Abstract
Circulating tumor cells detection and ARV7 expression are associated with worse clinical outcomes in metastatic Castration-Resistant Prostate Cancer (mCRPC) undergoing Androgen Receptor Targeted Agents. ARFL, PSMA and PSA may help to refine prognostic models. In our institution, a prospective observational trial testing CTC detection in mCPRC undergoing I line ARTA therapy terminated the planned enrollment in 2020. Here, we present a pre-planned interim analysis with 18 months of median follow-up. RT-qPCR was used to determine the CTC expression of PSA, PSMA, AR and ARV7 before starting ARTA. PSA-drop, Progression-Free and Overall Survival (PFS and OS) and their correlation with CTC detection were reported. Forty-four patients were included. CTC were detected in 43.2% of patients, of whom 8.94% expressed PSA, 15.78% showed ARV7, 63.15% and 73.68% displayed ARFL and PSMA, respectively. Biochemical response was significantly improved in CTC + vs CTC− patients, with median PSA-drop of 18.5 vs 2.5 ng/ml (p = 0.03). After a median follow-up of 18 months, 50% of patients progressed. PFS was significantly longer in CTC- patients (NR vs 16 months). Eight (18.2%) patients died, a non-significant trend in terms of OS was detected in favor of CTC− patients (NR vs 29 months, p = 0.05). AR, PSA and PSMA expression in CTC + had no significant impact on PSA-drop, PFS or OS. PRIMERA-trial confirmed the CTC detection predictive importance in mCRPC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Androgen deprivation therapy (ADT) is one of the cornerstones of treatment for metastatic prostate cancer (PCa), but a substantial proportion of patients will eventually progress into metastatic castration-resistant prostate cancer (mCRPC) status. Since 2004, mCRPC prognosis improved thanks to the advances in systemic treatment available in this setting [1]. Taxane based therapy was implemented in treatment algorithm after publication of TAX327 and TROPIC trials [2, 3]. COU-AA 302 and PREVAIL trials showed overall survival (OS) improvement after treatment with androgen receptor targeted agents (ARTA) in chemotherapy-naive mCPRC patients [4, 5]. Another treatment option for mCRPC treatment is represented by radiomethabolic therapies. Both bone-targeted agents (i.e. Radium 223) and [177Lu] LuPSMA-based treatment have been tested in mCRPC patients and yielded promising results if compared to standard of care [6,7,8]. DNA damage repair (DDR) mutations and PTEN-loss are the first druggable targets in this panorama [9, 10]. Given the multiple treatment options available, most effective 1st line treatment for mCRPC is not known, and treatment sequencing is still to be defined [11]. For these reasons, biomarkers able to refine selection criteria and predict response to treatment are eagerly awaited. Circulating tumor cells (CTC) and CTC-based androgen receptor splicing variants 7 (ARV7) detection are promising tools for patients undergoing ARTA therapy. Indeed, ARV7 + CTC detection has been shown to be related to worse clinical outcomes after treatment after abiraterone and enzalutamide [12, 13]. Other promising tools in this setting, potentially helpful to predict treatment response to ARTA, are represented by full-length androgen receptor (ARFL) [14]. Prostate-specific membrane antigen (PSMA) expression could be important for both its diagnostic and therapeutic implications, and may be related to biological aggressiveness of disease [15]. However, many translational studies enrolled mixed cohorts of chemo-naive and post taxane patients, and data about prognostic impact of CTC molecular features within homogeneous populations (e.g. I line mCRPC undergoing ARTA treatment) may be helpful to better understand value of these biomarkers for treatment selection. PRIMERA (NCT04188275) is a prospective trial conducted in our institution, testing the prognostic impact of CTC detection and their expression of ARV7, ARFL, PSMA and PSA. Recently, an early report about this trial showing relationship between Time to Castration Resistance (TTCR) and CTC detection was published [16]. Here, we present a pre-planned interim analysis after enrollment of the complete planned cohort and 18 months of median follow-up, focusing on impact of CTC detection and ARV7, ARFL, PSMA and PSA expression on biochemical response to ARTA therapy and patients survival.
