Abstract
SMARCB1/INI1 deficiency is seen in several malignant tumors including malignant rhabdoid tumor (MRT), a highly aggressive pediatric malignancy. Loss of SMARCB1/INI1 function alters diverse oncogenic cellular signals, making it difficult to discover effective targeting therapy. By utilizing an in vitro drug screening system, effective therapeutic agents against SMARCB1/INI1-deficient tumors were explored in this study. In the in vitro drug sensitivity test, 80 agents with various actions were screened for their cytotoxicity in a panel of five SMARCB1/INI1-deficient tumor cell lines. The combination effect was screened based on the Bliss independent model. The growth-inhibitory effect was determined in both the conventional two-dimensional culture and the collagen-embedded three-dimensional culture system. Survivin expression after agent exposure was determined by Western blot analysis. All five cell lines were found to be sensitive to YM155, a selective survivin inhibitor. In the drug combination screening, YM155 showed additive to synergistic effects with various agents including chrysin. Chrysin enhanced YM155-induced apoptosis, but not mitochondrial depolarization upon exposure of SMARCB1/INI1-deficient tumor cells to the two agents for 6 h. YM155 and chrysin synergistically suppressed survivin expression, especially in TTN45 cells in which such suppression was observed as early as 6 h after exposure to the two agents. Survivin is suggested to be a therapeutic target in MRT and other SMARCB1/INI1-deficient tumors. Chrysin, a flavone that is widely distributed in plants, cooperatively suppressed survivin expression and enhanced the cytotoxicity of YM155.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant rhabdoid tumor (MRT) is a rare pediatric tumor affecting various anatomic sites such as the kidney (rhabdoid tumor of the kidney), brain (atypical teratoid/rhabdoid tumor), or soft tissues. MRT is a highly aggressive tumor. Long-term survival can be expected after complete surgical resection; however, the prognosis of patients with unresectable tumors or metastatic diseases is extremely poor with an expected long-term survival rate of less than 10% among patients with soft tissue MRT who were treated nonoperatively [1, 2]. MRT may initially respond to chemotherapy to some degree; however, it eventually acquires resistance [3].
Loss of expression of SMARCB1/INI1 protein is a characteristic feature of MRT, and pathological determination of INI1 expression in the tumor is useful for its diagnosis [4]. Loss of SMARCB1/INI1 protein expression is not specific for MRT; it is seen in other tumors as well, including some cases of epithelioid sarcoma [5]. SMARCB1/INI1 has been shown to act as a tumor suppressor gene, and loss of function of both alleles gives rise to SMARCB1/INI1-deficient tumors [6, 7]. Loss of SMARCB1/INI1 function leads to dysregulation of several cellular processes associated with oncogenesis such as the CDK4/CDK6/cyclinD1, Sonic Hedgehog pathway, and WNT/β-catenin pathway. Alterations in multiple cell signal pathways resulting from the loss of SMARCB1/INI1 function hamper the development of a specific signal inhibition therapy for MRT and other SMARCB1/INI1-deficient tumors.
Survivin is a member of the inhibitor of apoptosis (IAP) family of proteins, and is highly expressed in a broad range of solid tumors including childhood cancers such as ependymoma, malignant peripheral nerve sheath tumor, and hepatoblastoma [8,9,10]. Survivin has been identified in different cellular subfractions conferring various cellular processes including proliferation and maturation. Mitochondrial survivin plays a role in protecting cells from apoptosis [11]. Because survivin is not expressed in differentiated normal tissue, survivin can be one of the candidates as a therapeutic target of cancers. Survivin expression was reported in rhabdoid tumor of the kidney [12]; however, it has not been evaluated in other SMARCB1/INI1-deficient tumors.
In this study, we examined the in vitro growth-inhibitory effects of 80 agents including YM155, a survivin inhibitor, in five cell lines derived from MRT or other SMARCB1/INI1-deficient tumors. We also evaluated the combined effects of YM155 and other agents to discover a new therapeutic approach against SMARCB1/INI1-deficient tumors.
Materials and methods
Cell lines and cell culture
TTN45, RTK (GIF), and RTK (J)-4N are cell lines derived from tumors that were clinically diagnosed as MRT [13]. YCUS-5 derived from epithelioid sarcoma has been reported previously [14]. KCS1 is a cell line that we newly established from the recurrent tumor in the mediastinum of a 4-year-old girl. The tumor in this patient was initially considered to be pleuropulmonary blastoma. However, the typical pathological features of pleuropulmonary blastoma were not seen in the recurrent tumor, and it was pathologically diagnosed as an INI1-deficient tumor without rhabdoid feature. We confirmed loss of SMARCB1 expression by RNAseq in these five cell lines (data not shown). Cells were maintained in RPMI1640 medium (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) supplemented with 10% fetal bovine serum (FBS) in an atmosphere with 5% CO2 at 37 °C.
Reagents
YM155 was purchased from Selleck Chemicals (Houston, TX, USA) and dissolved in sterile water (to a final stock solution concentration of 200 μM). Chrysin dimethylether (chrysin), which was purchased from EXTRASYNTHESE S.A. (Lyon, France), was dissolved in DMSO (to a final stock solution concentration of 10 mg/ml). Stock solutions were stored at − 80 °C.
Drug sensitivity screening
Drug sensitivity screening, which we previously performed in leukemic cells, was performed to evaluate the drug sensitivity of SMARCB1/INI1-deficient tumor cell lines [15]. Briefly, 80 agents with several different classes of action were dissolved in DMSO or deionized water according to the manufacturer’s instructions, and diluted in FBS-free RPMI1640. Ten μl of agent-containing medium and its 5−1, 5−2, and 5−3 serially diluted media were loaded in a 384-well plate (drug-store plate). In the control wells, RPMI1640 without agent was added. The list of agents and their highest concentrations used in the assay are shown in Supplemental Table 1.
