was expressed under the mutated TA29 promoter; therefore, no transcription and no male sterility. But in construct-II, TBPm3 protein bind at mutated TA29 promoter, which leads to the transcription of BECLIN1 gene and induction of male sterility. And in case of construct-III, TBPm3 was expressed as a fusion protein with GR-LBD and remained associated with HSPs in cytoplasm. But in the presence of dexamethasone, HSPs get released from TBPm3-GR-LBD fusion protein, which binds with mutated TA29 promoter resulting in transcription of BECLIN3 and male sterility in transgenic plants [34]
Construct-III was further modified by fusing 131 amino acid N-terminal domain of Long hypocotyl in far red 1 (HFR1) with TBPm3 [35]. HFR1, a basic helix-loop-helix (bHLH) transcription factor, participates in the seedling response to far-red light (Fig. 4a). Transgenic plants expressing construct-III resulted in male sterile plants. Fertility restoration was achieved in these male sterile plants by crossing with the pollen of transgenic plant expressing constitutive photo-morphogenesis 1 (COP1), a repressor of light-mediated photo-morphogenesis, under Arabidopsis tapetum-specific A9 promoter [35]. COP1 interacts with N-terminal of HFR1 and degrades it including fused protein TBPm3. Therefore, TBm3 becomes unavailable for mutated TA29 promoter (TA29TGTA) and repressed the transcription of BECLIN1 which leads to restoration of fertility in the F1 hybrid (Fig. 4b) [35].
Schematic explanation of male sterility–fertility restoration developed by modifying construct-III. 131 amino acid N-terminal domain of Long hypocotyl in far red 1 (HFR1NT131) was fused with TBPm3 instead of GR-LBD in construct-III. HFR1-TBPm3 complex binds with mutated TA29 promoter which leads to transcription of BECLIN1 gene and caused male sterility in transgenic plants. Further, male fertility was restored through crossing of male sterile transgenic plants with the pollen of COP1-expressing transgenic plants. COP1 protein interacts with the HFR1NT131 protein and degrade the HFR1-TBPm3 complex resulted in no transcription from BECLIN1 gene through mutated TA29 promoter and restored fertility in F1 progeny [35]
Guerineau et al. [36] expressed the temperature-sensitive diphtheria toxin A-chain polypeptide gene sequence under the tapetum-specific A9 promoter and generated transgenic Arabidopsis plants. Resultant transformed plants were fully fertile at 26 °C, but when the temperature was decreased to 18 °C, male sterility was induced [36].
Kriete et al. [37] developed an inducible male sterility in plants through the expression of the bacterial gene argE. Gene argE encodes a deacetylase enzyme, N-acetyl l-ornithine deacetylase and it produces a phytotoxic herbicide l-phosphinothricine (ppt, gluphosinate) from the non-phytotoxic compound, N-acetyl phosphinothricine (N-ac-pt) through the removal of the acetyl group. Since the argE gene coding region was brought under the control, the tapetum-specific promoter (TA29) and transformed tobacco plants were raised. Foliar treatment of non-toxic compound N-ac-pt on these plants leads to early expression of l-phosphinothricine in tapetal cells resulting in male sterility, but female reproduction remained unaffected [37]. Similarly, Rao et al. [38] developed inducible male sterility in rice through expression of argE gene in the pollen grains of rice through pollen allergen (OSIPA) promoter, and transgenic rice plants expressing argE displayed complete male sterility on application of N-ac-pt.
Male Sterility via Post-transcriptional Gene Silencing
Post-transcriptional gene silencing is defined as destroying or blocking of mRNA of a particular gene after its transcription. Gene silencing is based on the homology of the target sequence and the double-stranded RNA (dsRNA) directed against the transcript of the target gene or its promoter region [39, 40]. The discovery of gene silencing mechanism has expanded the horizon for research on control of gene expression [40, 41]. In the recent past, it has got a lot of importance as an approach toward inducing male sterility.
