Abstract
The aim of this study was to explore prognostic factors for non-small cell lung cancer (NSCLC) patients with brain metastases (BM) on the basis of EGFR mutation status. Among 779 consecutive NSCLC patients who underwent EGFR mutation screening, all 197 patients with BM were divided according to the EGFR mutation status. The prognostic factors, including patient characteristics at the time of BM diagnosis, treatment history, and radiologic features, were analyzed. Of 197 patients with BM, 108 had wild-type EGFR and 89 had EGFR mutation. The patients with EGFR mutation presented longer overall survival after BM diagnosis (OS) than those with wild-type EGFR, regardless of whether BM was synchronous or metachronous. For the patients with EGFR mutation, favorable prognostic factors in multivariate analysis were age <65 (p = 0.037), good performance status (PS) (p < 0.0001), cranial radiotherapy (p = 0.020), previous chemotherapy ≤1 regimen (p = 0.009), stable extracranial disease at BM diagnosis (p = 0.022), and erlotinib therapy after BM diagnosis (p = 0.0015). On the other hand, favorable prognostic factors for the patients with wild-type EGFR were only good PS (p = 0.0037) and cranial radiotherapy (p = 0.0005). Among patients treated with erlotinib after BM diagnosis, the patients with exon 19 deletion showed longer OS than those with exon 21 point mutation (p = 0.019). The prognostic factors for NSCLC patients with BM were different according to the EGFR mutation status. Particularly in NSCLC patients with EGFR mutation and stable extracranial disease, regular cranial evaluation for detecting asymptomatic BM would lead to good prognosis. In addition, erlotinib therapy would be preferable in NSCLC patients with BM and EGFR mutation, especially those with exon 19 deletion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer remains the leading cause of cancer-related death worldwide. More than 25 % patients with lung cancer develop brain metastases (BM) during the clinical courses [1, 2]. Despite treatment with systemic chemotherapy and/or local radiotherapy, the prognosis of lung cancer patients with BM was extremely poor [3–5].
Recently, epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), including gefitinib and erlotinib, have been reported effective for non-small cell lung cancer (NSCLC) with EGFR mutation [6, 7]. In addition, those drugs have been reported to present a positive effect on BM from NSCLC with EGFR mutation [8]. Therefore, EGFR-TKIs have become an efficient therapeutic option for BM from NSCLC with EGFR mutation. To date, the favorable prognostic factors for NSCLC patients with BM reported the presence of EGFR mutation, stable extracranial disease at the time of BM diagnosis, and eastern cooperation oncology group (ECOG) performance status (PS) ≦1 [9, 10]. However, there are no reports to investigate prognostic factors on the basis of EGFR mutation status. In our previous study, we reported that patients with BM from NSCLC with exon 19 deletion had multiple small BM with scarce brain edema, although this study included only patients with BM detected at the time of NSCLC diagnosis [11]. We suppose that BM from NSCLC with EGFR mutation is clinically and radiographically distinct from those with wild-type EGFR. If so, prognostic factors for patients with BM may be different according to the EGFR mutation status. To clarify these questions, we performed the present study.
Materials and methods
Patients
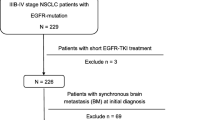
We retrospectively investigated all consecutive non-squamous NSCLC patients who underwent EGFR mutation screening at Kanagawa Cardiovascular and Respiratory Center (KRCC) and Ibarakihigasi National Hospital from March 2005 through September 2012. Among them, all patients with BM, which were detected at the time of NSCLC diagnosis (synchronous BM) or developed during clinical course (metachronous BM), were included in this study. The patients with minor and/or double mutations were excluded because the aim of this study was to reveal the prognostic factors for patients with BM from NSCLC with exon 19 deletion and exon 21 point mutation, which accounts for 90 % of EGFR mutations [6, 7, 12]. This study was approved by the Institutional Human Ethics Committees of KRCC and Ibarakihigashi National Hospital.