Cells were suspended in RPMI1640 medium with 20% FBS at a concentration of 1 × 105 live cells/ml, and 10 μl of the cell suspension was injected into each well of a 384-well plate (cell-culture plate). After 1-day incubation in a humidified environment at 37 °C under 5% CO2, the agent-containing medium was transferred to the cell-culture plate from the agent-store plate at 10 μl/well. After incubation in a humidified environment at 37 °C under 5% CO2 for 3 days, the cell viability in each well was measured using the CellTiter-Glo luminescent assay (Promega, Madison, WI, USA).
The effect of the drug or agent was expressed as the drug effect score (DES) proposed by Szulkin et al. as previously reported [15, 16]. DES was calculated based on the different degrees of sensitivity at each different concentration, by weighted counting of the survival percentage and the drug concentration as follows: DES = {(100 − % survival at 5−3 dilution)*ln(125) + (100 − % survival at 5−2 dilution)*ln(25) + (100 − % survival at 5−1 dilution)*ln(5) + (100 − % survival at no dilution)}/{ln(125) + ln(25) + ln(5) + 1}. The reference DES is the mean value when the assay was performed using peripheral blood mononuclear cells from healthy volunteers. The cell line was considered to be sensitive to a drug or agent when its DES was larger than the corresponding reference DES.
Drug combination assay
To screen the effects of the combination of YM155 with other agents, cell lines were applied onto 2 wells of 384-well plates for drug sensitivity screening. In one of the wells, YM155 at 10 nM (final concentration) and the partner agent were added; in the other well, only the partner agent was added. Cell survival in each well was measured as described above. For the validation study, cell survival after exposure to YM155 at eight serially diluted concentrations with or without chrysin at 4 μg/ml was measured in the same way. The effect of the combination of the two agents was expressed as the combination index (CI) based on the Bliss independence model, which is one of the most popular models to assess the combined effects of drugs [17]. The Bliss independence model has a limitation that the model does not take into account heterogeneity of drug actions; however, the methodological simplicity is suitable for the screening of the combined effects. The CI was calculated as follows: CI = (EA + Eb − EAEb)/EA+b, where EA or Eb is the effect (1 − survival rate) of agent A or B at concentration a or b, respectively, and EA+b is the effect of the combination of agent A at concentration a and agent B at concentration b. When the CI was equal to, less than, or greater than 1.0, the combination was judged to be additive (CI = 1.0), synergistic (CI < 1.0), or antagonistic (CI > 1.0), respectively.
Collagen-gel-embedded three-dimensional culture of cell lines
Recent studies suggest that the three-dimensional (3D) culture system may provide a better tool to mimic physiological drug function [18, 19]. The growth-inhibitory effects of YM155 and chrysin were evaluated in the collagen-gel-embedded 3D-culture, using a collagen gel culture kit (Nitta Gelatin, Osaka, Japan). Collagen gel was constituted by a mixture of Cellmatrix I-A, ten times Ham’s F-12 medium, and reconstruction buffer at a ratio of 8:1:1. Cells were suspended in the collagen gel at a density of 2 × 105 cells/ml, and 50 μl of the gel was injected into each well of a Falcon 96-well plate (Corning, Corning, NY, USA). After semi-solidification of the gel by incubation at 37 °C for 20 min, 40 μl of RPMI1640 medium with 20% FBS was added in each well. After 1-day incubation, 10 μl of medium containing serially diluted YM155 and/or chrysin at 4 μg/ml was added onto each well. After incubation for 72 h, cell survival was evaluated by the CellTiter-Glo luminescent assay.
Apoptotic change of nuclei after agent exposure
Cells were seeded onto a 24-well glass bottom plate (IWAKI, Shizuoka, Japan) at a density of 5 × 104 cells/500 μl with control medium (0.1% DMSO), medium containing YM155 alone at 10 nM, medium containing chrysin alone at 4 μg/ml, or medium containing a combination of YM155 at 10 nM and chrysin at 4 μg/ml. These concentrations were determined by selecting the concentration that caused 50% growth inhibition in the drug sensitivity screening test. After incubation for 48 h, cellular nuclei were stained with NucBlue Live ReadyProbes Reagent (ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s instructions. Cells were observed for apoptotic change of nuclei under fluorescence microscopy (KEYENCE, Osaka, Japan).
JC-1 assay
To evaluate mitochondrial damage after agent exposure, the MitoPT JC-1 assay (ThermoFisher Scientific, Waltham, MA) was performed. Cells were seeded onto a 24-well glass bottom plate at a density of 5 × 104 cells/500 μl. After incubation for 6 h in control medium (0.1% DMSO), medium containing 2-[2-(3-chlorophenyl) hydrazinylyidene] propanedinitrile (CCCP; as an inducer of mitochondrial depolarization) at 50 μM, medium containing YM155 alone at 10 nM, medium containing chrysin alone at 4 μg/ml, or medium containing the combination of YM155 and chrysin at the indicated concentrations, cells were stained with MitoPT JC-1 at 37 °C for 30 min. Because mitochondria depolarization is indicated by a decrease in red fluorescence and an increase in green fluorescence in the JC-1 assay, the numbers of intact (JC-1 red) and damaged (JC-1 green) cells were counted under fluorescence microscopy. The analysis by JC-1 was performed in triplicate.