The plant hormone, jasmonic acid (JA), participates in many developmental signaling processes in plants including senescence, fruit ripening, anther dehiscence and pollen maturation [42,43,44,45,46]. In the JA biosynthetic pathway, allene oxide synthase (AOS) catalyzes the dehydration of 13-hydroxypenoxylinolenic acid to 12–13 epoxy linolenic acid [43]. Bae et al. [47] reported the induction of male sterility through RNA silencing of OsAOS1 and OsAOS2 activity with the promoter of anther-specific genes Osc4 and Osg6b separately. Four constructs, P-Osc4:: OsAOS1-RNAi (pSK230), P-Osc4:: OsAOS2-RNAi (pSK123), P-Osg6b:: OsAOS1-RNAi (pSK231) and P-Osg6b:: OsAOS1-RNAi (pSK124), were developed and transformed into rice calli individually. The level of sterility in transformed plants with pSK230 was approximately 8% (3 of 37), with pSK231 about 28% (10 of 36) and with pSK123 23% (9 of 40). Complete sterility was found in 47% (9 of 19) of the plants from pSK124. It was concluded that OsAOS2-RNAi vector driven by Osg6b promoter is potent enough for generating male sterility in rice [47].
Woo et al. [48] established a link between abnormal transcript and ms-h genic male sterility in rice. Analysis of ms-h gene through fine mapping and nucleotide sequencing revealed that there is single nucleotide substitution in the UDP-glucose pyrophosphorylase1 (UGPase1, EC 2.7.7.9) gene at the 3′ splice junction of the 14th intron. This substitution resulted in aberrant splicing, which gives rise to two abnormal sizes transcripts of UGPase 1 having no activity. Further, the role of UGPase activity in male sterility was demonstrated by suppressing the UGPase activity via RNAi construct having a 473 bp of UGPase1 cDNA in wild plants and overexpressing the UGPase1 in mutant ms-h plant. Down-regulation of UGPase1 gene gave rise to two male sterile lines having similar developmentally retarded phenotype to the ms-h mutant. Restoration of male fertility was observed in transformed ms-h mutant plants overexpressing UGPase1 gene. This report also suggested that UGPase1 plays a critical role in pollen development as well as seed carbohydrate metabolism [48].
Nawaz-ul-Rehman et al. [49] silenced the TA29 gene with a hairpin RNAi construct, which resulted in about 10 out of 13 transgenic tobacco lines exhibiting male sterility.
Yui et al. [50] reported the inhibition of mitochondrial pyruvate dehydrogenase (PDH)-E1α subunit gene in tapetum resulting in male sterility which was similar to the sugar beet CMS phenotype. Pyruvate dehydrogenase is crucial for tricarboxylic acid (TCA) cycle. Two subunits of PDH, E1α1 and E1α2 were identified from sugar beet flower buds, and it was found that the PDH_E1α1 expression in the tap root was high, whereas bvPDH_E1α2 in flower buds. GFP fusion with bvPDH_E1α1 revealed its mitochondrial targeting property, and a 300 bp of bvPDH_E1α1 cDNA sequence (from +620 to +926) was used for silencing PDH activity in mitochondria. The construct was prepared by fusing TA29 promoter with 300 bp of bvPDH_E1α1 in reverse orientation and transformed into tobacco plants. Seven independent transformants were obtained of which, three transformants, designated as #1, #2 and #3, respectively, exhibited very less viable pollen grains compared to the non-transgenic tobacco plants. Their production of viable pollen grains in different transgenic plants was 15.0% (#1), 47.1% (#2) and 65.5% (#3), respectively, and the four plants were able to produce viable pollen grains at more than 80% efficiency. It was premised that the conversion of pyruvate into acetyl CoA was blocked due to the inhibition of PDH activity in tapetum, and it adversely affected the operation of the TCA cycle, which was unable to fulfill the need of tapetum leading to male sterility [50].
Heiser et al. [51] induced male sterility in transgenic potato through antisense suppression of mitochondrial NADH-binding subunit of complex I. An antisense construct was prepared by cloning the gene encoding 55 kDa NADH-binding protein in reverse orientation with 35S CaMV promoter. Antisense construct was further transformed into the potato. Transgenic potato displayed 33% reduction in transcripts for 55 kDa subunit compared to wild-type level, and the amount of 55 kDa protein in mitochondrial extract has also gone down to about 50% in transformed plants. It disturbed the pollen maturation but did not affect the vegetative growth and tuber formation. It was argued that down-regulation of NADH-binding component of respiratory complex caused an insufficient mitochondrial respiratory chain [51].