EGFR mutation detection
EGFR mutation status was assessed by the polymerase chain reaction (PCR) clamp method at Mitsubishi Chemical Medience Corporation (Tokyo, Japan) or the cycleave method at SRL Inc. (Tokyo, Japan). EGFR mutation analysis was conducted using tissue specimens from primary tumor and metastatic lesion, or cytologic materials such as bronchial lavage fluid and pleural effusion [13].
Patient characteristics, treatment history, and extracranial disease activity
Patient characteristics including gender, age, smoking history, histology, ECOG PS, staging, and presence of neurological symptoms at the time of BM diagnosis, and treatment history including cranial radiotherapy and EGFR-TKIs, were investigated. For cranial radiotherapy, whole brain radiotherapy or radiosurgery was performed based on the condition of BM such as size and number. For EGFR-TKIs, gefitinib and erlotinib were initially administered at 250 and 150 mg, respectively, daily on a continuous basis. In addition, we evaluated extracranial disease activity at the time of BM diagnosis, referring to the previous report by Eichler et al. [9]. Extracranial disease activity at the time of BM diagnosis was defined as “active” if new sites of extracranial disease and/or progression at already known sites of disease including primary tumor were observed by chest, abdomen, and pelvis CT; chest radiograph; and/or bone scan [9]. In all patients with synchronous BM, extracranial disease was considered “active”.
Radiographic analysis
All patients with BM evaluated with Gd-enhanced magnetic resonance imaging (MRI) were included for radiographic analysis. Referring to the previous paper [11], three brain tumor variables were measured: (1) number and (2) size of the brain tumors on T1-weighted Gd-enhanced imaging and (3) size of associated peritumoral brain edema (PTBE) on T2-weighted imaging or fluid-attenuated inversion recovery (FLAIR) sequence imaging. For the patients with multiple BM, the two largest tumors were evaluated for the tumor and PTBE sizes, following the new RECIST version 1.1 [14]. The PTBE size was defined by the subtraction of the diameter measured on T1-weighted Gd-enhanced imaging from the diameter on T2 or FLAIR imaging. If PTBE was absent, the PTBE size was defined as zero. Because many papers have reported that PTBE size is related with tumor size [15–17], we defined the relationship between tumor and PTBE sizes as the PTBE-index described in our previous report [11]. The PTBE-index was calculated by dividing PTBE size by tumor size to minimize the influence of tumor size on PTBE size. All measurements were performed by at least one board-certified radiologist blinded to the clinical data and EGFR mutation status.
Statistical analysis
Fisher’s exact test was used to compare categorical variables such as gender, histology, smoking history, ECOG PS, staging, extracranial disease activity, and neurological symptoms. The Mann–Whitney U test was performed to compare continuous variables, including age, number of BM, tumor size, PTBE size, and PTBE-index. Overall survival (OS) was evaluated as the period from the detection of BM to the day of death from any cause. The outcome was censored if a patient had not progressed or died at the time of the last follow-up. Survival duration was estimated using the Kaplan–Meier method, with differences between the groups compared using the log-rank test. Prognostic factors affecting OS were analyzed using the Cox proportional regression (hazards) model. A p value of <0.05 was considered statistically significant, and only those variables with p values of <0.05 in univariate analysis were included in multivariate analysis. JMP 10 software (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Patient characteristics
Of the 779 consecutive NSCLC patients who underwent EGFR mutation screening, 210 had synchronous or metachronous BM. Thirteen patients were excluded because they harbored minor and/or double EGFR mutations. Therefore 197 patients (89 with synchronous BM and 108 with metachronous BM) were finally included in this study. Of the 89 patients with synchronous BM, there were 50 patients with wild-type EGFR and 39 patients with EGFR mutation (24 with exon 19 deletion and 15 with exon 21 point mutation). Of the 108 patients with metachronous BM, there were 58 patients with wild-type EGFR and 50 patients with EGFR mutation (26 with exon 19 deletion and 24 with exon 21 point mutation). According to the EGFR mutation status, the patients were divided into the two groups: the EGFR mutation group and the wild-type group. The characteristics of the both groups are listed in Tables 1 and 2.