Western blotting
Cells were seeded onto a 6-well plate at a density of 1 × 105 cells/ml. Then, YM155 at 10 nM, chrysin at 4 μg/ml, or their combination was added to each well. DMSO at 0.1% without agents was added in the control wells. Cells were incubated for 6 or 24 h. Cellular proteins were extracted in RIPA buffer (ThermoFisher Scientific). Twenty-five μg of protein was loaded onto 4–12% SDS–PAGE gels and blotted onto a nitrocellulose membrane using the iBlot 2 Dry Blotting System (ThermoFisher Scientific). Blots were blocked and probed with antibodies against survivin (1:500 dilution; Abcam, Cambridge, UK) or with antibodies against β-actin (1:10,000 dilution; Sigma‑Aldrich, St. Louis, MO, USA). The blots were incubated with horseradish peroxidase-conjugated secondary antibodies and detected using iBind Western System (ThermoFisher Scientific). Finally, the protein bands were scanned with a Gel imaging Instrument (KURABO, Osaka, Japan).
Proteins extracted from Jurkat cells were used as a positive control for survivin (data not shown).
Statistical analysis
The statistical significance of differences between the control and treated samples was evaluated by Student’s t test. Statistical analyses were performed using the analysis function of SigmaPlot 14 software (Systat Software Inc., San Jose, CA).
Results
Drug sensitivity screening
In the high-throughput drug sensitivity screening, a cell line was considered as being sensitive to the tested agent when its DES was larger than the corresponding reference DES (the value in peripheral blood mononuclear cells obtained from healthy volunteers). We found that all cell lines were sensitive to several agents as shown in supplemental Fig. 1. In Table 1, the mean DES among five cell lines for each drug after subtracting the corresponding reference DES is presented. Among the tested agents, YM155, a survivin inhibitor, and topotecan, a topoisomerase inhibitor, were potentially effective agents with the relatively high DES and with the broadest spectrum. As the therapeutic benefit of topotecan has been well established in refractory or recurrent solid tumors of children [20, 21], we proceeded with further examination of YM155 to explore its therapeutic potential for SMARCB1/INI1-deficient tumors in this study.
Dose response curves of YM155 with or without Chrysin in the 2D or 3D-culture system. a To validate the combination effect between YM155 and chrysin, cell lines were applied onto 2 of 384-well plates, in one of which chrysin was added to each tested well at 4 μg/ml (final concentration). YM155 at eight serially diluted concentrations was loaded onto the wells, and cell survival in each well was measured by CellTiter-Glo luminescent assay. An open or closed circle in the figure indicates cell survival with or without chrysin, respectively. b The growth-inhibitory effects of YM155 and chrysin were evaluated in the collagen-gel-embedded 3D-culture, using a collagen gel culture kit (Nitta Gelatin, Osaka, Japan). Cells suspended in the collagen gel were injected into each well of a 96-well plate. After 1-day incubation, medium containing serially diluted YM155 and/or chrysin at 4 μg/ml was added onto each well. After 72 h incubation, cell survival was evaluated by the CellTiter-Glo luminescent assay
Drug combination screening
The combination therapy by cytotoxic chemotherapy and targeting drugs is a promising approach for cancer therapy. Thus, as the next step, we screened the combination effects of YM155 with other agents. The CIs between a agent at four serially diluted concentrations and YM155 at 10 nM were calculated based on the Bliss independence model. As a result (Supplemental Fig. 2, Table 2), the CI values were around 1.0, including some values that were less than 1.0, in all 79 tested agents, suggesting that combined use of YM155 may provide additive to synergistic effects to a wide range of anticancer agents. Among those agents that had additive to synergistic combination effects with YM155 in the screening assay, we next focused on chrysin as a partner of YM155, because chrysin was previously shown to antagonize the cytotoxic effects of various anticancer drugs [22].
To validate the results of the drug combination screening, the combination effect of YM155 and chrysin was evaluated using the CellTiter-Glo luminescent assay (Fig. 1a). MRT and epithelioid sarcoma cells were seeded onto a 384-well plate, and then exposed to 1.5 times serially diluted YM155 at concentrations of up to 40 nM, with or without chrysin at 4 μg/ml. The CIs of YM155 and chrysin were around 1.0 across the tested YM155 concentrations in all cell lines but there were several combinations that were judged as synergistic (CI < 1.0). The CIs at each concentration of YM155 and chrysin at 4 μg/ml are shown in Table 3. The combination effect of YM155 and chrysin was also evaluated by collagen-gel-embedded 3D-culture assay (Fig. 1b). Compared to the two-dimensional (2D) culture, the combination effects of YM155 and chrysin in the 3D-culture system were not consistent. In RTK (J)-4N and KCS1 cells, the combination effects were considered to be rather antagonistic (CI > 1.0) at lower concentrations of YM155. However, otherwise, the CIs were around 1.0 or < 1.0, especially in TTN45 or RTK (GIF), in which the CIs were less than 1.0 at all tested concentrations.
Induction of apoptosis by YM155 and chrysin
It has been shown that YM155 induces apoptosis in cancer cells by activating the mitochondrial apoptotic pathway [23]. To evaluate if chrysin enhances YM155-induced apoptosis, we evaluated the nuclear morphological change in TTN45 and RTK (GIF) cells after exposure to YM155 and/or chrysin by nuclear staining with NucBlue (Supplemental Fig. 3). We found that YM155 exposure resulted in cells with apoptotic features, which were further enhanced in concert with chrysin, although chrysin alone did not induce morphological apoptotic features.
