Abstract
Lysosome-associated protein transmembrane-4 beta (LAPTM4B) is a novel oncogene, which has been indicated to be dramatically overexpressed in various malignant tumors. The aims of this study were to detect LAPTM4B protein expression in patients with non-small cell lung cancer (NSCLC) and then analyze the relationships of LAPTM4B protein with clinicopathologic factors, tumor angiogenesis and prognosis with SPSS statistical software. Immunohistochemistry was used to examine the expression of LAPTM4B and CD34 proteins in NSCLC tissues, and its results showed that LAPTM4B protein expression in NSCLC tissues was significantly higher than that in normal lung tissues (P < 0.001). Of the186 NSCLC cases, 129 (69.35 %) had strong expression of LAPTM4B protein, which was associated with histopathologic differentiation (P = 0.017), lymph node metastasis (P = 0.001) and TNM stage (P = 0.046), as well as the microvessel density (MVD) (P = 0.019). Kaplan–Meier survival analysis revealed that patients with strong LAPM4B protein expression and high MVD might have poor overall survival (OS; P = 0.001, P = 0.002, respectively) and disease-free survival (DFS; P = 0.002, P = 0.038, respectively). Multivariate analysis demonstrated that LAPTM4B protein was an independent prognostic marker for OS and DFS of NSCLC patients (P = 0.037, P = 0.046, respectively). These findings illustrated that LAPTM4B protein was closely associated with NSCLC progression, angiogenesis and poor prognosis, suggesting that LAPTM4B protein could be applied not only in predicting patient’s outcome, but also in antiangiogenic therapy as a possible novel target molecule.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the most commonly diagnosed malignant tumor in the world. It is reported that the number of deaths of lung cancer amounts to 1.5 million, occupying 19 % of all cancer-related deaths in 2010, which was the leading cause of cancer-related deaths worldwide [1]. Among all the cases, non-small cell lung cancer (NSCLC) accounts for 80 % [2]. In the past decades, the 5-year survival rate of NSCLC has remained at 15 % [3], and conventional treatments are not effective enough to improve the prognosis of the disease. It is widely accepted that the molecular carcinogenesis of NSCLC is characterized by multiple alterations of gene expression, which leads to abnormal changes in signaling pathways and biological behaviors. Based on the theory, drugs targeting certain gene mutations have been identified to modify the host responses to tumors or directly inhibit tumor cell growth, but the therapeutic effect is far from satisfactory. Hence, there is a need to identify novel molecules for the diagnosis and therapy of NSCLC.
Lysosome-associated protein transmembrane-4 beta (LAPTM4B), a novel oncogene candidate, was initially identified in hepatocellular carcinoma. According to BLAST program analysis, it was located in chromosomes 8q22 and composed of seven exons separated by six introns [4, 5]. Previous report indicated that LAPTM4B might play important roles in the initiation, promotion and metastasis of tumors [6]. Evidences from various literatures have shown that LAPTM4B protein was dramatically overexpressed in various malignant tumors [7–13]. However, there has not been research reporting its role in NSCLC, and the clinicopathologic significance of the LAPTM4B protein in NSCLC remains unclear.
Tumor angiogenesis, the formation of new capillary blood vessels, is an essential requirement for carcinogenesis, progression and metastasis of malignant tumors [14, 15]. The microvessel density (MVD) is now widely used to evaluate the degree of tumor angiogenesis and has been proven to be associated with the metastasis and prognosis of a wide range of human tumors [16–18]. Recent study showed that the tissues with LAPTM4B protein overexpression had significantly much more new blood vessels than those of weak expression in a mouse model of liver cancer, indicating that LAPTM4B protein might play potential roles in tumor angiogenesis [19]. Thus, it is really of great importance to investigate whether there is a possible angiogenic property of LAPTM4B in NSCLC.
In the present study, we firstly detected and compared the expression of LAPTM4B protein in 186 NSCLC tissues and 73 normal control tissues using immunohistochemistry, and then the relationships between LAPTM4B protein expression and clinicopathologic parameters were investigated. Furthermore, survival analysis was adopted to evaluate LAPTM4B protein as a biomarker with regard to the prognosis. In addition, we also explored the roles of LAPTM4B protein in tumor angiogenesis. Our study attempted to discover the potential function of LAPTM4B protein in the development of NSCLC.
