Abstract
Sarcoidosis is a multisystem granulomatous disorder characterized by helper T cell inflammation. Sarcoid-like reaction (SLR) is a well-defined entity and may be related with several malignant disorders and/or their therapies. SLR has been reported more than 20 years ago and in recent years in cases treated by checkpoint inhibitors (CPIs). Better outcome has been reported in cases developing granulomatous reaction and/or SLRs during CPI treatments. However, these lesions clinically may be thought as disease progression and may cause to stop treatment or alterations. These therapeutic manipulations may be harmful for the patients. Clinicians should be aware of SLRs in cases treated by CPIs and tissues must be sampled and reviewed by an experienced pathologist to avoid misdiagnosis and also unnecessary CPI treatment cessations.
Significance Statement
Clinicians should be aware of sarcoid-like reactions in cases treated by checkpoint inhibitors and tissues must be sampled and reviewed by an experienced pathologist to avoid misdiagnosis and CPI treatment stops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Definition and history
Sarcoidosis (S) is a systemic disease characterized by inflammation-associated helper T cell reaction. Sarcoid-like reaction (SLR) is a term used to describe granulomatous inflammation detected in the setting of active cancer or after effective anti-neoplastic treatments [1]. Firstly, this terminology has been used in cases with acquired immunodeficiency syndrome (AIDS) treated by anti-retroviral treatment. After anti-retroviral treatment in cases with AIDS, this entity has been reported in cases with cancer treated by interferon, anti-neoplastic drugs, and lastly in cases treated by checkpoint inhibitors, CPIs [2, 3]. Interferon and intravesical BCG are other drugs causing granulomatous reactions [4, 5]. First case of CPI-related SLR has been reported in 2008 in a case with malignant melanoma (MM) treated by ipilimumab [6]. PD-1 inhibitor nivolumab-related SLR has been reported at 2016 [7].
Frequency
There is no clear data about the frequency of SLR in cases treated by CPIs. The cause of this situation is the low awareness of this entity among clinicians and also misdiagnosis of this entity in many patients due to mimicking progression in posttreatment imagings, especially in FDG PET/CT scans. In a retrospective study, sarcoid-like lymphadenopathy has been reported in 5% of 147 patients treated by ipilimumab for MM [8]. However, higher frequency of this entity will be diagnosed in next years and clinicians must be aware of this unique complication/adverse effect in cases treated by CPIs.
Pathogenesis
Sarcoidosis (S) is a systemic inflammatory disease and its pathogenesis is not clear enough. However, there is a hypothesis that T cells cause inflammation by the production of proinflammatory cytokines including interferon gamma and interleukin-17 [9, 10]. When we look the history of SLR, severe pulmonary granulomatous reaction named as sarcoid-like process has been reported in cases with AIDS. This entity has been reported more than 20 years ago after successful anti-retroviral treatment in 2 cases with AIDS at the time of peripheral blood CD4(+) T cell recovery. This condition has been a suggested relationship between highly active anti-retroviral treatment and sarcoid-like process. Typical CT findings showing micronodules with diffuse and perilymphatic distribution in a case with AIDS after highly active anti-retroviral treatment have been shown in Fig. 1 [11].
Chest CT showing micronodules with a diffuse and perilymphatic distribution in case with AIDS after successful HAART treatment. Figure has been reprinted from [11]
The exact mechanism of SLR is not known. Granulomatous inflammation associated with active cancer suggests the struggle of the host against the cancer. For example, granulomatous inflammation in a case with lymphoma after chemotherapy suggests the immune reconstitution of the patient [12]. Chemokine receptors have been shown to play a role in the pathogenesis of S. It has been shown that CCR6, CCR4, CCR10, CXCR3, and CXCR are expressed on T cells and produce proinflammatory cytokines [13, 14]. T cells with CCR6(+)CCR4(−)CXCR3(+), CCR10(−) phenotype named as Th17.1 have been found in cases with S and these cells have been proposed for the pathogenesis of S [15]. Interestingly, Th17.1 cells with this phenotype have been shown in 15 melanoma cases developing SLR associated with CPI treatment [16].
