Abstract
Tomato leaf curl virus (ToLCV) disease is a serious threat for tomato cultivation in the tropics and subtropics. Despite serious efforts no immune commercial varieties or F1 hybrids are available till date. In this study, the interaction between Solanum lycopersicum and ToLCV was characterized on molecular and biochemical basis. RNA silencing mediated by short interfering RNA (siRNA) and reactive oxygen species (ROS) has been proposed as central components of plant adaptation to several stresses. A comparative RNA interference study between two contrasting tomato genotypes, LA1777 (tolerant) and 15SBSB (susceptible) infected with Tomato Leaf Curl New Delhi Virus (ToLCNDV) revealed relatively higher accumulation of siRNA in the leaves of tolerant genotype. In LA1777, ToLCNDV produced chlorotic as well as necrotic areas at the inoculation sites 5–10 days post-inoculation. Caspase-9- and caspase-3-like activities were significantly increased in response to ToLCNDV infection in LA1777 at inoculated region. Activities of antioxidant enzymes involved in the detoxification of ROS were examined in both systemic and localized area of infection, and their expression level was further validated through quantitative real-time PCR of the corresponding transcripts. Expression patterns of three genes encoding pathogenesis-related proteins showed higher accumulation in tolerant genotype. Tolerance against the ToLCNDV in LA1777 can be attributed to the higher siRNA accumulation, localized cell death, altered levels of antioxidant enzymes and activation of pathogenesis-related genes at different durations of virus infection. Based on these direct and indirect evidences, we have proposed a putative mechanism for ToLCNDV tolerance in the tolerant genotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato (Solanum lycopersicum L.) is the second most important and popular vegetable crop after potatoes in the world. Tomato leaf curl disease (ToLCD), caused by the Tomato leaf curl virus, a large group of viruses, is one of the most devastating diseases of tomato, especially in tropical and subtropical climates and considered as a serious threat to tomato yield and production [1]. The typical symptoms of the infected susceptible plants are severe leaf curling, shrinking of leaves and stunted plant growth. The disease is reported to be caused by different species of genus begomovirus (Family-Geminiviridae), which are transmitted by whitefly (Bemisia tabaci Genn.). It has mono-/bi-partite genome and in some cases needs a betasatellite molecule for either establishing their infection or increasing the severity of the symptom manifestation. Monopartite begomoviruses infecting tomato have been reported from different parts of southern India [2]. On contrary, bipartite begomoviruses causing ToLCD have been reported from northern and western part of India [3]. Among these different begomoviruses, Tomato leaf curl New Delhi virus (ToLCNDV) reported from New Delhi region of India has very wide distribution and infects many other crops. The severity can vary in intensity, from very mild symptoms to very severe symptoms and plant death [4].
As viruses and hosts interact, a series of plant defense mechanisms are activated to protect themselves from viral infection, such as post-transcriptional gene silencing [5, 6], activation of hypersensitive responses (HR) and onset of systemic acquired resistance (SAR) [7]. These responses are accompanied by changes in gene-expression patterns of pathogenesis-related (PR) proteins and of several other proteins involved in cell signaling [8]. Plants can reduce virus infection by RNA silencing; a mechanism in which small RNAs direct the degradation or inhibition of larger RNA translation molecules [9, 10]. The triggering of resistance (R) gene pathway generates massive cellular ion influxes directing intracellular pH changes and an oxidative burst. This leads to the accumulation of reactive oxygen species (ROS), cell-wall thickening near the infection site, nitric oxide production, and the synthesis of antimicrobial products including phytoalexins and PR proteins [11]. The oxidative burst associated with HR includes production of ROS, which damages both the host and the pathogen. ROS accumulation causes oxidative damage through actions such as lipid peroxidation with membrane destruction, protein inactivation or DNA mutation. Fortunately, plants, similar to other aerobic organisms, are endowed with efficient ROS-scavenging mechanisms, which include both enzyme and chemical antioxidant systems.
The key antioxidant enzymes are superoxide dismutase (SOD; catalyzing the dismutation of O2 − to H2O2 and O2), catalase (CAT; dismutating H2O2 to O2 and H2O) and ascorbate peroxidase (APX; reducing H2O2 to H2O by utilizing Ascorbate) [12]. These enzymatic systems function to protect cells against oxidative damage. Moreover, other enzymes involved in the ascorbate–glutathione ASC–GSH cycle [glutathione reductase (GR) and dehydroascorbate reductase (DHAR)] as well as glutathione-S-transferases (GST) are important in protecting cells against oxidative stress [13]. Significant changes in the activities of the antioxidant enzymes have been previously detected in response to virus infection [12]. Peroxidases show increased activity in Cucurbita pepo plants infected with Cucumber mosaic virus (CMV) [14] and Zucchini yellow mosaic virus (ZYMV) [12, 15].