Xu et al. [52] observed that the down-regulation of Bcp 1 gene in transgenic Arabidopsis plants leads to the arrest of pollen development. The Bcp 1 gene from Brassica oleracia was identified as an anther-specific gene, which is developmentally regulated and expressed temporally in diploid tapetal cells and haploid microspores during pollen maturation. To confirm the role of Bcp1 gene, two antisense constructs were prepared. In first construct (Bcp1 promoter-antisense construct), 0.5 kb of a Bcp 1cDNA was kept under the 0.77 kb of Bcp1 promoter in reverse orientation. In the second construct (LAT52 promoter-antisense construct), LAT52 promoter from tomato was fused with antisense of 0.5 kb of a Bcp1cDNA and resultant constructs were transformed into Arabidopsis separately. About 36% (16 out of 42) in Bcp1 promoter-antisense Bcp1 construct carrying transgenic plants were completely male sterile. It was found that in T2 generation, homozygous transgenic plants were completely sterile. Transformed plants having LAT52-antisense Bcp1 construct had 1:1 segregation of viable/aborted pollen grains, which conferred the gametophytic control of Bcp 1 gene [52].
Flavonoids and Male Sterility
Flavonoids are well-known flower pigments controlling flower color. In addition to this, they are also involved in disease-related mechanisms and plant reproduction [53,54,55,56]. There are reports that any disturbance in flavonoid synthesis also leads to altered pigmentation and male sterility in the plants.
Stilbene synthase gene (STS), which has been reported to enhance disease resistance, can also induce male sterility when it was expressed in tapetum. Fischer et al. [57] observed that the expression, a stilbene synthase (STS) gene from grape vine (VstI), driven by 35S RNA promoter with duplicated enhancer region and tapetum-specific promoter (Tap1) of Antirrhinum majus separately, caused alteration in flower pigmentation and male sterility. Similar observations were also made when STS was expressed using male cone-specific promoter (PrMALE1) from Pinus radiata (radiate pine) [58]. However, the mechanism of induced male sterility through STS is not clear. It was argued that the introduction of STS strives for the substrates, 4-coumaroyl CoA and malonyl CoA along with endogenous CHS. 4-coumaroyl CoA and malonyl CoA are necessary for sporopollenin and fatty acid biosynthesis. It was also hypothesized that there was a decrease in p-coumaroyl availability, which results in impaired sporopollenin production and pollen wall formation. This eventually caused male sterility.
van der Meer et al. [59] demonstrated that down-regulation of chalcone synthase (CHS) gene under the control of modified 35S CaMV promoter carrying an anther box, a homologous sequence present in the flavonoid-specific gene and active during the early stages of anther development leads to the down-regulation of pigmentation and arrest of male gametophyte development in the anthers of transgenic tobacco plant.
RNA Editing as a Tool for Inducing Male Sterility
RNA editing has been described as a modification occurring in the nucleotide sequence of RNA, which makes it distinct from the DNA sequence that encoded it. RNA editing involves changes in nucleotide or insertion of a nucleotide, which alternatively results in changed amino acid sequence of the polypeptide. In plant mitochondria, RNA editing process involves nucleotide changes mostly from C to U, but sometimes from U to C [60,61,62,63]. In CMS plants, male sterility is linked with mitochondrial DNA rearrangement creating new chimeric open reading frames (ORFs), which leads to dysfunction of mitochondria [64], for example the chimeric gene pcf-S of petunia [65], ORF-B and ORF224 of polima in rapeseed [66]. The transcripts of all these chimeric genes are edited in vivo in the normal transcripts formed by RNA fragment [67]. Thus, the chimeric proteins PCF and ORF-B have originated from edited transcripts and are involved in petunia and rapeseed cms, respectively [67].
Similarly, it was reported that overexpressing unedited mitochondrial orfB gene in transgenic line of indica rice exhibited a reduction in ATPase activity of F1F0-ATP synthase, which resulted in male sterility in a dose-dependent manner [68]. Fertility was restored in these transgenic lines through RNAi-mediated silencing of orfB gene expression [68].
Hernould et al. [69] expressed an unedited ATP9 in mitochondria, which caused male sterility in transgenic plants.
Chloroplast Engineering and Male Sterility
In recent years, genetic transformation of plastid genome has become a popular tool in the field of biotechnology. This technology offers several advantages, which include high level expression of transgene, maternal inheritance of the transgene, expression of multigene operons and expression of bacterial genes without codon optimization [70]. Its maternal inheritance feature has allowed the induction of male sterility via plastid transformation, which also displayed the cms trait, i.e., production of a uniform population of male sterile plants through cross-pollination. Contrast to this, the pollen sterility via nuclear transformation cannot be true breeding as the male sterile plants must be propagated through pollination with wild type, which results in segregation into sterile and fertile types.