Mitochondrial depolarization after incubation of SMARCB1/INI1-deficient tumor cells with YM155 and/or chrysin was measured by the MitoPT JC-1 assay (Fig. 2, Supplemental Fig. 4). YM155 at 10 nM significantly induced loss of mitochondria transmembrane potential which was indicated by the decreased ratio of JC-1 red (intact) cells/JC-1 green (damaged) cells in TTN45, RTK (GIF), and KCS1 cells, although significant differences were not observed in RTK (J)-4N and YCUS-5 cells upon exposure for 6 h. The effect of chrysin was inconsistent among the cells. Chrysin at 4 μg/ml seemed rather protective in TTN45, RTK (J)-4N, and YCUS-5 cells, showing a significant difference in YCUS-5 cells. In TTN45 cells, chrysin showed a significant protective effect against YM155-induced mitochondrial damage. These results suggest that the mitochondrial pathway is involved in YM155-induced apoptosis in SMARCB1/INI1-deficient tumor cells; however, it is not likely that chrysin directly enhances YM155-induced mitochondrial apoptosis.
Loss of mitochondrial transmembrane potential after agent exposure. Cells were seeded onto a 24-well glass bottom plate. After 6 h incubation in control medium, medium containing 2-[2-(3-chlorophenyl) hydrazinylyidene] propanedinitrile (CCCP; as a positive control) at 50uM, medium containing YM155 alone at 10 nM, medium containing chrysin alone at 4 μg/ml, or medium containing the combination of YM155 at 10 nM and chrysin at 4 μg/ml, cells were stained with MitoPT JC-1 (ThermoFisher Scientific, Waltham, MA). The numbers of cells with intact or damaged mitochondrial transmembrane potential (JC-1 red or green, respectively) were counted under fluorescence microscopy. The bar with the error bar indicates the mean ratio of JC-1 red/green cells with standard deviation in triplicate counting. *P < 0.05, **P < 0.01
Combination of YM155 and chrysin reduced survivin expression
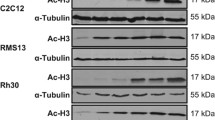
Survivin expression was evaluated by Western blotting in SMARCB1/INI1-deficient cell lines after 6 or 24 h exposure to YM155 at 10 nM, chrysin at 4 μg/ml, or their combination (Fig. 3). YM155 has been characterized as an inhibitor of survivin expression; however, upon 24 h exposure, YM155 noticeably reduced survivin expression in only YCUS-5 cells. In contrast, when YM155 was combined with chrysin, survivin expression was repressed in all cell lines. Such change was observed as early as 6 h after incubation with the two agents in TTN45 cells. Chrysin alone did not affect survivin expression. Thus, the mechanism by which the combination of YM155 and chrysin reduced the viability of the cancer cell lines includes synergistic inhibition of survivin expression.
Western blot analysis of survivin expression after agent exposure. Cells were incubated in medium containing YM155 at 10 nM, chrysin at 4 μg/ml, or their combination for 6 or 24 h. Proteins were extracted and applied to Western blot analysis to detect the expression of survivin and β-actin as a control. Y + C, combination of YM155 at 10 nM and chrysin at 4 μg/ml
Discussion
MRT is a rare, aggressive soft tissue sarcoma that mostly develops in infants [24]. Loss of SMARCB1/INI1 expression is a characteristic of MRT, but is not an exclusive feature of MRT [4]. SMARCB1/INI1 is a core subunit of the SWI/SNF (BAF) chromatin-remodeling complex, and loss of function of SMARCB1/INI1 has been shown to lead to several cellular events associated with proliferation such as Cyclin D1 expression, activation of the Hedgehog pathway, and activation of the WNT/β-catenin pathway [6, 7]. Loss of SMARCB1/INI1 is thought to be a driver event of oncogenesis in MRT and other SMARCB1/INI1-deficient tumors [6, 7]. However, a specific therapy targeting SMARCB1/INI1 loss has not been developed because loss of wild-type SMARCB1/INI1 functions results in diverse cellular signal alterations.
In this study, we performed in vitro drug sensitivity screening in an attempt to discover a novel therapy against SMARCB1/INI1-deficient tumors. We found that YM155, a survivin inhibitor, effectively inhibited the survival of MRT and other SMARCB1/INI1-deficient tumor cell lines. Recently, EZH2 inhibition was suggested to counteract the epigenetic alterations caused by SMARCB1/INI1 loss and to have a therapeutic potential in MRT [25]. Tazemetostat, a specific EZH2 inhibitor, was included in our tested agent panel; however, tazemetostat did not reduce the viability of SMARCB1/INI1-deficient tumor cells in vitro at concentrations of up to 1 μM in our assay.
Survivin is a member of the IAP family, which plays roles in regulation of cell proliferation and cell death [11]. Survivin is overexpressed in various types of cancers including rhabdoid tumor of the kidney [12], although its expression is not seen in most normal differentiated tissues, suggesting that survivin is an attractive therapeutic target of cancer. Survivin inhibits apoptotic and autophagic cell death and its overexpression is associated with the aggressive phenotype and reduced drug sensitivity of cancer cells [11]. YM155, a small molecule inhibitor of survivin expression, has anti-tumor effects in several cancers, and results of clinical trials of YM155 in patients with non-small cell lung cancer [26], lymphoma [27], breast cancer [28], melanoma [29], or prostate cancer [30] have been reported, although the effects of YM155 in MRT and other SMARCB1/INI1-deficient tumors had not been determined. Based on our results, YM155 is expected to have a therapeutic effect against SMARCB1/INI1-deficient tumors. YM155 has been reported to be able to induce cancer cell death via the pathways independent from survivin inhibition [31]. Because survivin inhibition was not evident by YM155 alone except for YCUS5 cells, other pathways than survivin inhibition might be involved in YM155-induced SMARCB1/INI1-deficient tumor cell death. However, LQZ-7I, a survivin dimerization inhibitor [32], also inhibits growth of RTK (J)-4N or YCUS5 cells at 50% inhibitory concentration of 7–8 µM (data not shown), supporting survivin can be a therapeutic target of SMARCB1/INI1-deficient tumors.