It is well known that PD-1/PD-L1 pathway controls T cell/Th17 cell balance. Blockade of this pathway may cause Th17 cell hyperactivity and an increase in IL-17 expression [17]. In pre-clinical studies, it has been shown that PD-1 blockade reinvigorates exhausted T cells which have been suppressed by repeated exposure to PD-L1 ligand. It is suggested that this phenomenon has a capacity to restore T cell cytotoxicity and other immune functions [18]. PD-1 blockade causes an increase in mixed lymphocyte reaction and interferon gamma release which are essential for granuloma formation [19]. These results suggest that PD-1 blockade increases interferon gamma secretion from memory T cells and causes SLR. PD-1 blockade also awakens the memory T cells against previous targets and reactivates previous granulomatous disease [20]. Reactivation of previous granulomatous reaction has been reported in some cases developing SLR after CPI and this is an interesting point and requires further evaluation [20,21,22,23]. However, if this hypothesis is completely true, so far we had to see too much SLRs associated with PD-1 blockers. In summary, CPIs block the inhibitory signal of CD4(+) Th1 cells and this causes T cell activation and proliferation, and also macrophage activation, aggregation, and induction of granuloma formation [24]. On the other hand, it has been proposed by Saidha S et al. that sarcoidal reaction can be a response of the host to cancer antigens. These antigens are secreted to the blood or adverse effect of the chemotherapy causes damage to tissues. Granulomatous inflammation may develop as a result of this tissue damage [19].
Demographic findings
Age for SLR has been found between 26 and 83 with female predominance. SLR has been reported most commonly in cases with MM and this is followed by non-small cell lung cancer (NSCLC). There are very little cases with ovarian cancer, urothelial cancer, Hodgkin lymphoma, endometrial cancer, leiomyosarcoma, breast cancer, and urinary bladder cancer [20, 25,26,27,28,29,30,31]. There may be two reasons for this condition: firstly, MM is the most common disease treated by CPIs from the beginning of CPI era, and secondly, MM is one of the most immunogenic tumors among malignant tumors and there is high potential for the development of immune events like SLR.
The most commonly used CPIs causing SLR at the beginning was ipilimumab and in recent years pembrolizumab, nivolumab, and also combination of PD-1 and CTLA-4 blockade. Probably, these CPIs are the most commonly used in clinical practice and SLR has been reported most frequently with these agents. In a review covering 43 cases, the most commonly used CPI was pembrolizumab followed by ipilimumab, nivolumab, and ipilimumab–nivolumab combination. On the other hand, a few cases with SLR treated by PD-L1 blockers including durvalumab, atezolizumab, and avelumab have been reported [25,26,27,28].
Clinical findings
First cases with SLR have been presented associated with CTLA-4 blocker ipilimumab. SLR related with PD-1 blockers nivolumab and pembrolizumab have been reported in later years. The most commonly involved organs are lung, mediastinum, and skin. Multiple sites involving trunk, lymph nodes, extremities, and face have been reported in some cases treated by CPIs [22, 26, 32,33,34,35]. Indurated and tender papules and plaques on the lower legs have been detected in a case with endometrium cancer treated by pembrolizumab [34]. Cutaneous granulomatous reaction involving trunk, extremities, and face after 18 months of ipilimumab treatment has been presented by Cervantes. Figure 2 shows facial lesions associated with ipilimumab [33]. Arthralgia may be seen [32]. Renal injury is a late complication of CPIs and granulomatous reactions may be seen. Critical point is the early detection of this reaction and to prevent the irreversible changes in kidney [36]. Dry eye and parotid and salivary gland involvements have been reported. Isolated spleen involvement has been reported in a case treated with ipilimumab after 20 months of therapy [37].
Grouped crusty inflammatory papules distributed symmetrically on the face. Figure has been reprinted from [30]
In some cases, reactivation of previous granulomatous reaction has been reported after CPI treatment [20,21,22,23]. Granulomatous inflammation has been reported in a case with squamous cell cancer at the site of previously treated tumor and the presence of keratinous material within the granulomas. This finding may suggest an immune response against the tumor [36]. These points are important for the clinicians during CPI treatment.
Nine SLR cases with MM treated by ipilimumab have been reviewed by Danlos et al. Pulmonary involvement has been found to be the most prominent finding in these cases: 8 cases had lung lesions, 4 had skin lesions, 1 had cerebral, and 1 had spleen involvement. Granulomatous reactions have been found to be developed between 1 and 20 months of CPI treatments [7]. Lastly, 20 cases with SLR associated with ipilimumab have been reviewed by Cervantes et al. Sixteen of these cases had MM and one case in each: colon cancer, urothelial cancer, castration resistant prostate cancer, and NSCLC. Among these cases, 12 had skin, 14 had mediastinal and hilar lymph nodes, and 9 had skin involvement. In some of these cases, multiple organ involvements including lymph nodes, bronchial epithelium, spleen, liver, cella, and kidney have been found in 9 cases [30].