Ruiz and Daniell [71] demonstrated for the first time a promising approach for inducing cytoplasmic male sterility via plastid engineering. In this approach, phaA gene from Acinetobacter sp. which encodes a β-ketothiolase was expressed in chloroplast via chloroplast transformation. It was found that the transgenic lines showed hyper-expression of β-ketothiolase in leaves, flower and anther, which interferes with the pollen development and subsequently leads to male sterility. Further, male fertility was restored by keeping transgenic male sterile plants under continuous illumination, which allows acetyl CoA carboxylase (ACCase) to use available acetyl CoA that restores the normal fatty acid synthesis and reduced production of PHB via β-ketothiolase [71].
Heterologous Expression
It was a well-established fact that CMS is associated with novel chimeric ORF in the mitochondrial DNA sequences and mitochondria dysfunction. However, the molecular mechanism of male sterility was not completely understood until it was validated that the expression of a chimeric sequence of mitochondrial DNA sequences could interfere with the pollen development through the genetic engineering approach.
Nizampatnam et al. [72] developed transgenic tobacco plants expressing, a pet1-CMS-associated mitochondrial gene of sunflower, orfH522, which was targeted to mitochondria and driven by TA29 promoter. About 35% of transformed tobacco plants were completely sterile [72]. Later, the male fertility was restored through suppression of orfH522 transcripts using RNAi method [73].
Yamamoto et al. [74] investigated the translational product of male sterile I-12 CMS cytoplasm derived from beets by combining it with the normal fertile cytoplasm. In this study, the presence of 12 kDa novel polypeptide encoded by unique open reading frame (orf129) was observed in I-12 cms mitochondria. To investigate the functional role of orf129, transgenic plants expressing orf129 targeted to mitochondria and driven by Arabidopsis apetala 3 promoter were raised. The resultant plants expressing orf129 were male sterile [74].
Kim et al. [75] expressed the orf456 fused with coxIV presequence in Arabidopsis thaliana and found that approximately 45% of the transgenic plants exhibiting the male sterility. This male sterility was shown to be associated with exine layer deformity and vacuolated pollen phenotypes [75].
Restoration of Male Fertility
Good fertility restoration mechanism is essential if the seed is the economic part of the plants and the crop is predominantly self-fertilized one. Several reports were published on the development of male sterility, but there are only a few reports on the restoration of male fertility via the development of restorer lines. Restoration was obtained using cysteine protease–cystatin system [22, 23], barnase–barstar system [17, 18] at protein level, gene silencing approach [73, 76, 77] at RNA level and site-specific recombination system (CRE-LOX) [78, 79] at DNA level. It is essential for proper restoration that restorer line must be capable of nullifying the function of the transgene in induced male sterility.
Conclusions and Future Prospects
Genetic engineering has given us an opportunity to develop several new engineered male sterile systems for controlling pollination that can be useful for the production of hybrids. The barnase–barstar system is still a popular system even though it is associated with ethical and environmental concern as both genes are from a prokaryotic system. Several approaches have been used in last decade to develop efficient pollination control systems as alternatives to the barnase–barstar system. However, the steroid-inducible and tapetum-specific three-component system of conditional male sterility–fertility restoration and the one employing the cysteine protease–cystatin system (plant gene-based male sterility–fertility restoration system) have shown to be promising approaches as alternative systems to the barnase–barstar system. However, both of them need to be tested in crop plants for their commercial exploitation.
Propagation of the male sterile line has a limiting factor in the commercial production of hybrids. Every time, the farmer has to buy hybrid seed as heterosis of the plants gets lost in the second generation (F 2) because of segregation and recombination. Thus, it is important that future research should focus on the development of a strategy of maintenance of true-to-type hybrids by combining the feature of apomixis with the hybrids so that the farmer would not be forced to buy the hybrid seed generation after generation. A step in this direction, the haploid inducer line (chimeric CENH3 or GEM line) was developed for clonal seed production [80, 81]. These haploid inducer lines for engineering apomixis appear to be a promising approach. However, basic research work is required to continue for making this concept a success.
It could be probably possible by associating inducible male sterility with apomixis so that after fixing trait, fertile plants could be generated. It is also important to ensure that apomixis should also be inducible, which would allow reversion of the apomixis to sexual reproduction so that the breeder would have the option of improvement in the hybrids further by developing suitable combiner lines.
In conclusion, environmental and ecological safety should be our top agenda during the development of genetically engineered crops.
References
FAO, U. (2009). How to feed the world in 2050. In Rome: Highlevel expert forum.