Combined use of drugs with different modes of action is a clinically promising approach to enhance the effect on the target and to disperse organ toxicities. Since survivin inhibition was suggested to be therapeutic in SMARCB1/INI1-deficient tumors, we screened agents for their synergy with YM155. Among 79 agents with various modes of action, most of the agents had more than additive effects upon simultaneous addition of YM155. Among these agents, we decided to perform further validation studies with chrysin, since the result seemed to be contradictory to the findings of our previous study [22]. In the validation study with a fixed dose of chrysin in contrast to the drug combination screening where a fixed dose of YM155 was used, the CIs were approximately 1.0 at various concentrations of YM155 in all tested cell lines. Thus, chrysin was shown to have at least an additive effect in combination with YM155. The 3D-culture system provides a more physiological in vitro condition to cells than the 2D-culture system, and cancer cells in the 3D-culture respond to chemotherapeutic drugs differently compared to cancer cells in the conventional 2D-culture [18, 19]. In this study, we utilized the collagen gel droplet embedded-drug sensitivity test (CD-DST) [33,34,35] with some modifications to apply the method to the high-throughput drug screening. In the CD-DST, the various drugs added in the culture medium have been successfully evaluated for their cytotoxicity to 3D-cultured tumor cells in collagen gel droplet. Using the modified CD-DST, the combination effects of YM155 and chrysin were also judged as being mostly additive to synergistic, except for some data points at lower concentrations of YM155 where the combination effects were judged as antagonistic, presumably due to the computational problem derived by decreased cytotoxic effects of chrysin in the 3D-culture.
Chrysin, a bioactive natural flavone, has been shown to have several bioactivities including antioxidant, anti-inflammatory, and anti-tumor effects [36]. We previously showed that 5,7-dimethoxyflavone which is a bioavailable derivative of chrysin induced cell cycle arrest in acute lymphoblastic leukemia cells and antagonized cytotoxic effects of simultaneously added chemotherapy drugs [22]. In contrast, another study showed that chrysin increased the sensitivity of pancreatic cancer cells to gemcitabine by inhibiting the activity of carbonyl reductase 1 which is associated with resistance to gemcitabine [37]. Thus, the combination effect conferred by chrysin might be different depending on the partner drug or the tumor type.
In this study, chrysin enhanced YM155-induced apoptosis in SMARCB1/INI-1-deficient tumor cells, although chrysin at 4 μg/ml alone did not induce apparent apoptotic features upon 48 h exposure. YM155 is thought to induce both intrinsic and extrinsic apoptosis by inhibiting survivin expression. Because chrysin has been reported to induce apoptosis in some cancers by activating the mitochondrial apoptotic pathway [38], the changes in mitochondrial transmembrane potential in SMARCB1/INI-1-deficient tumor cells were determined after exposure to YM155 and chrysin. We found that YM155 at 10 nM induced mitochondrial damage upon incubation with SMARCB1/INI-1-deficient tumor cells for 6 h; however, further enhancement by adding chrysin was not observed at this time point. Among its various biological activities, chrysin has been shown to act as a histone deacetylase inhibitor (HDACi) [39]. HDACi represses nuclear factor-kappa b targeting gene expression including survivin expression; in fact, chrysin was reported to decrease survivin expression in melanoma cells [40]. In the present study, chrysin significantly enhanced YM155-induced suppression of survivin expression especially inTTN45 cells, where an apparent decrease in survivin expression was seen after exposure to the combination of YM155 and chrysin for as short as 6 h. These results suggest that synergistic suppression of survivin expression underlies the anti-tumor effect of the combination of YM155 and chrysin in SMARCB1/INI-1-deficient tumor cells.
In summary, our data suggest that survivin can be a therapeutic target in MRT and other SMARCB1/INI-1-deficient tumors. Chrysin, a dietary flavonoid, suppressed survivin expression in concert with YM155. Considering poor bioavailability of chrysin [41], these results are not applicable to clinic practice instantly, however, can provide important suggestions leading development of effective and less toxic therapy.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Morgan KM, Siow VS, Strotmeyer S, Gow KW, Malek MM. Characteristics and outcomes in pediatric non-central nervous system malignant rhabdoid tumors: a report from the National Cancer Database. Ann Surg Oncol. 2022;29:671–8. https://doi.org/10.1245/s10434-021-10370-x.
Enault M, Minard-Colin V, Corradini N, Leverger G, Thebaud E, Rome A, Proust S, Marie-Cardine A, Defachelles AS, Sarnacki S, Philippe-Chomette P, Delattre O, Masliah-Planchon J, Lacour B, Ferrari A, Brennan B, Orbach D, Bourdeaut F. Extracranial rhabdoid tumours: results of a SFCE series of patients treated with a dose compression strategy according to European Paediatric Soft Tissue Sarcoma Study Group recommendations. Eur J Cancer. 2022;161:64–78. https://doi.org/10.1016/j.ejca.2021.10.025.
Brennan B, De Salvo GL, Orbach D, De Paoli A, Kelsey A, Mudry P, Francotte N, Van Noesel M, Bisogno G, Casanova M, Ferrari A. Outcome of extracranial malignant rhabdoid tumours in children registered in the European Paediatric Soft Tissue Sarcoma Study Group Non-Rhabdomyosarcoma Soft Tissue Sarcoma 2005 Study-EpSSG NRSTS 2005. Eur J Cancer. 2016;60:69–82. https://doi.org/10.1016/j.ejca.2016.02.027.