Materials and methods
Patients and specimens
We collected primary tumor specimens from 197 patients who underwent surgical resection and were diagnosed as lung adenocarcinoma or squamous cell carcinoma at the Department of Thoracic Surgery, Qilu Hospital, Shandong University between March 2006 and December 2008. Large-cell carcinoma, adenosquamous carcinoma and other NSCLC subtypes were not included in this study. Meanwhile, we also randomly obtained 73 normal lung tissues taken more than 5 cm from the margin of the tumors. For all patients, histological type of lung cancer was determined by the World Health Organization classifications, and pathological staging was based on the international staging system revised in 2009 [20]. None of the patients received radiotherapy or chemotherapy before surgical resection, and all patients were treated with routine chemotherapy after the operation. Of these 197 patients, 11 cases were excluded (six cases lost to follow-up, five cases died of perioperative complications), so a total of 186 patients were enrolled in the retrospective study. Each patient signed informed consent according to the Helsinki Declaration, and this study was approved by the Ethics Committee of Qilu Hospital.
Immunohistochemistry staining
Tissue samples were fixed with 10 % formaldehyde solution (pH 7.4) and embedded in paraffin wax. The paraffin-embedded serial tissues were sectioned at a thickness of 4 μm and stained with hematoxylin–eosin for tumor confirmation. Sections adjacent to the hematoxylin–eosin-stained sections were used for immunohistochemical staining. Tissue sections were deparaffinized with xylene and rehydrated in graded alcohol concentrations under the standard procedures. Then, all the deparaffinized sections were immersed in 0.01 mol/L citrate buffer (pH 6.0) and heated (95 °C) for 15 min in a microwave oven. After cooling down, samples were incubated with 3 % hydrogen peroxide (H2O2) for 15 min to block endogenous peroxide activity and with 10 % normal goat serum for 30 min to block non-specific immunoglobulin binding. Subsequently, the sections were incubated at 4 °C overnight with primary rabbit polyclonal LAPTM4B antibody (1:200 dilution; provided by Dr. Rouli Zhou, Department of Cell Biology, Peking University Health Science Center) and primary rabbit monoclonal CD34 antibody (2150-1, 1:500 dilution; Epitomics), respectively. In the next day, the sections were rinsed with PBS and incubated for 30 min at 37 °C with biotin-labeled secondary antibody followed by horseradish peroxidase conjugated streptavidin for 30 min. At last, the sections were treated with 3,3′-diaminobenzidine tetrahydrochloride (Dako, Hamburg, Germany) in 0.01 % H2O2 for 7 min, then counterstained with hematoxylin for 60 s and mounted with neutral balsam. Immunohistochemistry was performed using an immunohistochemistry kit (OriGene, Beijing, China) according to the manufacturer’s instructions. Negative controls were performed by replacing the primary antibody with normal rabbit serum. The positive controls were ovarian carcinomas with positive expression of LAPTM4B protein [10].
Staining evaluation
For LAPTM4B protein expression degree, it was based on the percentage of positive stained cancer cells and the staining intensity. The percentage of immunoreactive cells was scored as follows: 0, <10 %; 1, 10–50 %; 2, >50 %, and the staining intensity was rated as follows: 0, negative staining or weak staining; 1, moderate staining; 2, intense staining. The overall score of LAPTM4B protein expression was the sum of scores of the staining intensity and the percentage of positive stained cancer cells. A total staining score of 0–4 was calculated and divided into two groups: a low-expression group with an overall score between 0 and 2 and a high-expression group with an overall score between 3 and 4 [21].
For intratumoral MVD assessment, microvessels were recorded by counting CD34-positive stained endothelial cells according to the international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid tumors [22]. Initially, sections were scanned at low power (100× magnification) to identify the areas with the highest vascularity. Subsequently, calculations of the stained microvessels were performed on three consecutive high-power (200× magnification) fields within this area. Any brown stained endothelial cell that was clearly separated from adjacent microvessels was considered as a single countable microvessel [23]. The value of MVD was evaluated by the average of three 200× field microvessel counts. Tumors with microvessels ≥90 were classified as high MVD, while tumors with microvessels <90 were classified as low MVD according to the previous reports [24, 25].
Tissue specimens were examined separately by two pathologists under double-blinded conditions without prior knowing of the clinical outcomes of the specimens. The independent scores assigned by the two pathologists were combined as a final score. In cases of significant disagreement, the contradictory scores were reviewed between the two pathologists by discussion.