Pinstrup-Andersen, P., & Cohen, M. (2000). Modern biotechnology for food and agriculture: Risks and opportunities for the poor. In G. J. Persley, & M. M. Lantin (Eds.), Agricultural biotechnology and the poor (pp. 159–172). Washington DC: Consultative Group on International Agricultural Research.
Sharma, H., Crouch, J., Sharma, K., Seetharama, N., & Hash, C. (2002). Applications of biotechnology for crop improvement: Prospects and constraints. Plant Science, 163, 381–395.
Myers, N. (1999). The next green revolution: Its environmental underpinnings. Current Science, 76, 507–513.
Miller, J. K., Herman, E. M., Jahn, M., & Bradford, K. J. (2010). Strategic research, education and policy goals for seed science and crop improvement. Plant Science, 179, 645–652.
Kempe, K., & Gils, M. (2011). Pollination control technologies for hybrid breeding. Molecular Breeding, 27, 417–437.
Acquaah, G. (2009). Principles of plant genetics and breeding. New York: Wiley.
Kaul, M. (1987). Male sterility in higher plants (vol. 1005, pp. 116–117). Berlin: Springer.
Budar, F., & Pelletier, G. (2001). Male sterility in plants: Occurrence, determinism, significance and use. Comptes Rendus de l’Académie des Sciences-Series III-Sciences de la Vie, 324, 543–550.
Chaudhury, A. M. (1993). Nuclear genes controlling male fertility. The Plant Cell, 5, 1277.
Horner, H. T., & Palmer, R. G. (1995). Mechanisms of genic male sterility. Crop Science, 35, 1527–1535.
Hanson, M. R. (1991). Plant mitochondrial mutations and male sterility. Annual Review of Genetics, 25, 461–486.
Budar, F., Touzet, P., & Pelletier, G. (2008). 7 Cytoplasmic male sterility. Annual Plant Reviews, Flowering and Its Manipulation, 20, 147.
Mackenzie, S., & McIntosh, L. (1999). Higher plant mitochondria. The Plant Cell, 11, 571–585.
Wise, R., Gobelman-Werner, K., Pei, D., Dill, C., & Schnable, P. (1999). Mitochondrial transcript processing and restoration of male fertility in T-cytoplasm maize. Journal of Heredity, 90, 380–385.
Bentolila, S., Alfonso, A. A., & Hanson, M. R. (2002). A pentatricopeptide repeat-containing gene restores fertility to cytoplasmic male-sterile plants. Proceedings of the National Academy of Sciences, 99, 10887–10892.
Mariani, C., De Beuckeleer, M., Truettner, J., Leemans, J., & Goldberg, R. B. (1990). Induction of male sterility in plants by a chimeric ribonuclease gene. Nature, 347, 737–741.
Mariani, C., Gossele, V., Beuckeleer, M. D., Block, M. D., Goldberg, R. B., Greef, W. D., & Leemans, J. (1992). A chimaeric ribonuclease-inhibitor gene restores fertility to male sterile plants. Nature, 357, 384–387.
Huang, J., Smith, A. R., Zhang, T., & Zhao, D. (2016). Creating completely both male and female sterile plants by specifically ablating microspore and megaspore mother cells. Frontiers in Plant Science, 7, 30.
Kumar, K. R. R., & Kirti, P. B. (2011). Differential gene expression in Arachis diogoi upon interaction with peanut late leaf spot pathogen, Phaeoisariopsis personata and characterization of a pathogen induced cyclophilin. Plant Molecular Biology, 75, 497–513.
Pande, S., Rao, J. N., & Dwivedi, S. (2002). Components of resistance to late leaf spot caused by Phaeoisariopsis personata in inter-specific derivatives of groundnut. Indian Phytopathology, 55, 444–450.
Shukla, P., Singh, N. K., Kumar, D., Vijayan, S., et al. (2014). Expression of a pathogen-induced cysteine protease (AdCP) in tapetum results in male sterility in transgenic tobacco. Functional & Integrative Genomics, 14, 307–317.
Shukla, P., Subhashini, M., Singh, N. K., Ahmed, I., et al. (2016). Targeted expression of cystatin restores fertility in cysteine protease induced male sterile tobacco plants. Plant Science: An International Journal of Experimental Plant Biology, 246, 52–61.
Engelke, T., Hirsche, J., & Roitsch, T. (2010). Anther-specific carbohydrate supply and restoration of metabolically engineered male sterility. Journal of Experimental Botany, 61, 2693–2706.