Keeping the above in view, in the present study, we monitor the changes in the HR-mediated programmed cell death (Caspase-9- and caspase-3-like activities), enzymatic antioxidant status, and lipid peroxidation in two contrasting genotypes of tomato in response to ToLCNDV infection. The results obtained after biochemical analyses were further validated by quantitative real-time PCR (qRT-PCR) of stress responsive genes.
Materials and Methods
Plant Growth Conditions
Tomato genotypes (LA1777 and 15SBSB) used in breeding programs aimed at introgression resistance to ToLCV from Solanum habrochaites have been obtained from Indian Institute of Horticultural Research (IIHR), Bangalore, India. Seeds were sown in pots containing agro-peat and vermiculite mixture (3:1) and kept in green house for germination. Temperature of green house was maintained at 25 ± 1°C.
Virus Treatment
Tomato genotypes LA1777 and 15SBSB were infected with Tomato Leaf Curl New Delhi Virus by agro-inoculation method. A construct of Agrobacterium tumefaciens harboring partial dimer of ToLCNDV-DNA (A and B) was agroinoculated at two-leaf stage by stem inoculation method. Wounds were made around the growing nodal region of the tomato stem by pricking it 5–6 times with 30-gaze needle. About 20 μl of Agrobacterium suspension was put on the wounds. For mock inoculation, Agrobacterium harboring only pCAMBIA vector was used. The virus inoculated, mock inoculated, and control tomato plants were kept separately in insect-free chambers of green house. Infectivity index of Tomato leaf curl New Delhi virus in genotypes LA1777 and 15SBSB is shown in Table 1.
Detection of Virus in Inoculated Plants
Detection of the viral replicon was carried out by Southern blot hybridization using a α32P dCTP-labeled ToLCNDV coat protein gene probe prepared with NEBlot kit (NEB, USA). Genomic DNA was isolated from the systemic leaves of tomato plants and separated on 0.8% (w/v) agarose gel by electrophoresis. DNA fragments from agarose gels were transferred to N+ Hybond membrane (Amersham-Pharmacia, USA), hybridized over-night at 60°C, washed at the moderate stringency conditions, and exposed to X-ray film (Kodak).
Detection of Virus-Specific siRNA
Virus-specific siRNAs were examined using the protocol described elsewhere [6]. In brief, 20 μg of low molecular weight RNA isolated from ToLCNDV- and mock-inoculated tomato plants was separated on a 15% polyacrylamide (19:1)/7 M urea gel. Separated RNAs were transferred to nylon and probed with the replication-specific gene fragments using DNA labeling kit (Roche, USA). Hybridization with the radiolabeled probes was performed for overnight at 42°C. Ethidium bromide stained 21 and 24 nucleotide long synthetic oligonucleotides were used as standard size markers.
Measurement of Caspase-Like Activity
Agroinfiltrated sites (local) and systemic leaves of control, mock treatment and ToLCNDV treatments from both genotypes were homogenized in caspase lysis buffer (G-biosciences, USA). Samples were incubated on ice for 15 min, supernatants were mixed with 50 μl of 2× caspase assay buffer with 150 μM LEHD-AFC (G-biosciences, USA) for caspase 9-like activity and 150 μM DEVD-AFC (G-biosciences, USA) for caspase-3-like activity, as peptide substrates. Fluorescence of AFC hydrolyzed from the peptide substrates was quantified by spectrofluorophotometer (Varian) using 400-nm excitation and 505-nm emission wavelengths. Enzymatic activity was normalized for protein concentration and expressed as fold activity present in control extracts.
Biochemical Assays
The level of lipid peroxidation in plant tissues (systemic and local) was determined with respect to 2-thiobarbituric acid (TBA) reactive metabolites chiefly malondialdehyde (MDA) [16]. Tissues were ground in 10 mM phosphate buffer (pH 7.4) and centrifuged, and supernatant was added to a reaction mixture containing 100 μl of 8.1% (w/v) SDS, 750 μl of 20% (w/v) acetic acid (pH 3.5), 750 μl of 0.8% (w/v) aqueous TBA, and 200 μl of distilled water. The level of lipid peroxidation was expressed as nmol of MDA formed by subtracting A600 from A535 for the non-specific turbidity using an extinction coefficient (ε) of 156/mM/cm. Glutathione Reductase (GR, EC 1.8.1.7) activity was measured spectrophotometrically by measuring the decline in A340 as NADPH (ε = 6.22/mM/cm) was oxidized [17]. The reaction mixture contained 50 mM Tris–Cl (pH 7.5), 3 mM MgCl2, 3 mM EDTA, 0.5 mM GSSG, and 0.15 mM NADPH. Guaiacol POX (EC 1.11.1.7) activity was determined spectrophotometrically by monitoring the formation of tetraguaiacol (ε = 26.6/mM/cm) from guaiacol at A470 in the presence of H2O2 [18]. The reaction mixture contained 50 mM sodium phosphate buffer (pH 6.5), 10 mM of H2O2 and was initiated by addition of 20 mM guaiacol. DHAR (EC 1.8.5.1) activity was measured by a direct spectrophotometric assay, monitoring the increase in A265 due to the formation of ascorbate from DHA [19]. The reaction mixture was composed of 5 mM GSH and 100 mM sodium phosphate buffer, pH 7.0 and 100 μg crude protein. The reaction was started by adding 0.2 mM DHA. The GST activity per individual was calculated using the published extinction coefficient (ε = 14/mM/cm). GST activity (EC 2.5.1.18) was assayed spectrophotometrically by the method of Li et al. [20]. Final substrate concentrations were 1 mM (CDNB) and 5 mM (GSH) in 50 mM sodium phosphate buffer (pH 7.5). The reaction was initiated by the addition of 1 mM GSH, and formation of S-(2,4-dinitrophenyl)glutathione was monitored in absorbance at 334. The GST activity per individual was calculated using the extinction coefficient (ε = 9.6/mM/cm).