Among the patients with synchronous BM, female gender, never-smoking status, and neurologically asymptomatic BM were more common in the EGFR mutation group than in the wild-type group (p = 0.0007, 0.0017, and 0.0034). All patients in the EGFR mutation group were evaluated with Gd-enhanced MRI, although 20 % patients in the wild-type group were evaluated with other radiographic modalities because of the presence of apparent neurological symptoms that required immediate evaluation (p = 0.0021).
Among the patients with metachronous BM, female gender, adenocarcinoma pathology, and never-smoking status were more common in the EGFR mutation group than in the wild-type group (p < 0.0001, 0.014, and <0.0001). The number of previous chemotherapy regimens at the time of BM detection tended to be greater in the EGFR mutation group than in the wild-type group although the difference was not significant (p = 0.081).
Radiographic analysis
We evaluated the brain tumor variables, dividing the EGFR mutation group into the exon 19 group and the exon 21 group. The EGFR mutation group and the exon 19 group had greater number of synchronous BM than did the wild-type group (p = 0.019, 0.017, Fig. 1a), although the difference was not significant in the number of metachronous BM (Fig. 1b). With regard to the tumor size, the EGFR mutation group and the exon 19 group had smaller-sized BM than the wild-type group, regardless of synchronous (p = 0.003 and 0.002; Fig. 1c) or metachronous BM (p = 0.023 and 0.03; Fig. 1d). In addition, the PTBE size in the EGFR mutation group and the exon 19 group was also smaller than that in the wild type, regardless of synchronous (p < 0.0001 and <0.0001; Fig. 1e) or metachronous BM (p = 0.0017 and 0.0016; Fig. 1f). With regard to the PTBE-index, the same trend was observed in the synchronous (p = 0.0005 and <0.0001; Fig. 1g) and metachronous BM (p = 0.0078 and 0.0052; Fig. 1h). The exon 21 group presented the similar trend toward the wild-type group as the exon 19 group in each radiographic variable, although the difference was not significant. The results for patients with adenocarcinoma were identical to those for patients with NSCLC (data not shown).
Details of brain metastases (a, b number of brain tumors, c, d tumor size, e, f peritumoral brain edema size (PTBE), g, h PTBE-index). Data Box plots present median ± 25th and 75th percentiles (solid box) with the 10th and 90th percentiles shown by outside the box. Asterisk (*) a significant difference compared to the wild-type group (p < 0.05). BM brain metastases, PTBE peritumoral brain edema
Treatment history after BM diagnosis, and survival and prognostic analysis
Treatment history after BM diagnosis is shown in Table 3. Brain metastasectomy was performed in five patients of each group. Radiotherapy was more commonly performed in the wild-type group than in the EGFR-mutation group (p = 0.010). Conversely, EGFR-TKIs were more frequently used in the EGFR-mutation group (p < 0.0001).
With the median follow-up period of 315 days after BM diagnosis, OS in the EGFR mutation group (median: 451 days) was longer than that in the wild-type group (median: 228 days) (p < 0.0001, Fig. 2a). In addition, OS in the EGFR mutation group was longer than that in the wild-type group, regardless of the time of BM diagnosis: synchronous BM (OS in the EGFR mutation group: 484 days versus OS in the wild-type group: 218 days, p = 0.0016) and metachronous BM (OS in the EGFR mutation group: 449 days versus OS in the wild-type group: 237 days, p = 0.0041), respectively (Fig. 2b, c). When limited in patients with adenocarcinoma, the EGFR mutation group also presented better OS than the wild-type group, regardless of whether BM was synchronous or metachronous (p = 0.0035, 0.0071).