Hirsche, J., Engelke, T., Völler, D., Götz, M., & Roitsch, T. (2009). Interspecies compatibility of the anther specific cell wall invertase promoters from Arabidopsis and tobacco for generating male sterile plants. Theoretical and Applied Genetics, 118, 235–245.
Becker, T. W., Caboche, M., Carrayol, E., & Hirel, B. (1992). Nucleotide sequence of a tobacco cDNA encoding plastidic glutamine synthetase and light inducibility, organ specificity and diurnal rhythmicity in the expression of the corresponding genes of tobacco and tomato. Plant Molecular Biology, 19, 367–379.
Cren, M., & Hirel, B. (1999). Glutamine synthetase in higher plants regulation of gene and protein expression from the organ to the cell. Plant and Cell Physiology, 40, 1187–1193.
Dubois, F., Brugière, N., Sangwan, R. S., & Hirel, B. (1996). Localization of tobacco cytosolic glutamine synthetase enzymes and the corresponding transcripts shows organ-and cell-specific patterns of protein synthesis and gene expression. Plant Molecular Biology, 31, 803–817.
Ribarits, A., Mamun, A. N., Li, S., Resch, T., et al. (2007). Combination of reversible male sterility and doubled haploid production by targeted inactivation of cytoplasmic glutamine synthetase in developing anthers and pollen. Plant Biotechnology Journal, 5, 483–494.
Worrall, D., Hird, D. L., Hodge, R., Paul, W., et al. (1992). Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. The Plant Cell, 4, 759–771.
Chang, Z., Chen, Z., Wang, N., Xie, G., Lu, J., Yan, W., et al. (2016). Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene. Proceedings of the National Academy of Sciences, 113, 14145–14150.
Cigan, A. M., Singh, M., Benn, G., Feigenbutz, L., Kumar, M., Cho, M. J., et al. (2017). Targeted mutagenesis of a conserved anther-expressed P450 gene confers male sterility in monocots. Plant Biotechnology Journal, 15, 379–389.
Hawkes, T., Pline-Srnic, W., Dale, R., Friend, E., et al. (2011). d-glufosinate as a male sterility agent for hybrid seed production. Plant Biotechnology Journal, 9, 301–314.
Singh, S. P., Pandey, T., Srivastava, R., Verma, P. C., et al. (2010). BECLIN1 from Arabidopsis thaliana under the generic control of regulated expression systems, a strategy for developing male sterile plants. Plant Biotechnology Journal, 8, 1005–1022.
Singh, S. P., Singh, S. P., Pandey, T., Singh, R. R., & Sawant, S. V. (2015). A novel male sterility–fertility restoration system in plants for hybrid seed production. Scientific Reports, 5, 11274.
Guerineau, F., Sorensen, A. M., Fenby, N., & Scott, R. (2003). Temperature sensitive diphtheria toxin confers conditional male-sterility in Arabidopsis thaliana. Plant Biotechnology Journal, 1, 33–42.
Kriete, G., Niehaus, K., Perlick, A., Pühler, A., & Broer, I. (1996). Male sterility in transgenic tobacco plants induced by tapetum-specific deacetylation of the externally applied non-toxic compound N-acetyl-l-phosphinothricin. The Plant Journal, 9, 809–818.
Rao, G. S., Tyagi, A. K., & Rao, K. V. (2017). Development of an inducible male-sterility system in rice through pollen-specific expression of l-ornithinase (argE) gene of E. coli. Plant Science: An International Journal of Experimental Plant Biology, 256, 139–147.
Denli, A. M., & Hannon, G. J. (2003). RNAi: An ever-growing puzzle. Trends in Biochemical Sciences, 28, 196–201.
Mansoor, S., Amin, I., Hussain, M., Zafar, Y., & Briddon, R. W. (2006). Engineering novel traits in plants through RNA interference. Trends in Plant Science, 11, 559–565.
Souza, A. J. D., Mendes, B. M. J., & Mourão Filho, F. D. A. A. (2007). Gene silencing: Concepts, applications, and perspectives in woody plants. Scientia Agricola, 64, 645–656.
Wasternack, C., & Parthier, B. (1997). Jasmonate-signalled plant gene expression. Trends in Plant Science, 2, 302–307.
Creelman, R. A., & Mullet, J. E. (1997). Biosynthesis and action of jasmonates in plants. Annual Review of Plant Biology, 48, 355–381.
