Abstract
Triple-negative breast cancer (TNBC) represents 10–20 % of all mammary tumors. It is often found in younger women and has been associated with poor prognosis, due to aggressive tumor phenotype(s), early metastasis to visceral organ or brain after chemotherapy and present lack of clinically established targeted therapies. In recent years, a greater understanding of the biology of this disease has led to the development of numerous and varied therapeutic approaches, especially the trials on poly (ADP-ribose) polymerase inhibitors BSI-201 and olaparib, and antiangiogenic agents such as bevacizumab and sunitinib, which have raised hopes in the treatment for TNBC and BRCA1/2-positive disease. But should these trials fail, what are the new possible perspectives we have in our hand to fight this disease? In the current review, we will assess mainly the possible future targeted therapeutic strategies, which could be the answer to our question in TNBC. Recent studies have shown several markers, which have roles in TNBC that could be possible targets for therapy. Some of these markers are p53-induced miR-205, leptin receptor antagonist peptide, enhancer of zeste homolog 2 and Notch 1 pathway components, each of them could offer different mechanism for target therapy in TNBC. Last but not least, vaccinia virus GLV-1h153 has shown exciting result in treating and preventing metastatic triple-negative breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is a common disease with high incidence among female worldwide. Of the 230,480 breast cancers diagnosed in 2011, ~20 % was triple-negative breast carcinomas (TNBC) (2011 ACS Cancer Facts and Figures) and was more prevalent in black compared with white [1, 2]. TNBC, as the name implies, is defined as tumors that are negative for estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) [3]. Variations in morphology and biologic characteristics result in differences in clinical presentation and response to therapy. Whereas hormone-receptor-positive and HER2-positive breast cancers have had favorable outcomes with chemotherapy and targeted treatment, TNBC on the contrary has got mixed result and still lacking a targeted therapy [4, 5]. Although in the neoadjuvant setting, TNBC shows a better response to chemotherapy compared with non-TNBC [6], paradoxically, these patients are more likely to have recurrent disease (64.93 vs. 45.39 %) (P < .05) and have greater mortality versus metastatic non-TNBC patients [7]. Recently, the introduction of targeted PARP inhibitor and mTORs inhibitor has brought high hopes; however, they have both underachieved at some points in their clinical trials [8, 9].
So it has become imperative to find an effective treatment for TNBC. A better understanding of pathogenesis of TNBC onset and progression, including the still unclear association with BRCA1 mutations, and the causes of phenotypic heterogeneity will be the key to a new era in the treatment for this disease. Several experiments are being done in the urgency to find a cure for TNBC. While some have failed, others have brought light to an improved understanding of the biology of the disease and the tumor cell environment with the finding of new biomarkers and potential target treatments.
Oncosuppressive role of p53-induced miR-205
MicroRNAs involvement in human cancer and their roles in tumorigenesis and tumor suppression and their possible implication as biomarkers and therapeutic tools have been supported by experimental evidence [10]. Wu et al. [11] reported microRNA miR-205 is greatly under-expressed in human breast cancer, and another author demonstrated that miR-205 expression was restricted to the myoepithelial/basal cell compartment of normal mammary ducts and lobules and there was loss of expression when tumor arose. Recently, another report by Radojicic et al. [12] confirmed that low expression of miR205 was found in primary TNBC compared to normal breast tissue, arising the possibility that MiR-205 could be a possible target in the cure of TNBC.
MiR-205 oncosuppressive lost in breast cancer is directly transactivated by oncosuppressor P53. It inhibits growth both in vitro and in vivo. In an experiment conducted by Piovana et al., the group showed that in TNBC, miR-205 is downregulated and affects cell cycle progression and cellular senescence by targeting E2F1, a factor critically involved in cell cycle progression from G1 to S-phase [13, 14]. Furthermore, the group demonstrated that miR-205 directly targets LAMC1, a protein implicated in a wide variety of biological processes including cell adhesion, differentiation, proliferation, migration, signaling, neurite outgrowth and metastasis, and in breast cancer, it shows an inverse correlation with miR-205. Moreover, most importantly, MiR-205 expression is regulated and enhanced by the tumor suppressor TP53 and this effect is mediated by its direct binding on a responsive element located upstream this microRNA [13]. Considering the high frequency of BRCA1 (34 of 35, 97 %) and sporadic (35 of 38, 92 %) basal-like carcinomas [15], inactivating mutations of TP53 or reduced expression of TP53 lead to decrease levels or complete absence of MiR-205 in breast cancer. Furthermore, from the direct targeting of E2F1 mediated by miR-205, Piovana et al. [13] also identified that p53 activation blocks the release of E2F1 from its inhibitor pRB through the transcriptional activation of p21 (waf-1/cip-1), which in turn negatively regulates several complexes of cyclin/cyclin-dependent kinases involved in the negative regulation of pRB and in the cell cycle progression from the G1 to the S-phase leading to cell cycle arrest and influences the cell fate to an antiproliferative choice. Their conclusion also demonstrated that miR-205 could be novel transcriptional target of p53 and that it exerts a role as oncosuppressor in triple negative.