In the literature review, time to development of granuolmatous reaction has been found between 1 and 20 months. Informative excellent clinical/demographic findings for the clinicians using CPIs and development of SLR have been summarized in Tables of Hiraki’s and Cervantes’s papers [7, 25, 26, 30]. Median interval from the beginning of the therapy and the development of granulomatous reaction has been found as 3.2 months in a retrospective analysis. Resolution time of these reactions has been found as 3.1 months [8, 38].
Diffuse alveolar damage, pulmonary fibrosis, lymphocytic myocarditis, and also SLR has been found in central nervous system, liver, and bone marrow which were clinically unapparent and interestingly progressive disease has been found at small bowel, peritoneum, and brain in an autopsy case [39]. This case suggests that CPIs may cause widespread SLR not detected in clinical follow-up and clinicians must be aware of this situation and minimal symptoms must be alerted while their patients are receiving CPI.
Histopathologic findings
Histopathologic findings of granulomatous reaction and also terminology are highly variable: SLR, granulomatous panniculitis, granuloma annulare, granulomatous dermatitis, interstitial granulomatous dermatitis, granulomatous foreign body reactions, dermal granulomatous reaction, and non-necrotizing/non-caseating granulomas have been used. For this reason, SLR may be found to be lower than its real frequency due to chaotic terminology [7, 22, 25, 29, 30, 40].
Biopsy is essential point for diagnosis of SLR and shows sarcoid-like granulomas which include epithelioid histiocytes, lymphocytes, and multinucleated giant cells. There may be translucent amorphous crystal-like foreign bodies with 20–80 μm surrounded by histiocytes and phagocytized by giant cells [25, 32, 41]. Interestingly granulomatous melanosis mimicking underlying MM has been reported in a case treated with pembrolizumab. Figure 3 shows melanosis associated with CPI usage.
Dermal nodule consisting of well-formed sarcoid-like granulomas with pigment-laden melanophages consistent with tumoral melanosis. Melanocytes were absent. Figure has been reprinted from [47]
Histopathological findings have been evaluated in an autopsy study with an excellent detail by Koelzer et al. [39]: in myocardium,patchy fibrosis and diffuse mononuclear infiltrates up to 45 CD3(+) lymphocytes/mm2 (normal range 5.3 ± 5.7/mm2) and up to 18 CD68(+) macrophages (normal range 9.3 ± 4.3/mm2) have been found. CD8 expression has been detected in 65% of infiltrating lymphocytes: PD-1 expression has been detected 85% of T cells and TIA-1 expression has been found in 35% of these cells. These findings are similar to PD-1-associated myocarditis. These results may suggest that myocarditis is a type of SLR in cases treated by CPIs and clinically fatal myocarditis requires further evaluation with the possibility of SLR. In brain, severe aseptic lymphocytic meningitis and mononuclear infiltrates in brain parenchyma have been found. T cell subtype was CD8(+); up to 180 cells have been found in meninges and 15 cells in periventricular brain parenchyma; respectively. Among these cells, 45% had PD-1 and 60% showed TIA expression and concomitant CD68(+) histiocyte infiltration. Interestingly there was hypercellularity in bone marrow with diffuse lymphocytosis: 15% of these cells had CD3 and 10% had CD79 expression, and only 15% of these cells had CD8(+) expression. Lymphocytic infiltration in liver has been found.
Diagnosis
Diagnostic approach is biopsy from radiographically detected lesions and pathologic evaluation by a pathologist who is expert in sarcoidosis [25, 26]. Lymph node biopsy sample showing granulomas has been seen in Fig. 4. Of course in a case where granulomatous reaction and bacterial and fungal cultures are detected, polymerase chain reaction for viral agents and also gram stains must be done to exclude the infectious etiology. Silver stain for Pneumocystis jiroveci, auramine stain for acid-fast bacilli, and M Tuberculosis must be ruled out [7, 26, 30, 40]. Angiotensin-converting enzyme levels may be normal or high. CD4/CD8 ratios may be normal or high in bronchoalveolar lavage [26, 30, 31, 42, 43].