Prognostic factors for patients with BM were analyzed on the basis of EGFR mutation status (Table 4, 5). In the EGFR mutation group, univariate analysis revealed age <65, good ECOG PS, cranial radiotherapy, stable extracranial disease, previous chemotherapy ≤1 regimen, erlotinib therapy after BM diagnosis, and number of BM <5 as favorable prognostic factors (p = 0.035, <0.0001, 0.002, 0.016, 0.012, 0.020, and 0.027). In multivariate analysis, age <65, good ECOG PS, cranial radiotherapy, stable extracranial disease, previous chemotherapy ≤1 regimen, and erlotinib therapy after BM diagnosis still remained as favorable prognostic factors (p = 0.037, <0.0001, 0.020, 0.022, 0.009, and 0.0015). Among patients treated with erlotinib after BM diagnosis, the patients with exon 19 deletion showed significantly longer OS than those with exon 21 point mutation (p = 0.019, Fig. 2d). However, this trend was not observed in patients treated with gefitinib after BM diagnosis (p = 0.42, Fig. 2e). In the wild-type group, on the other hand, univariate analysis revealed that female gender, never-smoking status, good ECOG PS, absence of neurological symptom, cranial radiotherapy, previous chemotherapy ≤1 regimen, and number of BM <5 were favorable prognostic factors (p = 0.031, 0.033, <0.0001, 0.031, <0.0001, 0.0083, and 0.033), while multivariate analysis revealed only good ECOG PS and cranial radiotherapy to be favorable prognostic factors (p = 0.0037 and 0.0005).
Discussion
The present study revealed the clear OS superiority of EGFR mutation over wild-type EGFR in NSCLC patients with BM. To date, there is a well-known prognostic index used in NSCLC patients with BM, graded prognostic assessment (GPA), which comprises age, Karnofsky performance status, number of BM, and presence of extracranial metastases; EGFR mutation status is not taken into consideration in GPA [18]. To the best of our knowledge, this is the first report to reveal the prognostic factors for NSCLC patients with BM on the basis of EGFR mutation status, and the present study revealed that the prognostic factors were different according to the EGFR mutation status. Therefore, it would be worth to evaluate EGFR mutation status as prognostic factor in GPA prognostic index. The present study demonstrated the four major findings as follows: (1) Stable extracranial disease at BM diagnosis was a favorable prognostic factor for NSCLC patients with BM and EGFR mutation; (2) Erlotinib therapy after BM diagnosis was also a favorable prognostic factor for NSCLC patients with BM and EGFR mutation; (3) Among NSCLC patients treated with erlotinib after BM diagnosis, the patients with exon 19 deletion showed longer OS than those with exon 21 point mutation; and (4) NSCLC patients with exon 19 deletion had small-sized BM with scarce PTBE, regardless of whether BM was synchronous or metachronous. These results provide the following three clinical implications.
First, regular cranial evaluation for detecting asymptomatic BM would lead to good prognosis, particularly in NSCLC patients with EGFR mutation and stable extracranial disease. Our study revealed that stable extracranial disease at BM diagnosis was a favorable prognostic factor for NSCLC patients with EGFR mutation. To the best of our knowledge, only two research groups have investigated the prognostic factors for NSCLC patients with BM and have reported almost the same results as ours; however, the patients in those studies were not divided according to the EGFR mutation status [9, 10]. Interestingly, the present study revealed that stable extracranial disease was not a prognostic factor for NSCLC patients with wild-type EGFR. BM sometimes causes seizures and decreases quality of life. Therefore, early tumor detection is important to facilitate appropriate treatment before the development of neurological symptoms and a consequent poor PS [19–21]. Importantly, neurological symptom at metachronous BM diagnosis was absent in 14 (87.5 %) of our 16 patients with EGFR mutation and stable extracranial disease. Therefore, regular cranial evaluation for detecting asymptomatic BM would be meaningful, particularly in NSCLC patients with EGFR mutation and stable extracranial disease.