Materials and methods
Design, setting, and participants
Population
PRIMERA is a monocentric, prospective, observational trial enrolling mCRPC patients undergoing I line treatment with ARTA + ADT. All patients were treated with either Abiraterone acetate or Enzalutamide according to clinician choice. Patients previously treated with taxane-based chemotherapy or any other agents approved for PCa (except for ADT) were excluded from this trial. CRPC definition was based on European Association of Urology (EAU) guidelines [11]. The study was conducted according to the guidelines of the Declaration of Helsinki, was approved by the local ethical committee and registered on Clinicaltrials.gov (NCT04188275).
CTC enrichment and analysis
All patients underwent blood sampling for CTC detection when starting ARTA treatment at mCRPC occurrence. 10 ml of blood were collected before starting a new line of therapy into collection tubes BD vacutainer glass ACD solution B (Becton Dickinson, Franklin Lakes, New Jersey, USA). For CTC enrichment and characterization, AdnaTest Prostate Cancer Panel AR-V7 (Qiagen Gmbh, Hilden, Germany) was used. CTC isolation was obtained through immuno-magnetic beads recognizing epithelial and tumor-associated antigens (AdnaTest Prostate Cancer Select). Cell lysis and reverse transcription were performed according to the manufacturer’s instructions. Reverse Transcription-quantitative real-time PCR (RT-qPCR) was used to evaluate PSA, PSMA, ARFL and ARV7 expression. A sample was considered positive—indicating the presence of CTC—if at least one prostate cancer-associated transcript (PSA, PSMA, AR or ARV7) was detected.
Outcome measurements and statistical analysis
Descriptive analysis was performed to summarize patient- and CTC-related characteristics in the study population. Outcome explored in the current analysis were progression-free survival (PFS) and overall survival (OS), PSA at 8 weeks after ARTA therapy start, PSA-drop at 8 weeks (defined as the difference between PSA at 8 weeks after ARTA therapy start and baseline PSA), Overall PSA-drop (defined as the difference between last PSA registered and baseline PSA), PSA nadir (defined as PSA lowest value registered during ARTA therapy). Correlation between CTC detection and these outcomes was tested. Prevalence and prognostic impact of ARV7, ARFL, PSA and PSMA were evaluated in CTC + patients. A Chi-square test was performed to test the association between ARV7, ARFL, PSA and PSMA expression. Kaplan–Meier analysis was performed to assess the correlation of outcomes with CTC detection and expression of ARV7, ARFL, PSA and PSMA. Cox proportional hazard regression was performed to test the association between PFS, OS and PSA at CRPC diagnosis. All statistical analyses were performed with MedCalc version 18.9.
Results
Overall cohort, CTC detection rate and molecular profiling
Overall, 44 patients were included in the present cohort. Of these, CTC were detected at treatment start in 19 patients (43.2%), of whom 3 (15,78%), 12 (63.15%), 15 (78,94%) and 14 (73.68%) patients expressed ARV7, ARFL, PSA and PSMA, respectively. Principal characteristics and treatment outcomes measured in the overall population are summarized in Table 1. Median PFS and OS in the overall population were 20 months (95% CI 13–29) and NR, respectively. PFS was comparable in low vs high baseline disease burden according to CHAARTED criteria [17] (22 vs 16 months, p = 0.52) and in patients with baseline International Society of Urological Pathology (ISUP) score < vs > 3 (29 vs 17 months, p = 0.12). Moreover, OS was not influenced by baseline burden of disease (NR for both low or high, p = 0.58) or ISUP score (NR for both < 3 or > 3, p = 0.48). PSA at CRPC diagnosis had no impact on PFS or OS (p = 0.21 and p = 0.44, respectively).
AR, PSA and PSMA expression in CTC + patients
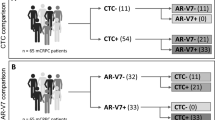
Figure 1 summarizes ARV7, PSMA, ARFL and PSA expression in the 19 CTC positive patients. Overall, 3 (15.8%), 9(63.1%), 15(78.9%) and 14(73.7%) patients had CTC expressing ARV7, ARFL, PSA and PSMA, respectively. No differences between ARV7 + and ARV7− CTC was detected in terms of ARFL, PSA and PSMA expression (p = 0.89, p = 032 and p = 0.76, respectively). Furthermore, PSMA + and PSMA− did not show any significant difference in terms of ARFL, PSA and ARV7 expression (p = 0.36, p = 0.22 and p = 0.76, respectively). PSA + and PSA− CTC showed no difference in terms of ARFL, PSMA and ARV7 expression (p = 0.58, p = 0.22 and p = 0.34, respectively). ARFL + and ARFL− CTC equally expressed PSA, PSMA and ARV7 (p = 0.58, p = 0.36 and p = 0.26, respectively).