Lymph node biopsy showing well-formed giant cell granulomas. Figure has been reprinted from [32]
Thirty papers published between 2009 and 2018 and presented as granulomatous/SLRs associated with CTLA-4 and PD-1 inhibitors have been reviewed by Hiraki et al. These lesions had been named as SLR in 4 cases, granulomatous panniculitis in 4 cases, granuloma annulare in 4 cases, granulomatous dermatitis in 1 case, and granulomatous foreign body reaction in 1 case. Table 1 of Hiraki’s paper is informative for clinicians and pathologists [25].
Radiologic findings
There are no specific radiologic findings. Mediastinal/hilar lymphadenopathies suggesting typical S are the most frequently seen findings. Septal thickening and nodules in subpleural and fissure regions have been reported [27, 31, 32]. An important point to differentiate from S may be the presence of pleural fluid which is not seen in classical S [20]. Pituitary involvement mimicking neurosarcoidosis in addition to mediastinal involvement due to granulomatous reaction has been reported in a case with MM treated by ipilimumab [44]. Figure 5 shows pituitary involvement.
Pituitary and infundibular lesions before and after steroid treatment (a and b). Figure has been reprinted from [44]
Prognostic importance
It is very well known that the majority of immune-related adverse events associated with CPIs have been found to be related with better clinical outcome. In a similar way, better outcome has been reported in cases developing granulomatous/SLRs during CPI treatments by many authors [17, 38, 44, 45]. In a review covering 43 cases, clinical response has been found in more than half of the cases (8 complete response, 3 partial response and 10 stable disease) and this is better than normal population [25]. However, these lesions clinically could be diagnosed as disease progression and can cause treatment cessations or alterations which are harmful for the patients. Clinicians must be aware of these reactions and have to avoid from unnecessary drug changes or discontinuations [7, 25, 38, 43, 44, 46].
Treatment
There is no specific treatment for SLR. Asymptomatic cases do not need treatment. Discontinuation is important choice to decrease the severity of reactions. Generally, granulomatous reactions resolve after cessation of CPI [26, 33, 48, 49]. However, some cases do not require to stop CPI treatment [41].
Steroids must be used in cases not responding to discontinuation of CPIs and/or having severe symptoms and signs. Steroids may be used by local, systemic, or intralesional route. Hydroxychloroquine may be a choice in cases not responding to steroid. Excision of local lesion may be an option in some cases. Outcome of SLR is generally improvement and/or resolution of the lesions in about all of the cases [7, 25, 30, 33, 43].
Clinical importance and future directions
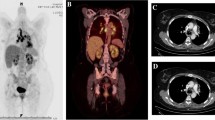
Clinicians should be aware of SLRs in cases treated by CPIs and tissues must be sampled and reviewed by an experienced pathologist to avoid misdiagnosis and CPI treatment stops. As a clinician we must consider that with the increased and widespread use of CPIs we will see more SLR and other immune reactions in clinical practice (Fig. 6).
PET/CT before and after 2 months of pembrolizumab treatment in a case showing hypermetabolic mediastinal and hilar lymph nodes. Figure has been reprinted from [32]
FDG PET/CT is the most frequently used imaging to measure the response to CPIs, but is not sensitive enough to predict atypical immune-related adverse events including pseudoprogression and SLR. At this point, newer imaging modalities including 18F-fluorothymidine (FLT) PET imaging may be useful. It is known that FLT is a substrate for thymidine kinase which is transported into the cell during DNA synthesis and trapped, but not incorporated into the DNA. With these properties, FLT uptake is a suitable marker of active DNA synthesis in vivo [50]. It has been hypothesized that FLT/PET is an important tool to show the proliferative activity of the tumor and has advantage to detect differentiate tumor progression from pseudoprogression associated with tumor infiltrating immune cells which have low proliferative capacity than tumor cells. This imaging has been used to detect the early response at 6 weeks to CPI than FDG/PET [51, 52]. By analogy, it can be proposed that FLT/PET may be used in cases with good clinical outcome in spite of progression detected by FDG/PET.
References
Hunt BM, Valliers E, Buduhan G, Aye R, Louie B. Sarcoidosis as a benign cause of lymphadenopathy in cancer patients. Am J Surg. 2009;197:629–32.
Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007;25:326–33.