Leptin receptor antagonist peptide
In recent years, interests have grown in the relationship between obesity and cancer. For breast cancer, a clear association between obesity and disease risk seems to have been established [16, 17], and new studies confirming this observation and investigating explanatory hypotheses for the relationship continue to appear regularly [18]. Obesity increases the risk of TNBC [19–21], and this is greatly due to the influence of adipokine leptin [21, 22]. Leptin is one of the most important adipose-derived hormones and it is manufactured by adipose tissue, but can also be synthesized in breast cancer cells in response to obesity-related stimuli [23–25]. In one study conducted by Chen et al. [26], no significant correlation was observed between leptin and leptin receptor (ObRb) expression for age, menopausal status, tumor size, pathological types, as well as the status of ER, PR and P53 (all, P > 0.05). Leptin and ObRb were detected by immunohistochemistry in invasive ductal carcinoma, invasive lobular carcinoma and intraductal carcinoma. Another study found that leptin was detectable in 86 % (59/69 cases) and ObR in 92 % (64/69) of TNBC biopsies examined. While the expression of leptin was found at 1+ (29 %), 2+ (42 %) and 3+ (29 %) levels, ObR was scored at 1+ (18 %), 2+ (62 %) and 3+ (20 %). The presence of ObR was highly correlated with the expression of leptin (P = 0.01). Both leptin and its receptor (ObR) are overexpressed in breast cancer cells, but are absent in normal epithelial breast tissues [22]. Leptin induces breast cancer cell proliferation as suggested by its association with high Ki-67 and affects survival by reducing the efficacy of breast cancer treatments as well as the anti-estrogen effect of tamoxifen [26, 27].
Recently, leptin and its receptor (ObR) have been suggested as possible targets for therapy in breast cancer [28]. ObR has been reported as being an independent factor in breast cancer having no relation with the ER/PR and HER2 and thus can provide an answer to the management of TNBC where up to date no optimal drug is available [29]. Laszlo Jr et al. [22] used a peptide called Allo-aca, a drug targeting the ObR, to see its effects in TNBC breast cancer xenograph model. Results were very promising and showed that Allo-aca at 1 mg/kg/day sc dose extended the average survival time of mice by 80 %.This result shows exciting prospects about a possible therapeutic target for TNBC.
Relevance of enhancer of zeste homolog 2 in triple-negative breast cancer
The Polycomb-group protein, enhancer of zeste homolog 2, is a transcriptional repressor involved in cell cycle regulation and has been linked to aggressive breast cancer [30]. Hussein et al. examined the clinical and biological significance of enhancer of zeste homolog 2 expression in triple-negative breast cancers. Among the 261 cases, 57 (21 %) cases were categorized as TNBC and 204 (79 %) as non-TNBC. High EZH2 expression was strongly associated with a triple-negative phenotype (P < .001) compared with all other non-triple-negative tumors. High EZH2 expression was noted in 41 (72 %) of 57 TNBCs versus 47 (23 %) of 204 non-TNBCs. In addition, high EZH2 expression was significantly more frequent in invasive ductal carcinoma and mixed ductal and lobular carcinoma compared with invasive lobular carcinoma (P < .001). High EZH2 expression was significantly associated with markers of poor prognosis such as high histological grade (P = .01), ER negativity (P < .001), PR negativity (P < .001) and high p53 expression (P < .001) which is characteristic of TNBC (30).