Correlation between CTC detection and selected outcomes
Baseline features as Gleason score, Disease Burden and ARTA treatment (Abiraterone vs Enzalutamide) were well balanced between CTC + and CTC− subgroups. CTC + patients had a significantly higher PSA at CRPC occurrence (22.7 vs 5.9 ng/ml, p = 0.01), see Table 2. After a median follow-up of 18 months, 22 (50%) patients progressed. PFS was significantly longer in CTC−patients (not reached vs 16 months, respectively, p = 0.02). CTC were not associated with ISUP score > 3 (p = 0.82) or higher burden of disease at diagnosis (p = 0.75). Conversely, higher PSA at mCRPC diagnosis was detected in CTC + patients (5.9 vs 22.7 ng/ml, respectively, p = 0.01). Eight (18.2%) patients died, a non-significant trend in terms of overall survival was detected in favor of CTC− patients (not reached vs 29 months, respectively, p = 0.05) (Fig. 2). Early biochemical response was not affected by CTC status, with no differences detected in terms of PSA at 8 weeks or PSA-drop at 8 weeks in CTC−vs CTC + patients (2.3 vs 3.5 ng/ml, p = 0.47 and − 2.1 vs − 6.3 ng/ml p = 0.16, respectively). However, overall biochemical response was significantly improved in CTC− vs CTC + patients, with median overall PSA-drop values of 18.5 vs 2.5 ng/ml (p = 0.03). PSA nadir showed no significant differences in the two groups, (0.87 vs 1.6 ng/ml in CTC− vs CTC + patients, respectively, p = 0.24). Table 3 summarizes treatment outcomes in the CTC− vs CTC + populations.
Correlation between AR, PSA and PSMA expression in CTC + patients and selected outcomes
Explorative analysis about impact of molecular profiling on treatment in CTC + population were conducted and are summarized in Table 4. ARV7, ARFL, PSMA and PSA expression on CTC had no impact on PFS of CTC + patients (p = 0.89, p = 0.99, p = 0.92 and p = 0.54, respectively), neither on OS (p = 0.33, p = 0.72, p = 0.37 and p = 0.96, respectively). Median PSA at 8 weeks and PSA nadir were comparable in ARV7 + vs ARV7− patients (p = 0.82 and p = 0.69, respectively), ARFL + vs ARFL− patients (p = 0.55 and p = 0.17, respectively), PSMA + vs PSMA− patients (p = 0.26 and p = 0.67, respectively) and PSA + vs PSA− patients (p = 0.84 and p = 0.27, respectively). ARFL + patients had a significant increase in terms of PSA-drop at 8 weeks (22.3 vs 1.9 ng/ml, p = 0.04), and overall PSA-drop (22.6 vs 3.2 ng/ml, p = 0.04). Overall, PSA-drop was significantly increased in PSMA + patients (20.5 vs 3.3 ng/ml, p = 0.04). ARV7 and PSA status had no significant impact on PSA-drop at 8 weeks (p = 0.21 and p = 0.16, respectively) and overall PSA-drop (p = 0.31 and p = 0.1, respectively).