Ishiguro, S., Kawai-Oda, A., Ueda, J., Nishida, I., & Okada, K. (2001). The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. The Plant Cell, 13, 2191–2209.
Sanders, P. M., Lee, P. Y., Biesgen, C., Boone, J. D., et al. (2000). The Arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. The Plant Cell, 12, 1041–1061.
McConn, M. (1996). The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. The Plant Cell, 8, 403–416.
Bae, H. K., Kang, H. G., Kim, G. J., Eu, H. J., et al. (2010). Transgenic rice plants carrying RNA interference constructs of AOS (allene oxide synthase) genes show severe male sterility. Plant Breeding, 129, 647–651.
Woo, M. O., Ham, T. H., Ji, H. S., Choi, M. S., et al. (2008). Inactivation of the UGPase1 gene causes genic male sterility and endosperm chalkiness in rice (Oryza sativa L.). The Plant Journal, 54, 190–204.
Nawaz-ul-Rehman, M. S., Mansoor, S., Khan, A. A., Zafar, Y., & Briddon, R. W. (2007). RNAi-mediated male sterility of tobacco by silencing TA29. Molecular Biotechnology, 36, 159–165.
Yui, R., Iketani, S., Mikami, T., & Kubo, T. (2003). Antisense inhibition of mitochondrial pyruvate dehydrogenase E1α subunit in anther tapetum causes male sterility. The Plant Journal, 34, 57–66.
Heiser, V., Rasmusson, A. G., Thieck, O., Brennicke, A., & Grohmann, L. (1997). Antisense repression of the mitochondrial NADH-binding subunit of complex I in transgenic potato plants affects male fertility. Plant Science, 127, 61–69.
Xu, H., Knox, R. B., Taylor, P. E., & Singh, M. B. (1995). Bcp1, a gene required for male fertility in Arabidopsis. Proceedings of the National Academy of Sciences, 92, 2106–2110.
Agati, G., Azzarello, E., Pollastri, S., & Tattini, M. (2012). Flavonoids as antioxidants in plants: Location and functional significance. Plant Science, 196, 67–76.
Agati, G., Matteini, P., Goti, A., & Tattini, M. (2007). Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytologist, 174, 77–89.
Brunetti, C., Di Ferdinando, M., Fini, A., Pollastri, S., & Tattini, M. (2013). Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. International Journal of Molecular Sciences, 14, 3540–3555.
Fini, A., Brunetti, C., Di Ferdinando, M., Ferrini, F., & Tattini, M. (2011). Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signaling & Behavior, 6, 709–711.
Fischer, R., Budde, I., & Hain, R. (1997). Stilbene synthase gene expression causes changes in flower colour and male sterility in tobacco. The Plant Journal, 11, 489–498.
Höfig, K. P., Möller, R., Donaldson, L., Putterill, J., & Walter, C. (2006). Towards male sterility in Pinus radiata—a stilbene synthase approach to genetically engineer nuclear male sterility. Plant Biotechnology Journal, 4, 333–343.
Van Der Meer, I. M., Stam, M. E., van Tunen, A. J., Mol, J., & Stuitje, A. R. (1992). Antisense inhibition of flavonoid biosynthesis in petunia anthers results in male sterility. The Plant Cell, 4, 253–262.
Covello, P. S., & Gray, M. W. (1989). RNA editing in plant mitochondria. Nature, 341, 662–666.
Gualberto, J. M., Weil, J.-H., & Grienenberger, J.-M. (1990). Editing of the wheat coxIII transcript: Evidence for twelve C to U and one U to C conversions and for sequence similarities around editing sites. Nucleic Acids Research, 18, 3771–3776.
Gualberto, J. M., Lamattina, L., Bonnard, G., Weil, J.-H., & Grienenberger, J.-M. (1989). RNA editing in wheat mitochondria results in the conservation of protein sequences. Nature, 341, 660–662.
Schuster, W., Hiesel, R., Wissinger, B., & Brennicke, A. (1990). RNA editing in the cytochrome b locus of the higher plant Oenothera berteriana includes a U-to-C transition. Molecular and Cellular Biology, 10, 2428–2431.
Hanson, M. R., & Bentolila, S. (2004). Interactions of mitochondrial and nuclear genes that affect male gametophyte development. The Plant Cell, 16, S154–S169.
Young, E. G., & Hanson, M. R. (1987). A fused mitochondrial gene associated with cytoplasmic male sterility is developmentally regulated. Cell, 50, 41–49.