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was isolated at 0, 5, 10, 15 and 20 dpi from the systemic leaves of ToLCNDV- and mock-inoculated tolerant (LA1777) and susceptible (15SBSB) tomato genotypes by TRIzol reagent (Sigma, USA). DNA contamination was removed from the RNA samples using RNase-free DNaseI (50 U/μl, Fermentas, USA). Total RNA (2 μg) was used to synthesize first-strand cDNA from each sample using superscript reverse transcriptase (Invitrogen) according to the supplier’s manual. The qRT-PCR was performed on a 7900HT Fast System Real-Time PCR (Applied Biosystems) using Power SYBR Green dye (Applied Biosystems). The primers for these transcripts were designed from the sequences of tomato obtained from NCBI database using Primer Express Version 3.0 (Table 2). PCR was performed for each sample in triplicate using α-tubulin, a constitutively expressed protein, as internal control (Table 2). The amount of transcript normalized to the internal control α-tubulin was analyzed using the 2−∆∆Ct method [21]. The PCR conditions were kept as 95°C for 10 min, 95°C for 15 s, 60°C for 1 min for 40 cycles, 95°C for 15 s and 60°C for 1 min and 95°C for 15 s. The experiments were repeated three times to check the reproducibility.
Statistical Analysis
The experiments were conducted in three replicates. Statistical analysis was carried out for all the measured parameters by one-way analysis of variance (ANOVA) using InStat Graphpad software. The data were presented as mean ± SD (n = 3), and significance was tested at P < 0.001.
Results and Discussion
Differential Symptom Manifestation in Tolerant and Susceptible Tomato Plants
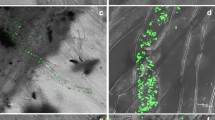
Two genotypes (LA1777 and 15SBSB) that differ in virus resistance were subjected to the symptom severity observation after ToLCNDV infection (Table 1). Leaves of susceptible genotype (15SBSB) showed symptoms of typical leaf curling and stunted growth after 5–7 dpi, whereas in LA1777, symptom development was delayed and started after 12–15 dpi. Only a few agro-injected LA1777 plants developed the disease symptoms, while the rest of plants had no typical leaf curling symptom, but the viral genomic DNA accumulation was observed in a very low amount as detected by Southern blot analysis (data not shown). In tolerant plants, necrosis development started at 5 dpi near the agro-injected site, whereas no such necrotic development was observed in susceptible plants (Fig. 1a). Additionally, to evaluate the cell death in ToLCNDV-infected nodal areas, Trypan blue staining was performed in both the genotypes (Supplementary Fig. S1). Patches of dead cell were clearly observed in the areas where the ToLCNDV infection was carried out in the tolerant genotype LA1777. Southern blot analysis was performed to evaluate the accumulation of viral genomic DNA in both genotypes. Accumulation of viral genomic components was highest at 10 dpi and further decreased to minimum until 20 dpi in LA1777 (Fig. 1b). Furthermore, in 15SBSB, the accumulation of viral genomic DNA component was higher in comparison with genotype LA1777 at 20 dpi of ToLCNDV infection (Fig. 1b). The formation of necrotic areas at the site of virus inoculation in LA1777 can be attributed to HR-induced cell death, which was not observed in 15SBSB plants. HR-induced cell death could be one reason of the delayed symptoms development in tolerant tomato plants. Hussain et al. [22] have shown previously that the nuclear shuttle protein of ToLCNDV, is an avirulence determinant, which induces HR in tomato and tobacco plants.