Paydas S, Yavuz S, Disel U, Zeren H, Hastürk S, Hanta I, Ergin M, Sahin B. Granulomatous reaction after chemotherapy for Hodgkin’s disease. Leuk Res. 2002;26:967–70.
Suditu N, Negru D. Bacillus Calmette–Guerin therapy-associated granulomatous prostatitis mimicking prostate cancer on MRI: a case report and literature review. Mol Clin Oncol. 2015;3:249–51.
Clerigo V, Castro A, Mourato T, Gomes C. A rare case of granulomatous pneumonitis due to intravesical BCG for bladder cancer. Acta Med Port. 2019;32:316–20.
Eckert A, Schoeffler A, Dalle S, Phan A, Kiakouama L, Thomas L. Anti-CTLA4 monoclonal antibody induced sarcoidosis in a metastatic melanoma patient. Dermatology. 2009;218:69–70.
Danlos FX, Pages C, Baroudjian B, Vercellino L, Battistella M, Mimoun M, Jebali M, Bagot M, Tazi A, Lebbe C. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:133–6.
Tirumani SH, Ramaiya NH, Keraliya A, Bailey ND, Ott PA, Hodi FS Nishino M. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res. 2015;3:1185–92.
Facco M, Cabrelle A, Teramo A, Olivieri V, Gnoato M, Teolato S, Ave E, Gattazzo C, Fadini GP, Calabrese F, Semenzato G, Agostini C. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax. 2011;66:144–50.
Ten Berge B, Paats MS, Bergen IM, van den Blink B, Hoogsteden HC, Lambrecht BN, Hendriks RW, Kleinjan A. Increased IL17A expression in granulomas and in circulating memory T cells in sarcoidosis. Rheumatology. 2012;51:37–46.
Naccache JM, Antoine M, Wislez M, Fleury-Feith J, Oksenhendler E, Mayaud C, Cadranel J. Sarcoid-like pulmonary disorder in human immunodeficiency virus-infected patients receiving antiretroviral therapy. Am J Respir Crit Care Med. 1999;159:2009–13.
Ishida M, Hodohara K, Furuya A, Okuno H, Yoshii M, Horinouchi A, Yoshida T. Sarcoidal granulomas in the mediastinal lymph nodes after treatment for marginal zone lymphoma of the esophagus: report of a case with review of the concept of the sarcoidosis-lymphoma syndrome. Int J Clin Exp Pathol. 2014;7(7):4428–32.
Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46.
Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, McCauley JL, Abreu MT, Unutmaz D, Sundrud MS. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med. 2014;211:89–104.
Ramstein J, Broos CE, Simpson LJ, Ansel KM, Sun SA, Ho ME, Woodruff PG, Bhakta NR, Christian L, Nguyen CP, Antalek BJ, Benn BS, Hendriks RW, van den Blink B, Kool M, Koth LL. IFN-gamma-producing T-helper 17.1 cells are increased in sarcoidosis and are more prevalent than T-helper type 1 cells. Am J Respir Crit Care Med. 2016;193:1281–91.
Lomax AJ, Mcguire HM, Mcneil C, Choi CJ, Hersey P, Karikios D, Shannon K, Van Hal S, Carr U, Crotty A, Gupta SK, Hollingsworth J, Kim H, Groth BF, Mcgill N. Immunotherapy-induced sarcoidosis in patients with melanoma treated with PD-1 checkpoint inhibitors: case series and immunophenotypic analysis. Int J Rheum Dis. 2017;20:1277–85.
Braun NA, Celada LJ, Herazo-Maya JD, Abraham S, Shaginurova G, Sevin CM, Grutters J, Culver DA, Dworski R, Sheller J, Massion PP, Polosukhin VV, Johnson JE, Kaminski N, Wilkes DS, Oswald-Richter KA, Drake WP. Blockade of the programmed death-1 pathway restores sarcoidosis CD4(+) T cell proliferative capacity. Am J Respir Care Med. 2014;190:560–71.
Zou W, Wolchok JD, Chen L. PD-L1 (B7eH1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers and combinations. Sci Transl Med. 2016;8:328.
Saidha S, Sotirchos ES, Eckstein C. Etiology of sarcoidosis: does infection play a role? Yale J Biol Med. 2012;85:133–41.
Al-dliw M, Megri M, Shahoub I, Sahay G, Limjoco TI, Shweihat Y. Pembrolizumab reactivates pulmonary granulomatosis. Respir Med Case Rep. 2017;22:126–9.