Second, erlotinib therapy would be preferable in NSCLC patients with BM and EGFR mutation. In fact, our results revealed that erlotinib therapy after BM diagnosis was a favorable prognostic factor in NSCLC patients with BM and EGFR mutation. To date, there were some reports which demonstrated the efficacy of erlotinib for BM [22, 23]. With regard to gefitinib, although the similar reports were present [24, 25], the current study did not show a positive effect of gefitinib on OS. Erlotinib was reported to have a slightly broader spectrum of kinase inhibitor compared with gefitinib [26], but the difference between erlotinib and gefitinib in terms of OS can primarily be attributed to the dose setting of these drugs. The approved daily dose of erlotinib is equivalent to the maximum tolerated dose (MTD) of erlotinib, while that of gefitinib is one-third of the MTD of gefitinib [27, 28]. In fact, the efficacy superiority of erlotinib over gefitinib has been reported in NSCLC patients with BM [29] and leptomeningeal metastases [30].
Third, the difference of characteristics of BM between NSCLC with exon 19 deletion and those with exon 21 point mutation definitely exists, and to differentiate these two mutations would be important at the point of therapeutic management of NSCLC patients with BM. In our study, the survival superiority of exon 19 deletion over exon 21 point mutation was observed in patients treated with erlotinib. Considering the results of radiographic variables, we do suggest the presence of radiologic and prognostic differences between exon 19 deletion and exon 21 point mutation. To date, some studies have showed the superiority of exon 19 deletion over exon 21 point mutation in NSCLC patients treated with erlotinib and/or gefitinib [31–33], while other studies have not [8, 34, 35]. Importantly, no reports have focused on the superiority of erlotinib over gefitinib for NSCLC patients with BM, as we pointed out in this study. Although NSCLC with exon 19 deletion has been reported to show a good response to lower concentrations of EGFR-TKIs than that with exon 21 point mutation [36], the present study showed survival superiority of exon 19 deletion over exon 21 point mutation only in patients treated with erlotinib, not gefitinib. Although erlotinib was reported to show a higher MTD compared with gefitinib [27, 28], the concentration of erlotinib was lower in the central nervous system (CNS) than in plasma [37]. Therefore, the concentration of erlotinib in the CNS may be high enough to treat BM from NSCLC with exon 19 deletion and not high enough to treat that with exon 21 point mutation.
Despite the important findings of the present study, it had several limitations. First, it was a small-sized retrospective study. Second, the timing and kinds of radiographic evaluation for BM were not precisely determined before this retrospective study. In particular, a bone scan was not performed in some part of patients, although chest, abdomen, and pelvis CT was done in almost all patients. Third, BM was not confirmed pathologically and genetically. In the present study, EGFR mutation status was evaluated, using the specimens from primary lesion in more than four-fifth of patients. Only in a patient, the specimen from BM was used for analyzing EGFR mutation status. More recently, discordance of EGFR mutation status between the primary and metastatic sites has been reported. The discordance rate for EGFR mutation status was reported to reach up to 27–28 % [38, 39]. Therefore, future studies using pathological specimens from BM will confirm our present study.
In conclusion, the prognostic factors for NSCLC patients with BM were different according to the EGFR mutation status. Particularly in NSCLC patients with EGFR mutation and stable extracranial disease, regular cranial evaluation for detecting asymptomatic BM would lead to good prognosis. Additionally, erlotinib therapy would be preferable in NSCLC patients with BM and EGFR mutation, especially those with exon 19 deletion. Accumulation of knowledge about prognostic factors based on EGFR mutation status will aid in approaches to the individual management of NSCLC patients with BM. Our results would be validated in future prospective study.
References
Burel-Vandenbos F, Ambrosetti D, Coutts M, Pedeutour F. EGFR mutation status in brain metastases of non-small cell lung carcinoma. J Neurooncol. 2013;111:1–10.
Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol. 2005;23:6207–19.
Mehta MP, Rodrigus P, Terhaard CH, Rao A, Suh J, Roa W, Souhami L, Bezjak A, Leibenhaut M, Komaki R, Schultz C, Timmerman R, Curran W, Smith J, Phan SC, Miller RA, Renschler MF. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529–36.
Sorensen JB, Hansen HH, Hansen M, Dombernowsky P. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6:1474–80.
Zabel A, Milker-Zabel S, Thilmann C, Zuna I, Rhein B, Wannenmacher M, Debus J. Treatment of brain metastases in patients with non-small cell lung cancer (NSCLC) by stereotactic linac-based radiosurgery: prognostic factors. Lung Cancer. 2002;37:87–94.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39.
Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500.
Park SJ, Kim HT, Lee DH, Kim KP, Kim SW, Suh C, Lee JS. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77:556–60.
Eichler AF, Kahle KT, Wang DL, Joshi VA, Willers H, Engelman JA, Lynch TJ, Sequist LV. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010;12:1193–9.
Hsiao SH, Lin HC, Chou YT, Lin SE, Kuo CC, Yu MC, Chung CL. Impact of epidermal growth factor receptor mutations on intracranial treatment response and survival after brain metastases in lung adenocarcinoma patients. Lung Cancer. 2013;81:455–61.
Sekine A, Kato T, Hagiwara E, Shinohara T, Komagata T, Iwasawa T, Satoh H, Tamura K, Kasamatsu T, Hayashihara K, Saito T, Takahashi H, Ogura T. Metastatic brain tumors from non-small cell lung cancer with EGFR mutations: distinguishing influence of exon 19 deletion on radiographic features. Lung Cancer. 2012;77:64–9.
Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–23.
Nagai Y, Miyazawa H, Huqun, Tanaka T, Udagawa K, Kato M, Fukuyama S, Yokote A, Kobayashi K, Kanazawa M, Hagiwara K. Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res. 2005;65:7276–82.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Gilbert JJ, Paulseth JE, Coates RK, Malott D. Cerebral edema associated with meningiomas. Neurosurgery. 1983;12:599–605.
Simis A, Pires de Aguiar PH, Leite CC, Santana PA, Jr, Rosemberg S, Teixeira MJ. Peritumoral brain edema in benign meningiomas: correlation with clinical, radiologic, and surgical factors and possible role on recurrence. Surg Neurol. 2008;70:471–477; discussion 477.
Tamiya T, Ono Y, Matsumoto K, Ohmoto T. Peritumoral brain edema in intracranial meningiomas: effects of radiological and histological factors. Neurosurgery 2001;49: 046–1051; discussion 1051–1042.
Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, Bhatt A, Jensen AW, Brown PD, Shih H, Kirkpatrick J, Schwer A, Gaspar LE, Fiveash JB, Chiang V, Knisely J, Sperduto CM, Mehta M. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–61.
Sundstrom JT, Minn H, Lertola KK, Nordman E. Prognosis of patients treated for intracranial metastases with whole-brain irradiation. Ann Med. 1998;30:296–9.
Herman ST. Epilepsy after brain insult: targeting epileptogenesis. Neurology. 2002;59:S21–6.
van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6:421–30.
Porta R, Sanchez-Torres JM, Paz-Ares L, Massuti B, Reguart N, Mayo C, Lianes P, Queralt C, Guillem V, Salinas P, Catot S, Isla D, Pradas A, Gurpide A, de Castro J, Polo E, Puig T, Taron M, Colomer R, Rosell R. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011;37:624–31.
Bai H, Han B. The effectiveness of erlotinib against brain metastases in non-small cell lung cancer patients. Am J Clin Oncol. 2013;36:110–5.