a, b Phenotypic and molecular analysis of ToLCNDV infection of LA1777 and 15SBSB genotypes. a Phenotypic analysis of tissue necrosis caused by Tomato Leaf Curl New Delhi Virus after inoculation in LA1777 and 15SBSB tomato plants. Arrows indicate site of agro-infiltration. b Southern blot analysis for the detection of viral particle accumulation in LA1777 and 15SBSB genotypes at different time intervals after virus inoculation. C control, dpi day post-inoculation
Short Interfering RNA Linked with Lower Viral DNA Accumulation
Emerging picture in past few years has suggested that the plant induces the virus-specific short interfering RNA accumulation as a defense response. Aiming to assess the tolerant behavior, we evaluated the virus-derived siRNA accumulation in both tomato genotypes at different time points of ToLCNDV infection. Although viral DNA accumulation was almost alike in both genotypes up to 10 dpi, variation in symptom development in the sensitive and tolerant genotypes could be correlated to their differential siRNA accumulation. The tolerant genotype LA1777 showed early accumulation of virus-derived siRNA which may be correlated to the late symptom development (Fig. 2a, b). Higher accumulation of virus-specific siRNA was also observed in tolerant genotype after 10 dpi also, which reduces the viral DNA accumulation and leads to the plant to undergo in recovery mode. On the other hand, however, no siRNA accumulation was observed in susceptible genotype at early stage even if it tended to increase gradually at later stages (Fig. 2b). Previously, we have postulated the co-relation among virus tolerance and siRNA accumulation [6]. Overall these results suggest that in LA1777, ToLCNDV tolerance is mediated by the rapid generation of siRNA.
a, b Accumulation of ToLCNDV Rep gene-specific siRNA in the tomato genotypes. a Accumulation of Rep gene-derived siRNA at different time points of infection separated on a 15% polyacrylamide/7 M urea gel; b Relative accumulation of Rep-specific siRNA. Ethidium bromide stained gel is shown for equivalent loading
Tomato Leaf Curl New Delhi Virus Infection Induces HR-Mediated Cell Death
Recent studies have shown the involvement of caspase-like proteases in control of cell death activation in plants under pathogenic and nonpathogenic responses [23, 24]. Synthetic fluorogenic substrates for caspase-3 (DEVD-AFC) and caspase-9 (LEHD-AFC) were used to detect caspase-like activity in both tomato genotypes at 10 dpi. We performed the caspase-like activity in both systemic and localized area of infection (Fig. 3a–d), but no significant difference was observed in the systemic region (Fig. 3c, d). On the other hand, localized region showed differential level of caspase-like activity in tolerant and susceptible genotypes. The activity of caspase-3 and caspase-9 significantly (P < 0.001) increased in LA1777 in comparison with 15SBSB (Fig. 3a, b). These results suggested that increased activity of caspase-3 and caspase-9 at the site of infiltration may lead to the HR-induced programmed cell death. This activation of cell death may further restrict the virus spread.
a–d Measurement of Caspase-like activity. a, b Caspase-3-like and caspase-9-like activity in locally infected nodes. c, d Caspase-3-like and caspase-9-like activity in systemic leaves. Extracts from genotypes LA1777 and 15SBSB infiltrated with ToLCNDV (V) along with the control (C) and mock (M) treatments were incubated with peptide substrates of the caspase-9 (LEHD-AFC) and caspase-3 (DEVD-AFC) in the caspase assay buffer, and their relative fluorescence were measured. Asterisks indicate the level of significance (P < 0.001)
Effect on Lipid Peroxidation and Antioxidant Activity in Systemic and Local Infection Caused by Tomato Leaf Curl New Delhi Virus
Lipid peroxidation (LP) products are produced during oxidative burst within the plants. The virus inoculation altered LP both locally (in the ToLCNDV-inoculated node) and systematically (leaves) with disease symptoms being associated with significantly (P < 0.001) high LP. The systemic leaves of 15SBSB accumulated 2.0-fold higher MDA at 5 dpi in comparison with LA1777 leaves, in which MDA content increased at 10 dpi (Fig. 4a). In a study with Cocksfoot mottle virus, Li and Burritt [25] had observed a four times increased LP in susceptible plants of Dactylis glomerata 7 days after virus inoculation.
a–e Biochemical analysis in local (L) and systemic (S) ToLCNDV-infected tissues of LA1777 and 15SBSB genotypes. a Lipid peroxidase (LP). b Glutathione reductase (GR). c Peroxidase (POX). d Dehydroascorbate reductase (DHAR). e Glutathione-s-transferase (GST). Bars indicate the standard deviations (±SD, n = 3). Asterisks indicate the level of significance (P < 0.001). M Mock, S Systemic, L Local
Glutathione reductase serve as key enzymes that help to maintain the cellular redox status of the plants by converting oxidized glutathione (GSSG) to reduced form (GSH) using NADPH as reducing power. The cellular membranes are protected from LP by consumption and degradation of GSH [26]. At 10 and 15 dpi, a significantly (P < 0.001) increased GR activity was observed in virus-infected systemic leaves of LA1777 as compared to 15SBSB (Fig. 4b). Hernández et al. [27] have reported increased GR activity in resistant cultivar of apricot (Prunus armeniaca) and not in susceptible cultivar after Plum pox virus (PPV) infection.