Handa, H., Gualberto, J. M., & Grienenberger, J.-M. (1995). Characterization of the mitochondrial orfB gene and its derivative, orf224, a chimeric open reading frame specific to one mitochondrial genome of the “Polima” male-sterile cytoplasm in rapeseed (Brassica napus L.). Current Genetics, 28, 546–552.
Araya, A., Zabaleta E., Blanc V., Bégu D., Hernould M., Mouras A., & Litvak S. (1998). RNA editing in plant mitochondria, cytoplasmic male sterility and plant breeding. Electronic Journal of Biotechnology, 1, 06–07.
Chakraborty, A., Mitra, J., Bhattacharyya, J., Pradhan, S., et al. (2015). Transgenic expression of an unedited mitochondrial orfB gene product from wild abortive (WA) cytoplasm of rice (Oryza sativa L.) generates male sterility in fertile rice lines. Planta, 241, 1463–1479.
Hernould, M., Suharsono, S., Litvak, S., Araya, A., & Mouras, A. (1993). Male-sterility induction in transgenic tobacco plants with an unedited atp9 mitochondrial gene from wheat. Proceedings of the National Academy of Sciences, 90, 2370–2374.
De Cosa, B., Moar, W., Lee, S.-B., Miller, M., & Daniell, H. (2001). Overexpression of the Bt cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nature Biotechnology, 19, 71–74.
Ruiz, O. N., & Daniell, H. (2005). Engineering cytoplasmic male sterility via the chloroplast genome by expression of β-ketothiolase. Plant Physiology, 138, 1232–1246.
Nizampatnam, N. R., Doodhi, H., Narasimhan, Y. K., Mulpuri, S., & Viswanathaswamy, D. K. (2009). Expression of sunflower cytoplasmic male sterility-associated open reading frame, orfH522 induces male sterility in transgenic tobacco plants. Planta, 229, 987–1001.
Nizampatnam, N. R., & Kumar, V. D. (2011). Intron hairpin and transitive RNAi mediated silencing of orfH522 transcripts restores male fertility in transgenic male sterile tobacco plants expressing orfH522. Plant Molecular Biology, 76, 557–573.
Yamamoto, M. P., Shinada, H., Onodera, Y., Komaki, C., et al. (2008). A male sterility-associated mitochondrial protein in wild beets causes pollen disruption in transgenic plants. The Plant Journal, 54, 1027–1036.
Kim, D. H., Kang, J. G., & Kim, B.-D. (2007). Isolation and characterization of the cytoplasmic male sterility-associated orf456 gene of chili pepper (Capsicum annuum L.). Plant Molecular Biology, 63, 519–532.
Hird, D. L., Paul, W., Hollyoak, J. S., & Scott, R. J. (2000). The restoration of fertility in male sterile tobacco demonstrates that transgene silencing can be mediated by T-DNA that has no DNA homology to the silenced transgene. Transgenic Research, 9, 91–102.
Zabaleta, E., Mouras, A., Hernould, M., & Araya, A. (1996). Transgenic male-sterile plant induced by an unedited atp9 gene is restored to fertility by inhibiting its expression with antisense RNA. Proceedings of the National Academy of Sciences, 93, 11259–11263.
Bayer, M., & Hess, D. (2005). Restoring full pollen fertility in transgenic male-sterile tobacco (Nicotiana tabacum L.) by Cre-mediated site-specific recombination. Molecular Breeding, 15, 193–203.
Cao, B., Huang, Z., Chen, G., & Lei, J. (2010). Restoring pollen fertility in transgenic male-sterile eggplant by Cre/loxp-mediated site-specific recombination system. Genetics and molecular biology, 33, 298–307.
Marimuthu, M. P., Jolivet, S., Ravi, M., Pereira, L., et al. (2011). Synthetic clonal reproduction through seeds. Science (New York, NY), 331, 876.
Ravi, M., & Chan, S. W. (2010). Haploid plants produced by centromere-mediated genome elimination. Nature, 464, 615–618.
Acknowledgements
The authors are grateful to Council of Scientific and Industrial Research, Government of India DST-FIST, UGC-SAP, Government of India, for the facilities provided to the Department of Plant Sciences, University of Hyderabad.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest.
Rights and permissions
About this article
Cite this article
Shukla, P., Singh, N.K., Gautam, R. et al. Molecular Approaches for Manipulating Male Sterility and Strategies for Fertility Restoration in Plants. Mol Biotechnol 59, 445–457 (2017). https://doi.org/10.1007/s12033-017-0027-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-017-0027-6