Follow-up
Each patient was scheduled for an examination every 3 months for the first 2 years and at 6-month intervals thereafter during the follow-up period, which included physical examination, blood analysis, chest X-ray, ultrasound examination, computed tomography. Tumor relapse was based on clinical, radiological or histological diagnosis, and the site and time of tumor relapse were both recorded. Follow-up was completed until December 2013 in 186 patients.
Statistical analysis
SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA) was used to perform statistical analysis. The chi-squared test or the Fisher’s exact was used to examine the association between LAPTM4B protein expression and various patients’ clinicopathologic factors. Survival curves were assessed by using the Kaplan–Meier method, and statistical differences were compared with a log-rank test. Multivariate Cox regression analysis was carried out to identify the potential prognostic factors of NSCLC patients. P value <0.05 was considered to be statistical significance.
Results
Up-regulation of LAPTM4B protein in NSCLC
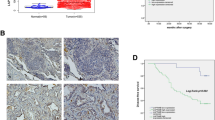
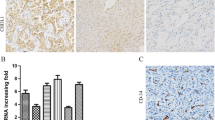
Immunohistochemical analysis of 186 NSCLC tissues and 73 normal lung tissues revealed that the staining of LAPTM4B protein was mainly located in the cell cytoplasm, and various intensities could be observed in different tissues shown in Fig. 1. Among the 186 patients, 124 patients (66.67 %) were men and 62 patients (33.33 %) were women, ranging in age from 32 to 80 years. Patients’ characteristics, such as gender, age, tumor size, TNM stage and tumor stage factors, were obtained from the medical records, which were summarized in Table 1. We found that 129 of 186 NSCLC tissues (69.35 %) showed LAPTM4B protein overexpression, and the other 57 cases (30.65 %) showed weak positive or negative LAPTM4B protein expression. In the normal lung tissues, most cases were negative LAPTM4B protein expression, while only 11 of 73 normal lung tissues (15.07 %) showed LAPTM4B protein expression positive. The difference between cancer tissues and normal lung tissues in LAPTM4B protein expression was statistically significant (P < 0.001) presented in Table 2.
Staining of LAPTM4B and CD34 proteins by immunohistochemistry. a, b Negative LAPTM4B staining in normal alveolar pneumocytes and bronchial epithelium. c Strong LAPTM4B staining in cancer cells. d Weak LAPTM4B staining in cancer cells. e Negative LAPTM4B staining in cancer cells. f Intratumoral microvessels were brown immunostained by the anti-CD34 antibody in NSCLC tissues. Original magnification, ×200
Correlation between LAPTM4B protein and clinicopathologic factors in patients with NSCLC
To investigate whether LAPTM4B protein was related to NSCLC progression, we analyzed the correlation of LAPTM4B protein expression with clinicopathologic factors in 186 NSCLC tissues by chi-squared test shown in Table 1. We found that high LAPTM4B protein expression was significantly associated with poor histopathologic differentiation (P = 0.017), positive lymph node metastasis (P = 0.001) and high TNM stage (P = 0.046), but not with gender, age, pathological type and smoking (P > 0.05).
Correlation between MVD and clinicopathologic factors in patients with NSCLC
Intratumoral MVD was quantified by counting CD34-positive endothelial cells in the same series of NSCLC tissues shown in Fig. 1 and the staining intensity of MVD ranged broadly from 27 to 158 microvessels/200× magnification field. Then, all the cases were divided into high-level group and low-level group according to the cutoff value of 90 microvessels/200× magnification field. As shown in Table 1, 99 cases (53.22 %) showed high MVD. Although a statistically significant difference was not detected, high MVD tended to be associated with poor histopathologic differentiation (P = 0.052) and positive lymph node metastasis (P = 0.069), and there was no statistically significant correlation between MVD and any other clinicopathologic factors (P > 0.1) (Table 1).
Correlation between LAPTM4B protein and MVD in patients with NSCLC
In LAPTM4B protein overexpression group, 76 of 129 cases (58.91 %) had high MVD, while in the other group, only 23 of 57 cases (40.35 %) showed high MVD. There was a positive correlation between LAPTM4B protein and MVD (P = 0.019) shown in Table 1.