The activity of another antioxidant enzyme, guaicol peroxidase, of the peroxidase family, was significantly increased (P < 0.001) at 10 and 15 dpi in LA1777 in contrast to susceptible genotype (Fig. 4c). Peroxidases were reported to be involved in resistance of Capsicum annuum to Cucumber mosaic virus [28]. Similar pattern of results was obtained in a study where increase in the peroxidase activity was correlated to the resistance in Phaseolus vulgaris against White clover mosaic virus [29]. Nadlong and Sequeira [30] also observed significant increase in peroxidase activity in Vigna mungo plants after inoculation with Urdbean leaf crinkle virus, respectively. Such results suggested that the increased POX-activity following virus infection was a reflection of physiological changes associated with, but not responsible for, induced resistance. The up-regulated peroxidases might be responsible for growth reductions and malformations in virus-infected plants [31].
A significant increase (P < 0.001) in DHAR activity was observed in 10 and 15 dpi in virus-infected 15SBSB plants in comparison with ToLCNDV-treated LA1777 and mock-treated 15SBSB (Fig. 4d). These results suggest that DHAR might contribute to the inhibition of HR-type necroses in 15SBSB. Our result was further supported by a previous observation, which showed that DHAR-overexpressing tobacco and maize plants exhibit elevated levels of reduced ascorbate and glutathione [32]. Glutathione imparts the reducing power to DHAR for recycling ascorbate that performs a crucial role in the detoxification of ROS [33]. It has been demonstrated that exogenously applied ascorbate could be linked to the elimination of necrosis during viral HR [34].
Glutathione is an integral part of an antioxidant system that includes GST and GR enzymes protecting plants from ROS which induces stress response. The overall GST activity was higher in ToLCNDV-inoculated LA1777 plants in comparison with virus-inoculated 15SBSB plants. The GST activity was significantly (P < 0.001) higher in virus-infected LA1777 at 15 and 20 dpi, respectively, in comparison with virus-infected 15SBSB genotypes at the same stages (Fig. 4e). Additionally, a decreased GST activity has been observed in ToLCNDV-inoculated 15SBSB in comparison with mock-inoculated 15SBSB, and the trend was maintained at all the stages of experiment. Similar observation was also reported in SAR against Tobacco mosaic virus (TMV) infection in tobacco [35].
A comparative study between local and systemic sites of infection was also performed (Fig. 4a–e). Relatively, more MDA was formed in locally infected tissues of LA1777 than in systemic tissues. Furthermore, this activity was also higher as compared to the same tissues from the sensitive one. Although no significant changes in DHAR activity were observed, the LA1777 local tissues maintained significantly (P < 0.001) lower activity of other antioxidants: GR, POX, and GST, in contrast to the systemic leaves as well as the nodal tissues of 15SBSB. The significantly (P < 0.001) enhanced LP activity at the local sites of infection could be correlated with increased ROS leading to advanced disintegration of membranes in infected area. Along with this, the lower antioxidant levels diminish the ROS scavenging capacity at that site. The increased ROS and membrane damage may permit cell death thereby checking further viral spread in the LA1777 genotype. However, many reports describe contradictory results with both induction and inhibition of antioxidant enzyme activities involved in free radical scavenging [29, 31, 36]. Our results reveal the complex nature of antioxidant enzymes as they are differentially expressed in both genotypes of tomato upon ToLCNDV infection.
Expression Analysis of Pathogen-Related Genes and ROS Scavenging Enzymes
Quantitative RT-PCR was performed to evaluate the expression of defense-related gene in genotype LA1777 (ToLCNDV tolerant) and 15SBSB (ToLCNDV susceptible) at 0, 5, 10, 15, and 20 dpi (Fig. 5a–g). The accumulation of PR1-protein transcripts started at 10 dpi and reached maximum at 15 dpi in LA1777 in comparison with 15SBSB after virus inoculation. The transcripts were more than 2-fold higher in LA1777 in comparison with 15SBSB at 15 dpi (Fig. 5a). A study by Fraser [37] reveals that PR proteins accumulate systemically in tobacco after infection with Tobacco mosaic virus. The Arabidopsis cpr1 mutants, overexpressing PR1, were less susceptible to Cabbage leaf curl virus infection [38]. Love et al. [39] have shown that delayed symptom phenotype for cpr1 plants infected with Cauliflower mosaic virus has a double-stranded DNA genome. The result suggested that increased level of PR1 accumulation can be correlated to the tolerance to ToLCNDV in tomato. At 15 dpi, the level of PR5 transcript accumulation was highest (>9 fold) in the ToLCNDV-tolerant genotype LA1777 in comparison with the mock LA1777 (Fig. 5b). ToLCNDV-susceptible 15SBSB also showed up-regulation (5.6 fold) in contrast to the mock 15SBSB (Fig. 5b). We have previously reported that a ToLCNDV-tolerant cv. H-88-78-1 showed differential expression of PR6 [6]. Keeping this in view, level of PR6 was also examined for the expression pattern in both genotypes with mock and ToLCNDV treatments. The level of PR6 transcript was up-regulated at 5 and 10 dpi in the ToLCNDV-tolerant genotype LA1777 (Fig. 5c). Induction of PR genes showed differential pattern of PRs, with PR5 expressing more at 10 to 15 dpi, while PR6 inducing early in ToLCNDV-tolerant genotype LA1777. These results suggest that the time-dependent expression pattern of pathogen-related genes in the tolerant genotype may have some plausible role in providing resistance/tolerance to ToLCNDV.