Discussion
The present analysis showed the prognostic impact of CTC detection in mCRPC patients undergoing ARTA treatment and enrolled in a prospective trial. Results show that PFS was significantly improved in CTC− patients, with a non-significant trend for OS in favour of this population. Overall biochemical response to ARTA further confirmed this trend, with CTC− patients showing increased response to I line therapy. These data confirm earlier results from a pre-planned analysis of PRIMERA trial [16]. Interestingly, other prognostic factors, such as baseline burden of disease, Gleason score and PSA at CRPC diagnosis, had no impact on clinical outcomes of this population, suggesting that CTC may help to refine selection criteria in this setting, regardless of other clinical features. Other studies showed worse clinical outcomes for CTC + patients after ARTA treatment in first or second-line setting [12]. However, our experience confirmed CTC predictive value for I line treatment, suggesting the importance of this biomarker in early clinical history of disease. Other series, including both patients treated in I and II line mCRPC, showed higher CTC detection rate in similar populations, ranging between 56 and 59% [12, 13]. Data from literature show that patients treated in later phases of disease may present higher CTC detection rate [18]. Thus, earlier phase of the disease explored in our analysis may as well explain the lower detection rate reported if compared to previous literature. Regarding CTC molecular profiling, results from PRIMERA trial suggest that none of the features analysed (ARV7, ARFL, PSMA or PSA expression) was mutually exclusive for the others. ARV7, PSMA, ARFL and PSA expression rate in CTC + patients may give an interesting snapshot about CTC molecular profiling of a cohort of mCRPC treated in I line setting. Interestingly, despite the limited cohort of CTC + patients in our analysis, results are in line with data from previous literature, with 15.8%, 63.1%, 78.9% and 73.7% of CTC + patients expressing ARV7, ARFL, PSA and PSMA, respectively. Indeed, ARV7 expression rate ranged between 12 and 24% of CTC + patients according to other authors [12, 13], while 71.4% of CTC + patients expressed ARFL according to Cattrini et al. [18]. Chung et al. reported a lower rate of PSA + /CTC + patients in a similar series (25%) [19], but it should be observed that this result came from the analysis of a multigene panel comprehensive of PSA, and this could explain the difference if compared to our analysis. Sixty-seven percent of CTC + patients were reported to express PSMA in a mixed cohort of metastatic prostate cancer patients [15]. Consistency of our results with literature data including both advanced and earlier metastatic disease may indicate that CTC + molecular profiling is a stable feature throughout disease clinical history, and may be useful to discriminate good vs poor prognosis patients, with different treatment strategies according to subgroup. For example, CTC molecular profiling of oligometastatic patients may help to maximise benefit of metastasis directed therapy in oligometastatic patients. In this setting, data from the randomized trial ARTO (NCT03449719) are awaited [20]. Of note, biochemical response was significantly improved in ARFL + /CTC + patients and PSMA + /CTC + patients. Predictive multigene scores on CTC + patients have been previously tested, Cho et al. validated a model comprehensive of ARV7, PSA, PSMA, EpCAM and KRT19 [21].
Moreover, given the interest about PSMA as a target for radiopharmaceutical conjugates [7, 8] clinical impact of PSMA expression is of particular importance. Of course, ARTA has been shown to be effective in earlier scenarios, such as high-risk non-metastatic prostate cancer [22], and hormone sensitive prostate cancer [23,24,25,26,27]. Furthermore, data from MAGNITUDE and PROPEL trial suggested that combination treatment with poly ADP ribose polymerase (PARP) inhibitors may be the preferred treatment option for I line treatment of mCRPC patients [28, 29]. Understanding the clinical relevance of results from this trial in this rapidly evolving panorama is challenging, but data about prognostic impact of CTCs may still be helpful to refine treatment tailoring and direct towards treatment intensification. Moreover, we believe that prognostic impact of CTCs may help to select patients who may still derive benefit from ARTA after I line progression, for example in combination with II line Taxane chemotherapy according to results from PRESIDE trial [30]
Conclusion
PRIMERA trial confirmed clinical impact of CTC detection within a prospectively enrolled, homogeneous cohort of mCRPC patients undergoing I line ARTA treatment. Comprehensive molecular profiling of CTC + patients, although explorative considering the limited sample, represent an important snapshot of CTC in a well-selected setting. Impact of ARFL and PSMA on biochemical outcome is hypothesis-generating and may be helpful to guide future considerations about implementation of multigene predictive models in this scenario.
References
Henríquez I, Roach M 3rd, Morgan TM, et al. Current and emerging therapies for metastatic castration-resistant prostate cancer (mCRPC). Biomedicines. 2021;9(9):1247. https://doi.org/10.3390/biomedicines9091247.
Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14(6):1756–64. https://doi.org/10.1200/JCO.1996.14.6.1756.
de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–54. https://doi.org/10.1016/S0140-6736(10)61389-X.
Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152–60. https://doi.org/10.1016/S1470-2045(14)71205-7.
Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–33. https://doi.org/10.1056/NEJMoa1405095.
Hoskin P, Sartor O, O’Sullivan JM, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014;15(12):1397–406. https://doi.org/10.1016/S1470-2045(14)70474-7.
Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797–804. https://doi.org/10.1016/S0140-6736(21)00237-3.
Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385(12):1091–103. https://doi.org/10.1056/NEJMoa2107322.
de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–102. https://doi.org/10.1056/NEJMoa1911440.
Sweeney C, Bracarda S, Sternberg CN, et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2021;398(10295):131–42. https://doi.org/10.1016/S0140-6736(21)00580-8.
Cornford P, Bellmunt J, Bolla M, et al. Guidelines on prostate cancer. part ii: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71(4):630–42. https://doi.org/10.1016/j.eururo.2016.08.002.
Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35(19):2149–56. https://doi.org/10.1200/JCO.2016.70.1961.
Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the prophecy study. J Clin Oncol. 2019;37(13):1120–9. https://doi.org/10.1200/JCO.18.01731.
Del Re M, Crucitta S, Sbrana A, et al. AR-V7 and AR-FL expression is associated with clinical outcome: a translational study in patients with castrate resistant prostate cancer. BJU Int. 2019. https://doi.org/10.1111/bju.14792.
Gorges TM, Riethdorf S, von Ahsen O, et al. Heterogeneous PSMA expression on circulating tumor cells: a potential basis for stratification and monitoring of PSMA-directed therapies in prostate cancer. Oncotarget. 2016;7(23):34930–41. https://doi.org/10.18632/oncotarget.9004.
Francolini G, Loi M, Salvestrini V, Mangoni M, Detti B, Di Cataldo V, Aquilano M, Pinzani P, Salvianti F, Desideri I, Mariotti M, Garlatti P, Stocchi G, Ciccone LP, Lucidi S, Salvatore G, Sottili M, Meattini I, Livi L. Prospective assessment of AR splice variant and PSMA detection on circulating tumor cells of mCRPC patients: preliminary analysis of patients enrolled in PRIMERA trial (NCT04188275). Clin Exp Metastasis. 2021;38(5):451–8.
Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–46. https://doi.org/10.1056/NEJMoa1503747.
Cattrini C, Rubagotti A, Zinoli L, et al. Role of circulating tumor cells (CTC), androgen receptor full length (AR-FL) and androgen receptor splice variant 7 (AR-V7) in a prospective cohort of castration-resistant metastatic prostate cancer patients. Cancers (Basel). 2019;11(9):1365. https://doi.org/10.3390/cancers11091365.
Chung JS, Wang Y, Henderson J, et al. Circulating tumor cell-based molecular classifier for predicting resistance to abiraterone and enzalutamide in metastatic castration-resistant prostate cancer. Neoplasia. 2019;21(8):802–9. https://doi.org/10.1016/j.neo.2019.06.002.
Francolini G, Garlatti P, Loi M, Detti B, Aquilano M, Allegra A, Guerrieri B, Salvestrini V, Pinzani P, Bellini C, Salvianti F, Stocchi G, Ciccone L, Salvatore G, Sottili M, Di Cataldo V, Desideri I, Mangoni M, Meattini I, Livi L. ARTO trial (NCT03449719), a randomized phase II trial enrolling oligometastatic castration-resistant prostate cancer patients treated with first-line abiraterone acetate with or without stereotactic body radiation therapy: preliminary results comprehensive of biochemical outcomes and circulating tumor cells analysis. J Clin Oncol. 2021;39(6_suppl):118–118. https://doi.org/10.1200/JCO.2021.39.6_suppl.118.
Cho H, Chung JI, Kim J, et al. Multigene model for predicting metastatic prostate cancer using circulating tumor cells by microfluidic magnetophoresis. Cancer Sci. 2021;112(2):859–70. https://doi.org/10.1111/cas.14745.
Attard G, Murphy L, Clarke NW, Cross W, Jones RJ, Parker CC, Gillessen S, Cook A, Brawley C, Amos CL, Atako N, Pugh C, Buckner M, Chowdhury S, Malik Z, Russell JM, Gilson C, Rush H, Bowen J, Lydon A, Pedley I, O’Sullivan JM, Birtle A, Gale J, Srihari N, Thomas C, Tanguay J, Wagstaff J, Das P, Gray E, Alzoueb M, Parikh O, Robinson A, Syndikus I, Wylie J, Zarkar A, Thalmann G, de Bono JS, Dearnaley DP, Mason MD, Gilbert D, Langley RE, Millman R, Matheson D, Sydes MR, Brown LC, Parmar MKB, James ND, Systemic Therapy in Advancing or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2022;399(10323):447–60.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, Sulur G, Luna Y, Li S, Mundle S, Chi KN. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686–700.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, Coskinas X, Frydenberg M, Hague WE, Horvath LG, Joshua AM, Lawrence NJ, Marx G, McCaffrey J, McDermott R, McJannett M, North SA, Parnis F, Parulekar W, Pook DW, Reaume MN, Sandhu SK, Tan A, Tan TH, Thomson A, Tu E, Vera-Badillo F, Williams SG, Yip S, Zhang AY, Zielinski RR, Sweeney CJ, ENZAMET Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–31.
Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, Alcaraz A, Alekseev B, Iguchi T, Shore ND, Rosbrook B, Sugg J, Baron B, Chen L, Stenzl A. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974–86.
Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, Kopyltsov E, Park CH, Alekseev B, Montesa-Pino Á, Ye D, Parnis F, Cruz F, Tammela TLJ, Suzuki H, Utriainen T, Fu C, Uemura M, Méndez-Vidal MJ, Maughan BL, Joensuu H, Thiele S, Li R, Kuss I, Tombal B, ARASENS Trial Investigators. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386(12):1132–42.
Chi KN, Chowdhury S, Bjartell A, Chung BH, de Santana Pereira, Gomes AJ, Given R, Juárez A, Merseburger AS, Özgüroğlu M, Uemura H, Ye D, Brookman-May S, Mundle SD, McCarthy SA, Larsen JS, Sun W, Bevans KB, Zhang K, Bandyopadhyay N, Agarwal N. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39(20):2294–303.
Chi Kim N, Rathkopf Dana E, Smith Matthew Raymond, Efstathiou Eleni, Attard Gerhardt, Olmos David, Lee Ji Youl, Small Eric Jay, Gomes Andrea Juliana, Roubaud Guilhem, Saad Marniza, Zurawski Bogdan, Sakalo Valerii, Mason Gary, del Corral Adam, Wang George C, Daphne Wu, Diorio Brooke, Gitlitz Angela Mennicke Lopez-, Sandhu Shahneen Kaur. Phase 3 MAGNITUDE study: First results of niraparib (NIRA) with abiraterone acetate and prednisone (AAP) as first-line therapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) with and without homologous recombination repair (HRR) gene alterations. J Clin Oncol. 2022;40(6):12–12.
Saad Fred, Armstrong Andrew J, Thiery-Vuillemin Antoine, Oya Mototsugu, Loredo Eugenia, Procopio Giuseppe, Janoski Juliana, de Menezes Gustavo, Girotto Colagiovanni, Arslan Cagatay, Mehra Niven, Parnis Francis, Brown Emma, Schlürmann Friederike, Joung Jae Young, Sugimoto Mikio, Poehlein Christian Heinrich, Harrington Elizabeth, Desai Chintu, Kang Jinyu, Clarke Noel. PROpel: Phase III trial of olaparib (ola) and abiraterone (abi) versus placebo (pbo) and abi as first-line (1L) therapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2022;40(6_suppl):11–11.
Merseburger AS, Attard G, Boysen G, Gourgioti G, Martins K, Chowdhury S. A randomized, double-blind, placebo (PBO)-controlled, phase 3b study of the efficacy and safety of continuing enzalutamide (ENZA) in chemotherapy-naïve, metastatic castration-resistant prostate cancer (mCRPC) patients (pts) treated with docetaxel (DOC) plus prednisolone (PDN) who have progressed on ENZA: PRESIDE. J Clin Oncol. 2022;40(6_suppl):15–15.
Funding
This study was sponsored under a grant from Ipsen. Ipsen had no input into the study design, analysis or interpretation of results. Ipsen reviewed this manuscript for scientific accuracy but had no input into the content.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No competing financial or non-financial interests have to be declared.
Ethical appoval
No conflict of interest has to be declared, Protocol was approved by local ethical committee (Comitato Etico Area Vasta Centro).
Informed consent
All patients signed informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Francolini, G., Loi, M., Ciccone, L.P. et al. Prospective assessment of AR splice variant and multi-biomarker expression on circulating tumor cells of mCRPC patients undergoing androgen receptor targeted agents: interim analysis of PRIMERA trial (NCT04188275). Med Oncol 39, 119 (2022). https://doi.org/10.1007/s12032-022-01756-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01756-2