Hussein et al. also showed the biological effect of EZH2 to modulate breast cancer growth in vivo. EZH2 gene silencing both in tumor and in endothelial cell resulted in 73 % reduction in tumor growth in the orthotopic MB-231 mouse model of triple-negative breast carcinoma (P < .01). In another experiment conducted by Chang et al. [31], the result showed that EZH2 promoted expansion of breast-tumor-initiating cells through the activation of RAF1-β-catenin signaling. Other studies have further confirmed EZH2 as a strong candidate oncogene in human tumors. In endometrial cancer, high EZH2 expression has been associated with tumor cell proliferation, migration and invasion with more aggressive biologic behavior [32, 33]. Cao et al. [34] reported ectopic overexpression of EZH2-regulated miRNAs attenuated cancer cell growth and invasiveness, and abrogated cancer stem cell (CSC) properties. In addition, Lu et al. [35] also reported that increased EZH2 expression in either tumor cells or tumor vasculature was predictive of poor clinical outcome and EZH2 silencing in the tumor-associated endothelial cells and tumor cells reduced ovarian cancer growth, suggesting potential for targeting EZH2 as an important therapeutic approach.
So the association of high EZH2 expression with TNBC has practical applications. Besides its prognostic value, its possibility as candidate for target therapy has been suggested in various studies [30–36]. Gonzalez et al. [37] recently showed that downregulation of EZH2 decreased the growth of ER-negative invasive breast carcinoma and required BRCA1. BRCA1 mutations and protein deficiency are frequent in TNBC. Downregulation of EZH2 in TNBC cell lines was also shown to be sufficient to restore BRCA1 protein levels in vivo and in vitro, suggesting that therapies targeting EZH2 protein may restore BRCA1 expression and function in TNBCs and may decrease tumor progression. So does the strong correlation between EZH2 and TNBC could lead to the much awaited new era of target therapy in TNBC.
Notch signaling molecules in breast cancer
Recently several authors have suggested that Notch has an implication in human breast cancers, associates with a poor prognosis, and mediates resistance to treatment and disease relapse. Notch signaling pathway proteins are known to play critical roles in maintaining the balance between cell proliferation, differentiation and apoptosis, and thus, it has been suggested that Notch may be responsible for the development and progression of human malignancies [38–41]. Numerous studies had demonstrated Notch 1, a member of the Notch family, as being involved in tumorigenesis in human and could be served as therapeutic target in several cancers including breast cancer [39–45]. Liu et al. [42] demonstrated that NOTCH1 functions as an oncogene by regulating the PTEN/PI3 K/AKT pathway in clear cell renal cell carcinoma, whereas Wang et al. [44] showed its oncogenicity in ovarian cancer cells and in both cancers, reduced signaling of Notch 1 was associated with tumor growth inhibition, which further strengthened the idea of Notch 1 being a possible therapeutic target.
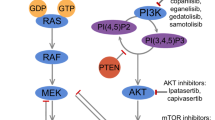
Many laboratories data have confirmed the correlation of breast cancer with Notch 1 protein and as a possible target for treatment. Recent data suggest that NOTCH1 contributes to mammary tumor-initiating activity and its inhibition in vivo results in mammary tumor regression and reduces mammary tumorsphere-forming activity in vitro, identifying Notch 1 as therapeutic agent in breast cancer [39]. Another study reported the correlation of breast cancer with chemosensitivity to Adriamycin (doxorubicin), and it had clearly demonstrated that MicroRNA-34a, a tumor suppressor, modulated chemosensitivity of breast cancer cells to Adriamycin by downregulating Notch 1, further consolidating the ideas of Notch 1 as a target option [41]. In a recent study published, results showed that specific inhibition of Notch 1 signaling in the TNBC, either alone or in combination with chemotherapeutic agent docetaxel, resulted in significant tumor growth inhibition due to multiple mechanisms, including induction of apoptosis and reduction in CSC frequency. These findings further suggested that Notch inhibitors can target cells with tumor-initiating features (like the CSC) leading the way to a targeted therapy for eliminating surviving cells, hence preventing tumor recurrence and improving long-term survival in triple-negative breast cancer patients [45] (Fig. 1).
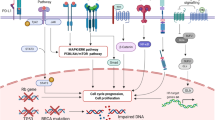
Binding of ligands (DLL4 delta like ligand 4, or Jagged 1) to EGF repeats of Notch receptor triggers downstream cleavages, which lead to activation of transcription factors (picture taken from http://www.healthvalue.net/notch.html)
Vaccinia virus as a potent oncolytic virus
Triple-negative breast cancer has long been described by several authors as being the most aggressive types of human breast cancers with high metastatic rate. Despite the growing advances in cancer treatment and metastatic prevention, no conventional therapy is yet available for treating TNBC except chemotherapy. However, recently a growing body of scientific evidence has reported amazing results, which could probably give an answer to the treatment for TNBC. Evidence suggests that oncolytic vaccinia virus carrying imaging genes may represent a new treatment strategy combining both tumor site-specific therapeutics and diagnostics (theranostics) [46].