a–g qRT-PCR analyses of the expression of transcripts in response to Tomato leaf curl New Delhi virus (ToLCNDV) infection in tolerant (LA1777) and susceptible (15SBSB) genotypes. a Pathogen-related protein 1 (PR1). b Pathogen-related protein 5 (PR5). c Pathogen-related protein 6 (PR6). d Peroxidase (POX). e Glutathione reductase (GR). f Dehydroascorbate reductase (DHAR). g Glutathione-s-transferase (GST). Bars indicate the standard deviations (±SD, n = 3). Asterisks indicate the level of significance (P < 0.001). M Mock, V Virus-inoculated
Plants can also produce H2O2 using peroxidases and oxidases whose expression can be regulated by biotic and abiotic stimuli [40]. Therefore, we evaluated the expression of a peroxidase gene (POX), which had been reported as inducible by Xanthomonas campestris in pepper [41]. In LA1777, more than 2-fold induction was observed at all time points of infection in comparison with 15SBSB (Fig. 5d). The level of GR transcripts was also examined, which showed the differential expression pattern in both the tomato genotypes in a time-dependent manner. Increasing transcript accumulation was observed in the ToLCNDV-infected LA1777, which showed maximum induction (~3 fold) in comparison with LA1777 mock. The level of GR transcripts in ToLCNDV-susceptible genotype 15SBSB was also observed but was lower than the accumulation in ToLCNDV-infected genotype LA1777 (Fig. 5e). DHAR is an important enzyme involved in ROS management and ascorbate-glutathion cycle. In this study, more than 3-fold higher expression level was observed in susceptible 15SBSB than in tolerant LA1777 after virus inoculation at 10 dpi, which was in agreement to DHAR enzyme assay (Fig. 5f). Activity of GST was also cross-confirmed by examining the expression pattern of GST in tomato genotypes LA1777 and 15SBSB. Significant difference (P < 0.001) was observed at 15 dpi in ToLCNDV-infected LA1777 where expression level of GST was highest (Fig. 5g). Our findings are in accordance with a previous report by Fodor et al. [35] where increased GST activity was found in response to TMV infection in tobacco.
Based on these experimental evidences, we have proposed a possible mechanism for ToLCNDV tolerance in LA1777 (Fig. 6). Apart from enzymatic action, siRNA-mediated defense represent the major defense pathway against the viruses. Further, activation of defense gene (such as PRs) and cell death are other phenomenon which renders LA1777 genotype tolerant to virus infection.
Conclusion
The production of ROS in plant cells in response to virus infection is well documented. ROS metabolism induces many changes in antioxidant enzyme systems which are still under discussion for either compatible or incompatible plant–virus interactions. On the basis of results obtained in this study, following conclusions and hypothesis could be postulated: (i) early accumulation of virus-derived siRNA may be linked to the late symptom appearance in the tolerant genotype; (ii) at the site of infection, plant activates the HR and directs the cell to undergo PCD by action of the plant caspase-like proteins. This restricts the viral movement toward healthy tissues; (iii) in response to ToLCNDV infection, oxidative stress occurred which was followed by the activation of differential antioxidant response. This study also corroborates differential expression patterns of genes like PR proteins in tomato subjected to Tomato leaf curl virus infection. The data herewith also coincided with the promising opinion that virus infection induced an intense transcriptional reprogramming in compatible and incompatible host plants, including key defense responses. Further, functional analyses will be necessary to confirm these hypotheses and to define in a more specific way the role of these differentially expressed genes in the virus–host interaction.
Abbreviations
- ToLCNDV:
-
Tomato Leaf Curl New Delhi Virus
- siRNA:
-
Short interfering RNA
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
References
Green, S. K., & Kalloo, G. (1994). Leaf curl and yellowing viruses of pepper and tomato: An overview. Technical Bulletin 21. Shanhua: Asian Vegetable Research and Development Center.
Muniyappa, V., Venkatesh, H. M., Ramappa, H. K., Kulkarni, R. S., Zeidan, M., Tarba, C. Y., et al. (2000). Tomato yellow leaf curl virus from Bangalore (ToLCV-Ban4): Sequence comparison with Indian ToLCV isolates, detection in plants and insects, and vector relationships. Archives of Virology, 145, 1583–1598.