Univariate and multivariate survival analyses
We conducted survival analysis in these 186 patients to investigate the impact of LAPTM4B protein on NSCLC prognosis. Of the 186 patients, 58 patients (31.2 %) were alive and 128 patients (68.8 %) had died of the disease within 5 years after operation. In univariate analysis, we first analyzed the association between the conventional clinicopathologic factors and patients’ outcomes. The related data were presented in Table 3. It was shown that poor histopathologic differentiation, positive lymph node metastasis and high TNM stage were associated with a significantly worse overall survival (OS) and disease-free survival (DFS). Then, we analyzed the prognostic significance of LAPTM4B protein and MVD. The survival analysis revealed that the OS rate of cases with LAPTM4B protein overexpression (24 vs. 47.4 %, P = 0.001) and high MVD (22.2 vs. 41.4 %, P = 0.002) was significantly lower than that of the remaining cases, respectively. In addition, Kaplan–Meier analysis of DFS also demonstrated poor 5-year survival rate in patients with LAPTM4B protein overexpression (14 vs. 33.3 %, P = 0.002) and high MVD (17.2 vs. 23 %, P = 0.038), respectively, which were shown in Fig. 2. To explore the possibility of crosstalk between LAPTM4B protein and MVD, we further examined the survival differences of patients stratified for low MVD and high MVD according to LAPTM4B protein expression status. Statistical analysis demonstrated that the OS in the low-MVD group was potentially higher than that in the high-MVD group (32.1 vs. 18.4 %, P = 0.03) shown in Table 4. However, the other three analyses showed no significant differences.
A multivariate Cox regression model adjusted for statistically significant prognostic factors was performed to analyze the independent factors on NSCLC prognosis. It was shown LAPTM4B protein expression (P = 0.037, P = 0.046, respectively), lymph node metastasis (P = 0.051, P = 0.049, respectively) and TNM stage (P = 0.033, P = 0.027, respectively) were identified as independent prognostic factors of patients’ OS and DFS in Table 5.
Discussion
NSCLC is the most aggressive and deadly tumor in solid tumors. Usually, the TNM stage is the crucial factor in the prognosis of patients with NSCLC, but it is also true that there are marked differences in survival of patients with the same pathologic stage of the disease, so a diverse range of genetic abnormalities may also be involved in the recurrence and early metastasis of NSCLC [26], it is of significance to explore new prognostic markers to screen patients for unfavorable prognosis, which could be utilized as possible therapeutic targets.
LAPTM4B belonging to the mammalian LAPTM family was originally identified as a hepatocellular carcinoma-associated gene [4]. Subsequently, it was found upregulated in many human cancers [6]; moreover, a number of studies confirmed its critical role in tumorigenesis, promotion, metastasis and prognosis in some human malignancies [7–10, 12, 13, 27]. Furthermore, extensive studies have been performed to account for such outcomes. Yang et al. [27] showed that the overexpression of LAPTM4B protein might promote carcinogenesis of L02 cell line which originated from normal human liver and enhance the growth and metastasis of the xenografts in nude mice. Later it was revealed that proliferation of the cells overexpressing LAPTM4B protein was out of control, which could be accounted for by the dysfunction of some proliferation-associated factors and apoptosis-related proteins [28, 29]. It was also found that LAPTM4B protein played a role in drug resistance. Recent literatures reported LAPTM4B protein sensitized cells to anthracycline and epirubicin resistance, which was likely to be involved in promoting drug efflux by activating PI3K/AKT signaling [30–32]. In addition, LAPTM4B was closely associated with cancer cell migration, invasion and autophage [33]. The structure of LAPTM4B protein was complicated, and many specific domains might serve as binding sites of signal molecules for the signaling transduction. According to the structure of LAPTM4B protein, we assumed that it could interact with some cancer-related proteins, such as protein phosphatase 2A and protein kinase C, to play a role in oncogenesis, which required further research.
In our study, we detected the expression of LAPTM4B protein and then analyzed the prognostic value of LAPTM4B protein in patients with NSCLC. Our results demonstrated significant associations of LAPTM4B protein with prognosis-related features, including histopathologic differentiation, lymph node metastasis and TNM stage. Thus, it was likely that LAPTM4B protein played important roles in NSCLC progression. Further analysis revealed the OS and DFS rate of patients with high LAPTM4B protein expression was worse than those with none or weak LAPTM4B protein expression, indicating that LAPTM4B protein overexpression was significantly related with poor 5-year survival rate. Multivariate survival analysis based on the Cox regression model manifested that, among all the factors analyzed, LAPTM4B was a significant independent prognostic factor for both OS and DFS of NSCLC patients following curative resection, suggesting its potential as a prognostic biomarker of NSCLC.