Margol AS, Judkins AR. Pathology and diagnosis of SMARCB1-deficient tumors. Cancer Genet. 2014;207:358–64. https://doi.org/10.1016/j.cancergen.2014.07.004.
Modena P, Lualdi E, Facchinetti F, Galli L, Teixeira MR, Pilotti S, Sozzi G. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res. 2005;65:4012–9. https://doi.org/10.1158/0008-5472.CAN-04-3050.
Kohashi K, Oda Y. Oncogenic roles of SMARCB1/INI1 and its deficient tumors. Cancer Sci. 2017;108:547–52. https://doi.org/10.1111/cas.13173.
Kim KH, Roberts CW. Mechanisms by which SMARCB1 loss drives rhabdoid tumor growth. Cancer Genet. 2014;207:365–72. https://doi.org/10.1016/j.cancergen.2014.04.004.
Preusser M, Wolfsberger S, Czech T, Slavc I, Budka H, Hainfellner JA. Survivin expression in intracranial ependymomas and its correlation with tumor cell proliferation and patient outcome. Am J Clin Pathol. 2005;124:543–9. https://doi.org/10.1309/PP2G5GAAFKV82DTG.
Karpinsky G, Krawczyk MA, Izycka-Swieszewska E, Fatyga A, Budka A, Balwierz W, Sobol G, Zalewska-Szewczyk B, Rychlowska-Pruszynska M, Klepacka T, Dembowska-Baginska B, Kazanowska B, Gabrych A, Bien E. Tumor expression of survivin, p53, cyclin D1, osteopontin and fibronectin in predicting the response to neo-adjuvant chemotherapy in children with advanced malignant peripheral nerve sheath tumor. J Cancer Res Clin Oncol. 2018;144:519–29. https://doi.org/10.1007/s00432-018-2580-1.
Uehara S, Oue T, Kawatsu M, Nara K, Fukuzawa M. Increased expression of survivin in hepatoblastoma after chemotherapy. Eur J Pediatr Surg. 2013;23:400–4. https://doi.org/10.1055/s-0033-1333637.
Rafatmanesh A, Behjati M, Mobasseri N, Sarvizadeh M, Mazoochi T, Karimian M. The survivin molecule as a double-edged sword in cellular physiologic and pathologic conditions and its role as a potential biomarker and therapeutic target in cancer. J Cell Physiol. 2020;235:725–44. https://doi.org/10.1002/jcp.29027.
Takamizawa S, Scott D, Wen J, Grundy P, Bishop W, Kimura K, Sandler A. The survivin:fas ratio in pediatric renal tumors. J Pediatr Surg. 2001;36:37–42. https://doi.org/10.1053/jpsu.2001.20000.
Takita J, Chen Y, Kato M, Ohki K, Sato Y, Ohta S, Sugita K, Nishimura R, Hoshino N, Seki M, Sanada M, Oka A, Hayashi Y, Ogawa S. Genome-wide approach to identify second gene targets for malignant rhabdoid tumors using high-density oligonucleotide microarrays. Cancer Sci. 2014;105:258–64. https://doi.org/10.1111/cas.12352.
Goto H, Takahashi H, Funabiki T, Ikuta K, Sasaki H, Nagashima Y. Brief report: neural differentiation of a novel cell line, YCUS-5, established from proximal-type epithelioid sarcoma of a child. Med Pediatr Oncol. 1999;33:137–8. https://doi.org/10.1002/(sici)1096-911x(199908)33:2%3c137::aid-mpo18%3e3.0.co;2-n.
Goto H, Yoshino Y, Ito M, Nagai J, Kumamoto T, Inukai T, Sakurai Y, Miyagawa N, Keino D, Yokosuka T, Iwasaki F, Hamanoue S, Shiomi M, Goto S. Aurora B kinase as a therapeutic target in acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2020;85:773–83. https://doi.org/10.1007/s00280-020-04045-9.
Szulkin A, Otvös R, Hillerdal CO, Celep A, Yousef-Fadhel E, Skribek H, Hjerpe A, Székely L, Dobra K. Characterization and drug sensitivity profiling of primary malignant mesothelioma cells from pleural effusions. BMC Cancer. 2014;14:709. https://doi.org/10.1186/1471-2407-14-709.
Foucquier J, Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect. 2015;3: e00149. https://doi.org/10.1002/prp2.149.
Zoetemelk M, Rausch M, Colin DJ, Dormond O, Nowak-Sliwinska P. Short-term 3D culture systems of various complexity for treatment optimization of colorectal carcinoma. Sci Rep. 2019;9:7103. https://doi.org/10.1038/s41598-019-42836-0.
Riedl A, Schlederer M, Pudelko K, Stadler M, Walter S, Unterleuthner D, Unger C, Kramer N, Hengstschläger M, Kenner L, Pfeiffer D, Krupitza G, Dolznig H. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J Cell Sci. 2017;130:203–18. https://doi.org/10.1242/jcs.188102.
Rubie H, Geoerger B, Frappaz D, Schmitt A, Leblond P, Ndiaye A, Aerts I, Le Deley MC, Gentet JC, Paci A, Chastagner P, Dias N, Djafari L, Pasquet M, Chatelut E, Landman-Parker J, Corradini N, Vassal G. Phase I study of topotecan in combination with temozolomide (TOTEM) in relapsed or refractory paediatric solid tumours. Eur J Cancer. 2010;46:2763–70. https://doi.org/10.1016/j.ejca.2010.05.004.