Chakraborty, S., Pandey, P. K., Banerjee, M. K., Kalloo, G., & Fauquet, C. M. (2003). Tomato leaf curl Gujarat virus, a new begomovirus species causing a severe leaf curl disease of tomato in Varanasi, India. Phytopathology, 93, 1485–1495.
Krause-Sakate, R., Redondo, E., Richard-Forget, F., Jadao, A. S., Houvenaghel, M. C., German-Retana, S., et al. (2005). Molecular mapping of the viral determinants of systemic wilting induced by a Lettuce mosaic virus (LMV) isolate in some lettuce cultivars. Virus Research, 109, 175–180.
Ding, S. W., & Voinnet, O. (2007). Antiviral immunity directed by small RNAs. Cell, 130, 413–426.
Sahu, P. P., Rai, N. K., Chakraborty, S., Singh, M., Prasanna, H. C., Ramesh, B., et al. (2010). Tomato cultivar tolerant to Tomato leaf curl New Delhi virus infection induces virus-specific short interfering RNA accumulation and defense-associated host gene expression. Molecular Plant Pathology, 11, 531–544.
Seo, Y. S., Gepts, P., & Gilbertson, R. L. (2004). Genetics of resistance to the geminivirus, Bean dwarf mosaic virus, and the role of the hypersensitive response in common bean. Theoretical Applied Genetics, 108, 786–793.
Culver, J. N., & Padmanabhan, M. S. (2007). Virus-induced disease: Altering host physiology one interaction at a time. Annual review of Phytopathology, 45, 221–243.
Carr, J. P., Lewsey, M. G., & Palukaitis, P. (2010). Signaling in induced resistance. Advances in Virus Research, 76, 57–121.
Llave, C. (2010). Virus-derived small interfering RNAs at the core of plant-virus interactions. Trends in Plant Science, 15, 701–707.
Cohn, J., Sessa, G., & Martin, G. B. (2001). Innate immunity in plants. Current Opinion in Immunology, 13, 55–62.
Radwan, D. E. M., Fayez, K. A., Mahmoud, S. Y., Hamad, A., & Lu, G. (2006). Salicylic acid alleviates growth inhibition and oxidative stress caused by Zucchini yellow mosaic virus infection in Cucurbita pepo leaves. Physiology & Molecular Plant Pathology, 69, 172–181.
Noctor, G., & Foyer, C. H. (1998). Ascorbate and glutathione: Keeping active oxygen under control. Annual Review of Plant Physiology Plant Molecular Biology, 492, 249–279.
Técsi, L. I., Smith, A. M., Maule, A. J., & Leegood, R. C. (1996). A spatial analysis of physiological changes associated with infection of cotyledons of marrow plants with cucumber mosaic virus. Plant Physiology, 111, 975–985.
Radwan, D. E. M., Fayez, K. A., Mahmoud, S. Y., Hamad, A., & Lu, G. (2007). Physiological and metabolic changes of Cucurbita pepo leaves in response to zucchini yellow mosaic virus (ZYMV) infection and salicylic acid treatments. Plant Physiology & Biochemistry, 45, 480–489.
Hodgson, R. A. J., & Raison, J. K. (1991). Lipid peroxidation and superoxide dismutase activity in relation to photo-inhibition induced by chilling in moderate light. Planta, 185, 215–219.
Schaedle, M., & Bassham, J. A. (1977). Chloroplast glutathione reductase. Plant Physiology, 59, 1011–1012.
Chance, B., & Maehley, A. (1955). Assay of catalases and peroxidases. Methods in Enzymology, 2, 764.
Stasolla, C., & Yeung, E. C. (2001). Ascorbic acid metabolism during white spruce somatic embryo maturation and germination. Physiologia Plantarum, 111, 196–205.
Li, Z. S., Zhen, R. G., & Rea, P. A. (1995). 1-chloro-2,4-dinitrobenzene-elicited increase in vacuolar glutathione-S-conjugate transports activity. Plant Physiology, 109, 177–185.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(−∆∆C (T)) method. Methods, 25, 402–408.
Hussain, M., Mansoor, S., Iram, S., Fatima, A. N., & Zafar, Y. (2005). The nuclear shuttle protein of Tomato leaf curl New Delhi virus is a pathogenicity determinant. Journal of Virology, 79, 4434–4439.
del Pozo, O., & Lam, E. (1998). Caspase and programmed cell death in the hypersensitive response of plants to pathogens. Current Biology, 8, 1129–1132.
Mlejnek, P., & Prochazka, S. (2002). Activation of caspase-like protease and induction of apoptosis by isopentenyladenosine tobacco BY-2 cells. Planta, 215, 158–166.