Several oncoviruses, such as herpes simplex virus-1 (JSI/34.5-/47-/GM-CSF) in melanoma therapy and poxvirus (JX-594) in various malignancies, have been investigated, and most importantly, adeno-associated virus type 2, parvovirus H-1 (H-1 PV) and vaccinia virus (GLV-1h68) have been undergoing laboratory trial and have had some moral boosting result [47–51]. However, recently some new studies published have been thrilling. In one study carried out by Li et al., they demonstrated the effect of oncolytic herpes simples virus (oHSV) vector G47∆ on breast cancer CD44+ CD24−/low stem cell both in vitro and in vivo. In vitro, the oHSV G47∆ took about 6 days to reduce viability of stem cells by over 90 %, whereas in vivo, in mouse model where the stem cells had undergone differentiation, over 90 % of the non-stem cells were killed by the day 4. In vitro, the virus inhibited the growth of established tumors by targeting the stem cells. However, in vivo the bulk tumor cells were more susceptible to G47∆. Thus, ability of oHSV to target breast CSCs efficiently is an important attribute that supports the possibility of successful clinical translation medicine [52]. In another study by Gholami et al., they used vaccinia virus GLV-1h53, a genetically modified strain from the parent virus GLV-1h68, for the treatment for TNBC. Results published showed that the virus successfully replicated in breast cancer cells and lysed the cell both in vivo and in vitro. In mice model, the virus not only decreased tumor size, but also prevented metastasis by killing the circulating tumor cells. Moreover, another interesting point suggested in the study was that the virus could be labeled with radioactive material and used for tumors imaging by PET scan. Hopefully, this oncolytic virus can be the future of breast cancers therapy, especially in TNBC where lack of hormonal expression and HER2 overexpression has rendered target therapy futile [53].
Conclusion
Although the aforementioned studies and findings need yet to be validated through other laboratory and clinical trials, they have brought new hopes in the treatment for TNBC and must not be ignored. Along with the emerging field of molecular imaging, recent scientific and technological advances nowadays provide a large inventory of candidate DNA, RNA and protein biomarkers, as well as a range of metabolites and networks of cell signaling pathways, all potential candidates for a better understanding of TNBC biology and finding new treatment. While some studies have already yield promising results, others are still being carried out with the hope to finally find the long awaited therapy in TNBC.
References
2011 ACS Cancer Facts and Figures. http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2011.
Swede H, Gregorio DI, Tannenbaum SH, et al. Prevalence and prognostic role of triple-negative breast cancer by race: a surveillance study. Clin Breast Cancer. 2011;11(5):332–41.
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–48.
Chen Q, Russo J. ERα-negative and triple negative breast cancer: molecular features and potential therapeutic approaches. Biochim Biophys Acta. 2009;1796:162–75.
Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest. 2011;121(10):3797–803.
Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81.
Baser O, Wei W, Xie L, et al. Retrospective study of patients with metastatic triple-negative breast cancer: survival, health care utilization, and cost. Community Oncol. 2012;9(1):8–14.
O’Shaughnessy J, Schwartzberg LS, Danso MA, et al. A randomized phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin (G/C) in metastatic triple-negative breast cancer (TNBC). J Clin Oncol. 2011; 29: (Abstr 1007, presented data—ASCO Annual Meeting 2011).
Gonzalez-Angulo AM, Green MC, Murray JL, et al. Open label, randomized clinical trial of standard neoadjuvant chemotherapy with paclitaxel followed by FEC (T-FEC) versus the combination of paclitaxel and RAD001 followed by FEC (TR-FEC) in women with triple receptor-negative breast cancer (TNBC). J Clin Oncol. 2011; 29: (Abstr 1016, presented data—ASCO Annual Meeting 2011).
Iorio MV, Croce CM. MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol. 2009;27(34):5848–56.
Wu H, Mo YY. Targeting miR-205 in breast cancer. Expert Opin Ther Targets. 2009;13(12):1439–48.
Radojicic J, Zaravinos A, Vrekoussis T, et al. MicroRNA expression analysis in triple-negative (ER, PR and Her2/neu) breast cancer. Cell Cycle. 2011;10(3):507–17.