Cotliar J, Querfeld C, Boswell WJ, Raja N, Raz D, Chen R. Pembrolizumab associated sarcoidosis. JAAD Case Rep. 2016;2:290–3.
Burillo-Martinez S, MoralesRaya C, Prieto-Barrios M, Rodriguez-Peralto JL, Ortiz-Romero P-L. Pembrolizumab-induced extensive panniculitis and nevus regression: two novel cutaneous manifestations of the post-immunotherapy granulomatous reactions spectrum. JAMA Dermatol. 2017;153:721–2.
Lopez AT, Khana T, Antonov N, Audrey-Bayan C, Geskin L. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664–9.
Wu J, Kwong BY, Martires KJ, Rieger KE, Chung WH, Iyer GV, Lacouture ME. Granuloma annulare associated with immune checkpoint inhibitors. J Eur Acad Dermatol Venereol. 2018;32:124–6.
Hiraki T, Hatanaka M, Arimura A, Kawahira H, Kirishimal M, Kitazono I, Horinouchi M, Higashi M, Kanekura T, Tanimoto A. Granulomatous/sarcoid-like reactions in the setting of programmed cell death-1 inhibition: a potential mimic of disease recurrence. J Cutan Pathol. 2020;47:154–60.
Mitchell MA, Hogan K, Amjadi K. Atezolizumab-induced sarcoid-like granulomatous reaction in a patient with urothelial cell carcinoma. Immunotherapy. 2018;10:1189–92.
Lafon M, Blaye C, Kind M, Bechade D, Chassaigne F, Italiano A, Grellety T. Sarcoidosis-like reaction in metastatic triple negative breast cancer treated by anti-PD-L1. Breast J. 2019;25:971–3.
Balestra R, Benzaquen S, Wang J. Sarcoidosis-like granulomatous lung reaction associated with anti-programmed death receptor-1 ligand therapy. Ann Am Thorac Soc. 2017;14:296–9.
Garcıa-Manso IG, del Mar Garda Rodenas M, Barroso Medel ME, Illân Gambın FJ. Sarcoidosis-like granulomatous reaction associated with pembrolizumab immunotherapy. Sci Lett/Arch Bronconeumol. 2018;54:592–3.
Cervantes J, Rosen A, Dehesa L, Dickinson G, Alonso-Llamazares J. Granulomatous reaction in a patient with metastatic melanoma treated with ipilimumab: first case reported with isolated cutaneous findings. Actas Dermosifiliogr. 2019;110(1):43–9.
Honigman AD, Lai F, Elakis J, Prall O, Goh M, McCormack C. Pembrolizumab-induced sarcoid granulomatous panniculitis and bullous pemphigoid in a single patient. Clin Case Rep. 2019;7:773–5.
Cousin S, Toulmonde M, Kind M, Cazeau AL, Bechade D, Coindre JM, Italiano A. Pulmonary sarcoidosis induced by the anti-PD1 monoclonal antibody pembrolizumab. Ann Oncol. 2016;27:1178–9.
Firwana B, Ravilla R, Raval M, Hutchins L, Mahmoud F. Sarcoidosis-like syndrome and lymphadenopathy due to checkpoint inhibitors. J Oncol Pharm Pract. 2017;23:620–4.
Reuss JE, Kunk PR, Stowman AM, Gru AA, Slingluff CL, Gaughan EM. Sarcoidosis in the setting of combination ipilimumab and nivolumab immunotherapy: a case report and review of the literature. J Immunother Cancer. 2016;4:94.
Wigenhof S, Mortion V, Seghers AC. Du four S, Wanderlinden E, Hanon S, Sarcoidosis in a patient with metastatic melanoma sequentially treated with anti-CTLA-4 monoclonal antibody and selective BRAF inhibitör. Anticancer Res. 2012;32:1355–9.
Izzedine H, Mateus C, Boutros C, Robert C, Rouvier P, Amoura Z, Mathian A. Renal effects of immune checkpoint inhibitors. Nephrol Dial Transplant. 2017;32:936–42.