It is well known that tumors are endowed with angiogenic capability, and their growth, invasion, and metastasis are all angiogenesis-dependent [34, 35]. In order to verify whether there was a relationship between LAPTM4B protein and tumor angiogenesis, we quantified the MVD, which was the standard method of measuring tumor angiogenesis and was closely related with tumor growth and postoperative prognosis. The findings of our study showed that LAPTM4B protein overexpression was significantly associated with increased angiogenic activity measured as CD34-determined intratumoral MVD, suggesting that LAPTM4B protein might promote tumor progression by the induction of tumor angiogenesis. Up to now, the exact molecular mechanisms by which LAPTM4B promotes the formation of intratumoral microvessels are far from clear yet; therefore, further studies are needed to elucidate the detailed molecular mechanisms.
In conclusion, our results provided evidences that LAPTM4B protein expression was higher in NSCLC tissues, and for the first time, revealed significant associations of LAPTM4B protein expression with various clinicopathologic characteristics and prognosis in patients with NSCLC. In particular, LAPTM4B protein was closely correlated with tumor angiogenesis and contributed to the formation of new tumor microvessels as an angiogenic factor. Moreover, survival analysis showed that LAPTM4B was an independent, negative prognostic factor for overall and DFS in NSCLC. All these findings suggested that LAPTM4B protein might have clinical potential not only as a promising prognostic predictor to identify individuals with poor outcomes but also as a novel therapeutic target in antiangiogenesis for NSCLC patients.
References
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128.
Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117(2):294–9.
Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell. 2002;1(1):49–52.
Liu X, Zhou R, Zhang Q, Zhang Y, Shao G, Jin Y, et al. Identification and characterization of LAPTM4B encoded by a human hepatocellular carcinoma-associated novel gene. J Peking Univ. 2003;35(4):340–7.
Shao GZ, Zhou RL, Zhang QY, Zhang Y, Liu JJ, Rui JA, et al. Molecular cloning and characterization of LAPTM4B, a novel gene upregulated in hepatocellular carcinoma. Oncogene. 2003;22(32):5060–9.
Kasper G, Vogel A, Klaman I, Grone J, Petersen I, Weber B, et al. The human LAPTM4b transcript is upregulated in various types of solid tumours and seems to play a dual functional role during tumour progression. Cancer Lett. 2005;224(1):93–103.
Zhang G, Liang Y, Huang Y, Chen Y, Zhou R. Elevated lysosome-associated protein transmembrane-4beta-35 is an independent prognostic marker in pancreatic carcinoma. J Int Med Res. 2012;40(4):1275–83.
Zhou L, He XD, Cui QC, Zhou WX, Qu Q, Zhou RL, et al. Expression of LAPTM4B-35: a novel marker of progression, invasiveness and poor prognosis of extrahepatic cholangiocarcinoma. Cancer Lett. 2008;264(2):209–17.
Yin M, Lou C, Zhang W, Meng F, Zhang H, Ning X, et al. LAPTM4B overexpression is a novel independent prognostic marker for metastatic ovarian tumors. Int J Gynecol Cancer. 2012;22(1):54–62.
Yin M, Xu Y, Lou G, Hou Y, Meng F, Zhang H, et al. LAPTM4B overexpression is a novel predictor of epithelial ovarian carcinoma metastasis. Int J Cancer. 2011;129(3):629–35.
Zhou L, He XD, Chen J, Cui QC, Qu Q, Rui JA, et al. Overexpression of LAPTM4B-35 closely correlated with clinicopathological features and post-resectional survival of gallbladder carcinoma. Eur J Cancer. 2007;43(4):809–15.
Yang H, Xiong FX, Lin M, Yang Y, Nie X, Zhou RL. LAPTM4B-35 overexpression is a risk factor for tumor recurrence and poor prognosis in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136(2):275–81.
Meng F, Luo C, Hu Y, Yin M, Lin M, Lou G, et al. Overexpression of LAPTM4B-35 in cervical carcinoma: a clinicopathologic study. Int J Gynecol Pathol. 2010;29(6):587–93.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Makrilia N, Lappa T, Xyla V, Nikolaidis I, Syrigos K. The role of angiogenesis in solid tumours: an overview. Eur J Intern Med. 2009;20(7):663–71.
Bremnes RM, Camps C, Sirera R. Angiogenesis in non-small cell lung cancer: the prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and blood. Lung Cancer (Amsterdam, Netherlands). 2006;51(2):143–58.
Murakami K, Kasajima A, Kawagishi N, Sekiguchi S, Fujishima F, Watanabe M, et al. The prognostic significance of vasohibin 1-associated angiogenesis in patients with hepatocellular carcinoma. Hum Pathol. 2014;45(3):589–97.