Hotta K, Kiura K, Ueoka H, Tabata M, Fujiwara K, Kozuki T, Okada T, Hisamoto A, Tanimoto M. Effect of gefitinib (‘Iressa’, ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer. 2004;46:255–61.
Shimato S, Mitsudomi T, Kosaka T, Yatabe Y, Wakabayashi T, Mizuno M, Nakahara N, Hatano H, Natsume A, Ishii D, Yoshida J. EGFR mutations in patients with brain metastases from lung cancer: association with the efficacy of gefitinib. Neuro Oncol. 2006;8:137–44.
Fabian MA, Biggs WH 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, Galvin M, Gerlach JL, Grotzfeld RM, Herrgard S, Insko DE, Insko MA, Lai AG, Lelias JM, Mehta SA, Milanov ZV, Velasco AM, Wodicka LM, Patel HK, Zarrinkar PP, Lockhart DJ. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–36.
Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Eek R, Horai T, Noda K, Takata I, Smit E, Averbuch S, Macleod A, Feyereislova A, Dong RP, Baselga J. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21:2237–46.
Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, Albain KS, Cella D, Wolf MK, Averbuch SD, Ochs JJ, Kay AC. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–58.
Hata A, Katakami N, Yoshioka H, Fujita S, Kunimasa K, Nanjo S, Otsuka K, Kaji R, Tomii K, Iwasaku M, Nishiyama A, Hayashi H, Morita S, Ishida T. Erlotinib after gefitinib failure in relapsed non-small cell lung cancer: clinical benefit with optimal patient selection. Lung Cancer. 2011;74:268–73.
Lee E, Keam B, Kim DW, Kim TM, Lee SH, Chung DH, Heo DS. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol. 2013;8:1069–74.
Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Ares L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sanchez JM, Bastus R, Mayo C, Bertran-Alamillo J, Molina MA, Sanchez JJ, Taron M. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–67.
Won YW, Han JY, Lee GK, Park SY, Lim KY, Yoon KA, Yun T, Kim HT, Lee JS. Comparison of clinical outcome of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations. J Clin Pathol. 2011;64:947–52.
Heon S, Yeap BY, Britt GJ, Costa DB, Rabin MS, Jackman DM, Johnson BE. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16:5873–82.
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8.
Zhu JQ, Zhong WZ, Zhang GC, Li R, Zhang XC, Guo AL, Zhang YF, An SJ, Mok TS, Wu YL. Better survival with EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small cell lung cancer patients is due to differential inhibition of downstream signals. Cancer Lett. 2008;265:307–17.
Togashi Y, Masago K, Masuda S, Mizuno T, Fukudo M, Ikemi Y, Sakamori Y, Nagai H, Kim YH, Katsura T, Mishima M. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70:399–405.
Gow CH, Chang YL, Hsu YC, Tsai MF, Wu CT, Yu CJ, Yang CH, Lee YC, Yang PC, Shih JY. Comparison of epidermal growth factor receptor mutations between primary and corresponding metastatic tumors in tyrosine kinase inhibitor-naive non-small-cell lung cancer. Ann Oncol. 2009;20:696–702.
Kalikaki A, Koutsopoulos A, Trypaki M, Souglakos J, Stathopoulos E, Georgoulias V, Mavroudis D, Voutsina A. Comparison of EGFR and K-RAS gene status between primary tumours and corresponding metastases in NSCLC. Br J Cancer. 2008;99:923–9.
Conflict of interest
Dr. Kato has received lecture fees from AstraZeneca, Boehringer Ingelheim and Chugai pharmaceutical, and research support from Boehringer Ingelheim and Chugai pharmaceutical. None of the remaining authors have any conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sekine, A., Satoh, H., Iwasawa, T. et al. Prognostic factors for brain metastases from non-small cell lung cancer with EGFR mutation: influence of stable extracranial disease and erlotinib therapy. Med Oncol 31, 228 (2014). https://doi.org/10.1007/s12032-014-0228-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0228-9