Hawkins DS, Bradfield S, Whitlock JA, Krailo M, Franklin J, Blaney SM, Adamson PC, Reaman G. Topotecan by 21-day continuous infusion in children with relapsed or refractory solid tumors: a Children’s Oncology Group study. Pediatr Blood Cancer. 2006;47:790–4. https://doi.org/10.1002/pbc.20739.
Goto H, Yanagimachi M, Goto S, Takeuchi M, Kato H, Yokosuka T, Kajiwara R, Yokota S. Methylated chrysin reduced cell proliferation, but antagonized cytotoxicity of other anticancer drugs in acute lymphoblastic leukemia. Anticancer Drugs. 2012;23:417–25. https://doi.org/10.1097/CAD.0b013e32834fb731.
Minoda M, Kawamoto T, Ueha T, Kamata E, Morishita M, Harada R, Toda M, Onishi Y, Hara H, Kurosaka M, Akisue T. Antitumor effect of YM155, a novel small-molecule survivin suppressant, via mitochondrial apoptosis in human MFH/UPS. Int J Oncol. 2015;47:891–9. https://doi.org/10.3892/ijo.2015.3077.
Oda Y, Tsuneyoshi M. Extrarenal rhabdoid tumors of soft tissue: clinicopathological and molecular genetic review and distinction from other soft-tissue sarcomas with rhabdoid features. Pathol Int. 2006;56:287–95. https://doi.org/10.1111/j.1440-1827.2006.01962.x.
Kurmasheva RT, Erickson SW, Earley E, Smith MA, Houghton PJ. In vivo evaluation of the EZH2 inhibitor (EPZ011989) alone or in combination with standard of care cytotoxic agents against pediatric malignant rhabdoid tumor preclinical models—a report from the Pediatric Preclinical Testing Consortium. Pediatr Blood Cancer. 2021;68: e28772. https://doi.org/10.1002/pbc.28772.
Shimizu T, Nishio K, Sakai K, Okamoto I, Okamoto K, Takeda M, Morishita M, Nakagawa K. Phase I safety and pharmacokinetic study of YM155, a potent selective survivin inhibitor, in combination with erlotinib in patients with EGFR TKI refractory advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2020;86:211–9. https://doi.org/10.1007/s00280-020-04112-1.
Papadopoulos KP, Lopez-Jimenez J, Smith SE, Steinberg J, Keating A, Sasse C, Jie F, Thyss A. A multicenter phase II study of sepantronium bromide (YM155) plus rituximab in patients with relapsed aggressive B-cell Non-Hodgkin lymphoma. Leuk Lymphoma. 2016;57:1848–55. https://doi.org/10.3109/10428194.2015.1113275.
Clemens MR, Gladkov OA, Gartner E, Vladimirov V, Crown J, Steinberg J, Jie F, Keating A. Phase II, multicenter, open-label, randomized study of YM155 plus docetaxel as first-line treatment in patients with HER2-negative metastatic breast cancer. Breast Cancer Res Treat. 2015;149:171–9. https://doi.org/10.1007/s10549-014-3238-6.
Kudchadkar R, Ernst S, Chmielowski B, Redman BG, Steinberg J, Keating A, Jie F, Chen C, Gonzalez R, Weber J. A phase 2, multicenter, open-label study of sepantronium bromide (YM155) plus docetaxel in patients with stage III (unresecn) or stage IV melanoma. Cancer Med. 2015;4:643–50. https://doi.org/10.1002/cam4.363.
Tolcher AW, Quinn DI, Ferrari A, Ahmann F, Giaccone G, Drake T, Keating A, de Bono JS. A phase II study of YM155, a novel small-molecule suppressor of survivin, in castration-resistant taxane-pretreated prostate cancer. Ann Oncol. 2012;23:968–73. https://doi.org/10.1093/annonc/mdr353.
Mondal A, Jia D, Bhatt V, Akel M, Roberge J, Guo JY, Langenfeld J. Ym155 localizes to the mitochondria leading to mitochondria dysfunction and activation of AMPK that inhibits BMP signaling in lung cancer cells. Sci Rep. 2022;12:13135. https://doi.org/10.1038/s41598-022-17446-y.
Peery R, Cui Q, Kyei-Baffour K, Josephraj S, Huang C, Dong Z, Dai M, Zhang JT, Liu JY. A novel survivin dimerization inhibitor without a labile hydrazone linker induces spontaneous apoptosis and synergizes with docetaxel in prostate cancer cells. Bioorg Med Chem. 2022;65: 116761. https://doi.org/10.1016/j.bmc.2022.116761.
Goto H, Kitagawa N, Sekiguchi H, Miyagi Y, Keino D, Sugiyama M, Sarashina T, Miyagawa N, Yokosuka T, Hamanoue S, Iwasaki F, Shiomi M, Goto S, Tanaka Y. The collagen gel droplet-embedded culture drug sensitivity test in relapsed hepatoblastoma. J Pediatr Hematol Oncol. 2017;39:395–401. https://doi.org/10.1097/MPH.0000000000000865.
Kii T, Sakuma K, Tanaka A. Optimal contact concentration of paclitaxel in the collagen gel droplet-embedded culture drug sensitivity test for human oral squamous cell carcinoma and evaluation of combination with cetuximab. Chemotherapy. 2021;65:147–57. https://doi.org/10.1159/000512542.
Kobayashi H. Development of a new in vitro chemosensitivity test using collagen gel droplet embedded culture and image analysis for clinical usefulness. Recent Results Cancer Res. 2003;161:48–61. https://doi.org/10.1007/978-3-642-19022-3_5.