Saponaro C, Malfettone A, Ranieri G, Danza K, Simone G, Paradiso A, et al. VEGF, HIF-1alpha expression and MVD as an angiogenic network in familial breast cancer. PLoS One. 2013;8(1):e53070.
Li L, Shan Y, Yang H, Zhang S, Lin M, Zhu P, et al. Upregulation of LAPTM4B-35 promotes malignant transformation and tumorigenesis in L02 human liver cell line. Anat Rec (Hoboken). 2011;294(7):1135–42.
Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 7th ed. Oxford: Blackwell; 2010.
Kang Y, Yin M, Jiang W, Zhang H, Xia B, Xue Y, et al. Overexpression of LAPTM4B-35 is associated with poor prognosis in colorectal carcinoma. Am J Surg. 2012;204(5):677–83.
Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002;38(12):1564–79.
Yuan A, Yu CJ, Shun CT, Luh KT, Kuo SH, Lee YC, et al. Total cyclooxygenase-2 mRNA levels correlate with vascular endothelial growth factor mRNA levels, tumor angiogenesis and prognosis in non-small cell lung cancer patients. Int J Cancer. 2005;115(4):545–55.
Li SH, Tian H, Yue WM, Li L, Li WJ, Chen ZT, et al. Overexpression of metastasis-associated protein 1 is significantly correlated with tumor angiogenesis and poor survival in patients with early-stage non-small cell lung cancer. Ann Surg Oncol. 2011;18(7):2048–56.
Kadota K, Huang CL, Liu D, Ueno M, Kushida Y, Haba R, et al. The clinical significance of lymphangiogenesis and angiogenesis in non-small cell lung cancer patients. Eur J Cancer. 2008;44(7):1057–67.
Coate LE, John T, Tsao MS, Shepherd FA. Molecular predictive and prognostic markers in non-small-cell lung cancer. Lancet Oncol. 2009;10(10):1001–10.
Yang Y, Yang H, McNutt MA, Xiong F, Nie X, Li L, et al. LAPTM4B overexpression is an independent prognostic marker in ovarian carcinoma. Oncol Rep. 2008;20(5):1077–83.
Liu X, Xiong F, Wei X, Yang H, Zhou R. LAPTM4B-35, a novel tetratransmembrane protein and its PPRP motif play critical roles in proliferation and metastatic potential of hepatocellular carcinoma cells. Cancer Sci. 2009;100(12):2335–40.
Yang H, Xiong F, Wei X, Yang Y, McNutt MA, Zhou R. Overexpression of LAPTM4B-35 promotes growth and metastasis of hepatocellular carcinoma in vitro and in vivo. Cancer Lett. 2010;294(2):236–44.
Li L, Wei XH, Pan YP, Li HC, Yang H, He QH, et al. LAPTM4B: a novel cancer-associated gene motivates multidrug resistance through efflux and activating PI3K/AKT signaling. Oncogene. 2010;29(43):5785–95.
Li Y, Zou L, Li Q, Haibe-Kains B, Tian R, Li Y, et al. Amplification of LAPTM4B and YWHAZ contributes to chemotherapy resistance and recurrence of breast cancer. Nat Med. 2010;16(2):214–8.
Zhou L, He XD, Yu JC, Zhou RL, Shan Y, Rui JA. Overexpression of LAPTM4B-35 attenuates epirubucin-induced apoptosis of gallbladder carcinoma GBC-SD cells. Surgery. 2011;150(1):25–31.
Li Y, Iglehart JD, Richardson AL, Wang ZC. The amplified cancer gene LAPTM4B promotes tumor growth and tolerance to stress through the induction of autophagy. Autophagy. 2012;8(2):273–4.
Ribatti D, Vacca A. The role of microenvironment in tumor angiogenesis. Genes Nutr. 2008;3(1):29–34.
Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev. 2010;10(2):138–46.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No. 30571844), the Science and Technology Development Foundation of Shandong Province (No. 2009GG10002007) and the National Natural Science Foundation of Shandong Province (No. ZR2013HM089). We would like to express my great gratitude to Dr. Rouli Zhou for providing LAPTM4B antibody and all the people participating in the study.
Conflict of interest
We declare that we have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, H., Tian, H., Yue, W. et al. Overexpression of LAPTM4B is correlated with tumor angiogenesis and poor prognosis in non-small cell lung cancer. Med Oncol 31, 974 (2014). https://doi.org/10.1007/s12032-014-0974-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0974-8