Li, Z., & Burritt, D. J. (2003). The influence of Cocksfoot mottle virus on antioxidant metabolism in the leaves of Dactylis glomerata L. Physiology & Molecular Plant Pathology, 62, 285–295.
Wise, R. R., & Naylor, A. W. (1987). Chilling-enhanced photooxidation. Plant Physiology, 83, 278–282.
Herna’ndez, J. A., Di’az-Vivancos, P., Rubio, M., Olmos, E., Ros-Barcelo, E., & Marti’nez-Go’mez, P. (2006). Long-term plum pox virus infection produces an oxidative stress in a susceptible apricot, Prunus armeniaca cultivar but not in a resistant cultivar. Physiologia Plantarum, 126, 140–152.
Riedle-Bauer, M. (1998). Activities of antioxidant enzymes in cucumber plants infected with Cucumber mosaic virus. Phyton-Annales Rei Botanicae, 38, 149–157.
Clarke, P. L., Guy, D. J., & Burritt, P. E. (2002). Changes in the activities of antioxidant enzymes in response to virus infection and hormone treatment. Physiologia Plantarum, 114, 157–164.
Nadlong, L., & Sequeira, L. (1980). Increases in peroxidase activities are not directly involved in induced resistance to Tobacco mosaic virus. Physiology & Molecular Plant Pathology, 16, 1–8.
Riedle-Bauer, M. (2000). Role of reactive oxygen species and antioxidant enzymes in systemic virus infections of plants. Journal of Phytopathology, 148, 297–302.
Chen, Z., Todd, E., Young, J. L., Su-Chih, C., & Daniel, R. G. (2003). Increasing vitamin C content of plants through enhanced ascorbate recycling. Proceedings of the National Academy of Sciences of the United States of America, 100, 3525–3530.
Asada, K. (1999). The water–water cycle in chloroplasts: Scavenging of active oxygen, dissipation of excess photons. Annual Review of Plant Physiology Plant Molecular Biology, 50, 601–639.
Farkas, G. L., Király, Z., & Solymosi, F. (1960). Role of oxidative metabolism in the localization of plant viruses. Virology, 12, 408–421.
Fodor, J., Gullner, G., Adam, A. L., Barna, B., Koemives, T., & Kiraly, Z. (1997). Local and systemic responses of antioxidants to Tobacco mosaic virus infection and to salicylic acid in tobacco. Plant Physiology, 114, 1443–1451.
Hao, Z., Wang, L., He, Y., Liang, J., & Tao, R. (2011). Expression of defense genes and activities of antioxidant enzymes in rice resistance to rice stripe virus and small brown planthopper. Plant Physiology & Biochemistry, 49, 744–751.
Fraser, R. S. S. (1982). Are ‘pathogenesis-related’ proteins involved in acquired systemic resistance of tobacco plants to tobacco mosaic virus? Journal of General Virology, 5, 305–313.
Bowling, S. A., Guo, A., Cao, H., Gordon, S., Klessig, D. F., & Dong, X. (1994). A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell, 6, 1845–1857.
Love, A. J., Laval, V., Geri, C., Laird, J., Tomos, D. A., Hooks, M. A., et al. (2007). Components of Arabidopsis defense- and ethylene-signaling pathways regulate susceptibility to Cauliflower mosaic virus by restricting long-distance movement. Molecular Plant Microbe Interaction, 20, 659–670.
Apel, K., & Hirt, H. (2004). Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55, 373–399.
Choi, H. W., Kim, Y. J., Lee, S. C., Hong, J. K., & Hwang, B. K. (2007). Hydrogen peroxide generation by the pepper extracellular peroxidase CaPO2 activates local and systemic cell death and defense response to bacterial pathogens. Plant Physiology, 145, 890–904.
Acknowledgements
We are grateful to the Director, National Institute of Plant Genome Research for providing facilities; to the Council for Scientific and Industrial Research, Govt. of India for providing a Senior Research Fellowship to Mr. Neeraj K Rai; to Dr. S. Chakraborty, SLS, JNU, New Delhi, India, for providing virus construct for agroinoculation and to Dr. A. T. Sadashiva, IIHR, Bangalore, India, for seed material. We gratefully acknowledge the financial support from the Department of Biotechnology, Government of India (Grant no. BT/PR/5274/AGR/16/464/2004).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
12033_2011_9481_MOESM1_ESM.doc
Supplementary Fig. S1 Trypan blue stained nodes of LA1777 and 15SBSB genotypes infected with Tomato leaf curl New Delhi virus at different day post-inoculation. (DOC 1518 kb)
Rights and permissions
About this article
Cite this article
Sahu, P.P., Rai, N.K., Puranik, S. et al. Dynamics of Defense-Related Components in Two Contrasting Genotypes of Tomato Upon Infection with Tomato Leaf Curl New Delhi Virus . Mol Biotechnol 52, 140–150 (2012). https://doi.org/10.1007/s12033-011-9481-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-011-9481-8