Garg A, Chaturvedi S. A comprehensive review on chrysin: emphasis on molecular targets, pharmacological actions and bio-pharmaceutical aspects. Curr Drug Targets. 2022;23:420–36. https://doi.org/10.2174/1389450122666210824141044.
Zhou L, Yang C, Zhong W, Wang Q, Zhang D, Zhang J, Xie S, Xu M. Chrysin induces autophagy-dependent ferroptosis to increase chemosensitivity to gemcitabine by targeting CBR1 in pancreatic cancer cells. Biochem Pharmacol. 2021;193: 114813. https://doi.org/10.1016/j.bcp.2021.114813.
Park W, Park S, Lim W, Song G. Chrysin disrupts intracellular homeostasis through mitochondria-mediated cell death in human choriocarcinoma cells. Biochem Biophys Res Commun. 2018;503:3155–61. https://doi.org/10.1016/j.bbrc.2018.08.109.
Ganai SA, Sheikh FA, Baba ZA. Plant flavone chrysin as an emerging histone deacetylase inhibitor for prosperous epigenetic-based anticancer therapy. Phytother Res. 2021;35:823–34. https://doi.org/10.1002/ptr.6869.
Pal-Bhadra M, Ramaiah MJ, Reddy TL, Krishnan A, Pushpavalli SN, Babu KS, Tiwari AK, Rao JM, Yadav JS, Bhadra U. Plant HDAC inhibitor chrysin arrest cell growth and induce p21WAF1 by altering chromatin of STAT response element in A375 cells. BMC Cancer. 2012;12:180. https://doi.org/10.1186/1471-2407-12-180.
Gao S, Siddiqui N, Etim I, Du T, Zhang Y, Liang D. Developing nutritional component chrysin as a therapeutic agent: bioavailability and pharmacokinetics consideration, and ADME mechanisms. Biomed Pharmacother. 2021;142: 112080. https://doi.org/10.1016/j.biopha.2021.112080.
Funding
This work was supported by Kanagawa Children’s Medical Foundation; Kanagawa Prefectural Hospital Organization.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Cell lines were established and maintained by HG, JT, and YH. Material preparation, data collection, and analysis were performed by YY, MI, YT, and MY. The first draft of the manuscript was written by YY and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflicts of interest related to the presented study.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Kanagawa Children’s Medical Center (09/14/2017, No. 105-8).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12032_2022_1843_MOESM1_ESM.tif
Supplementary file1 (TIF 250 KB)—Supplemental Figure 1. In vitro drug sensitivity screening in a panel of SMARCB1/INI1-deficient cell lines. Cells were injected onto a 384-well plate, and after 1-day incubation, 80 agents belonging to several different classes at 4 serially diluted concentrations were loaded onto the wells. After 4 days’ incubation, cell viability in each well was measured using the CellTiter-Glo luminescent assay (Promega, Madison, WI, USA). Cell survival rates in agent-containing wells were expressed as ratios of live cell-derived luminescence compared to the mean luminescence value in 5 agent-free control wells. To compare agent sensitivity among the samples, the drug effect score (DES) was calculated for each agent as follows: DES = {(100 − % survival at 5–3 dilution)*ln(125) + (100 − % survival at 5–2 dilution)*ln(25) + (100 − % survival at 5–1 dilution)*ln(5) + (100 − % survival at no dilution)}/{ln(125) + ln(25) + ln(5) + 1}. A cell line was defined to be sensitive to the tested agent if its DES was larger than the reference DES (i.e., the mean DES when peripheral blood mononuclear cells from 5 healthy volunteers were tested in the same drug sensitivity test). In the heat map of Fig. 1, the number represents the DES of the tested agent at a particular concentration after subtracting the corresponding reference DES. A red box indicates high DES; the more intense the redness, the higher the DES. Topotecan (red arrow) and YM155 (red arrow) showed relatively high DES values in the 5 cell lines. The highest concentrations of agents used in the screening assay are shown in Supplemental Table 1.
12032_2022_1843_MOESM2_ESM.tif
Supplementary file2 (TIF 698 KB)—Supplemental Figure 2. Drug combination screening. The combination indexes between YM155 at 10 nM and agents with various modes of action at 4 serial dilutions are shown. A red box indicates that the combination index is less than 1.0, suggesting a synergistic effect.
12032_2022_1843_MOESM3_ESM.tif
Supplementary file3 (TIF 464 KB)—Supplemental Figure 3. Apoptotic change of nuclei after agent exposure. Cells were seeded onto a 24-well glass bottom plate with control medium, medium containing YM155 alone at 10 nM, medium containing chrysin alone at 4 μg/ml, or medium containing the combination of YM155 at 10 nM and chrysin at 4 μg/ml. After 48 h incubation, cellular nuclei were stained with NucBlue Live ReadyProbes Reagent (ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s instructions. Nuclear blebbing indicating apoptotic change was observed upon incubation with YM155, but was not evident upon incubation with chrysin alone. (Original magnification: ×200).
12032_2022_1843_MOESM4_ESM.tif
Supplementary file4 (TIF 823 KB)—Supplemental Figure 4. Loss of mitochondrial transmembrane potential after agent exposure. Fluorescent microscopic pictures after JC-1 staining are shown. See Figure Legend 4 for details. (Original magnification: ×100).
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoshino, Y., Goto, H., Ito, M. et al. YM155 and chrysin cooperatively suppress survivin expression in SMARCB1/INI1-deficient tumor cells. Med Oncol 39, 234 (2022). https://doi.org/10.1007/s12032-022-01843-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01843-4