Andersen R, Norgaard P, Al-Jailawi MK, Svane IM. Late development of splenic sarcoidosis-like lesions in a patient with metastatic melanoma and long-lasting clinical response to ipilimumab. Oncoimmunology. 2014;3:954506.
van Baar MLM, Guminski AD, Ferguson PM, Martin LK. Pembrolizumab for cutaneous squamous cell carcinoma: report of a case of inoperable squamous cell carcinoma with complete response to pembrolizumab complicated by granulomatous inflammation. JAAD Case Rep. 2019;5:491–4.
Koelzer VH, Rothschild SI, Zihler D, Wicki A, Willi B, Willi N, Voegeli M, Cathomas G, Zippelius A, Mertz KD. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors—an autopsy study. J Immunother Cancer. 2016;4:1–8.
Cornejo CM, Haun P, English J, Rosenbach M. Immune checkpoint inhibitors and the development of granulomatous reactions. J Am Acad Dermatol. 2018. https://doi.org/10.1016/j.jaad.2018.07.051.
Trinidad C, Nelson KC, Glitza Oliva IC, Torres-Cabala CA, Nagarajan P, Tetzlaff MT, Ivan D, Hwu WJ, Prieto VG, Curry JL, Aung PP. Dermatologic toxicity from immune checkpoint blockade therapy with an interstitial granulomatous pattern. J Cutan Pathol. 2018;45:504–7.
Berthol G, Lazor R, Letonovic I, Romano E, Noirez L, Stalder JM, Speiser DE, Peters S, Michielin O. Pulmonary sarcoid-like granulomatosis induced by ipilimumab. J Clin Oncol. 2012;30:156–9.
Montaudie H, Pradelli J, Passeron T, Lacour JP, Leroy S. Pulmonary sarcoid-like granulomatosis induced by nivolumab. Br J Dermatol. 2017;176:1060–3.
Murphy KP, Kennedy MP, Barry JE, O’Regan KN, Power DG. New-onset mediastinal and central nervous system sarcoidosis in a patient with metastatic melanoma undergoing CTLA4 monoclonal antibody treatment. Oncol Res Treat. 2014;37:351–3.
Tetzlaff MT, Nelson KC, Diab A, Staerkel GA, Nagarajan P, Torres-Cabala CA, Chasen BA, Wargo JA, Prieto VG, Amaria RN, Curry JL. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer. 2018;6(1):14.
Zhang Y, Liu Z, Tian M, Hu X, Wang L, Ji J, Liao A. The altered PD-1/PD-L1 pathway delivers the ‘one-two punch’ effects to promote the Treg/Th17 imbalance in pre-eclampsia. Cell Mol Immunol. 2018;15:710–23.
Woodbeck R, Metelitsa AI, Naert KA. Granulomatous tumoral melanosis associated with pembrolizumab therapy: a mimicker of disease progression in metastatic melanoma. Am J Dermatopathol. 2018;40:523–6.
O’Kane GM, Labre C, Doherty MK, Young K, Albalâ H, Leigbl NB. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. 2017;22:70–80.
Weber JS, Postow M, Lao CD, Schadendorf D. Management of adverse events following treatment with anti-programmed death-1 agents. Oncologist. 2016;21:1230–40.
Crandall JP, Tahari AK, Juergens RA, Brahmer JR, Rudin CM, Esposito G, Subramaniam DS, Knopp MV, Hall NC, Gajwani P, Leal JP, Lodge MA, Joo HO, Gabrielson EW, Shankar LK, Wahl RL. A comparison of FLT to FDGPET/CT in the early assessment of chemotherapy response in stages IB–IIIA resectable NSCLC. EJNMMI Res. 2017;7:8.
Yeh R, Trager MH, Rizk ME, Finkel GG, Barker LW, Carvajal RD, Geskin LJ, Schwartz GK. FLT-PET at 6 weeks predicts response assessed by CT at 12 weeks in melanoma patients treated with pembrolizumab. Clin Nucl Med. 2020;45:267–77.
Li Z, Graf N, Herrmann K, Jünger A, Aichler M, Feuchtinger A, Baumgart A, Walch A, Peschel C, Schwaiger M, Buck A, Keller U, Dechow T. FLT-PET is superior to FDG-PET for very early response prediction in NPM-ALK-positive lymphoma treated with targeted therapy. Cancer Res. 2012;72:5014–24.
Funding
There is no financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paydas, S. Sarcoid-like reaction in cases treated by checkpoint inhibitors. Med Oncol 38, 29 (2021). https://doi.org/10.1007/s12032-021-01477-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01477-y