Piovan C, Palmieri D, Di Leva G, et al. Oncosuppressive role of p53-induced miR-205 in triple negative breast cancer. Mol Oncol. 2012;6:458–72.
Polager S, Ginsberg D. P53 and E2f: partners in life and death. Nat Rev Cancer. 2009;9:738–48.
Manié E, Vincent-Salomon A, Lehmann-Che J, et al. High frequency of TP53 mutation in BRCA1 and sporadic basal-like carcinomas but not in BRCA1 luminal breast tumors. Cancer Res. 2009;69(2):663–71.
Amir E, Cecchini RS, Ganz PA, et al. 25-Hydroxy vitamin-D, obesity, and associated variables as predictors of breast cancer risk and tamoxifen benefit in NSABP-P1. Breast Cancer Res Treat. 2012;133(3):1077–88.
Singh P, Kapil U, Shukla NK, et al. Association of overweight and obesity with breast cancer in India. Indian J Community Med. 2011;36(4):259–62.
von Drygalski A, Tran TB, Messer K, et al. Obesity is an independent predictor of poor survival in metastatic breast cancer: retrospective analysis of a patient cohort whose treatment included high-dose chemotherapy and autologous stem cell support. Int J Breast Cancer. 2011;2011:523276.
Davis AA, Kaklamani VG. Metabolic syndrome and triple-negative breast cancer: a new paradigm. Int J Breast Cancer. 2012;2012:809291.
Davis AA, Kaklamani VG, et al. Metabolic syndrome and triple-negative breast cancer: a new paradigm. Int J Breast Cancer. 2012;2012:809291.
Oh SW, Park CY, Lee ES, et al. Adipokines, insulin resistance, metabolic syndrome, and breast cancer recurrence: a cohort study. Breast Cancer Res. 2011;13(2):R34.
Otvos L Jr, Kovalszky I, Riolfi M, et al. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur J Cancer. 2011;47:1578–84.
Bartella V, Cascio S, Fiorio E, et al. Insulin-dependent leptin expression in breast cancer cells. Cancer Res. 2008;68:4919–27.
Cascio S, Bartella V, Auriemma A, et al. Mechanism of leptin expression in breast cancer cells: role of hypoxia-inducible factor-1a. Oncogene. 2008;27:540–7.
Cleary MP, Grossmann ME, Ray A. Effect of obesity on breast cancer development. Vet Pathol. 2010;47:202–13.
Chen X, Zha X, Chen W, et al. Leptin attenuates the anti-estrogen effect of tamoxifen in breast cancer. Biomed Pharmacother. 2013;67(1):22–30.
Jeong YJ, Bong JG, Park SH, et al. Expression of leptin, leptin receptor, adiponectin, and adiponectin receptor in ductal carcinoma in situ and invasive breast cancer. J Breast Cancer. 2011;14(2):96–103.
Ray A, Cleary MP. Leptin as a potential therapeutic target for breast cancer prevention and treatment. Expert Opin Ther Targets. 2010;14:443–51.
Xia XH, Gu JC, Bai QY, et al. Overexpression of leptin and leptin receptors in breast cancer positively correlates with clinicopathological features. Chinese Med J. 2009;122:3078–81.
Hussein YR, Sood AK, Bandyopadhyay S, et al. Clinical and biological relevance of enhancer of zeste homolog 2 in triple-negative breast cancer. Hum Pathol. 2012;43:1638–44.
Chang CJ, Yang JY, Xia W, et al. EZH2 promotes expansion of breast tumor initiating cells through activation of RAF1-β-catenin signaling. Cancer Cell. 2011;19(1):86–100.
Eskander R, Ji T, Huynh B, et al. Inhibition of enhancer of zeste homolog 2 (EZH2) expression is associated with decreased tumor cell proliferation, migration and invasion in endometrial cancer cell lines. Gynecol Oncol. 2012;127(1):S5.
Semaan A, Mert I, Solomon L, et al. Overexpression of enhancer of zeste homolog 2 (EZH2), focal adhesion kinase (FAK), and pFAK in high grade endometrial carcinoma. Gynecol Oncol. 2012;125(1):S164–5.
Cao Q, Mani RS, Ateeq B, Dhanasekaran SM, et al. Coordinated regulation of polycomb group complexes through microRNAs in cancer. Cancer Cell. 2011;20(2):187–99.
Lu C, Han HD, Mangala LS, et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell. 2010;18(2):185–97.
Hua WF, Fu YS, Liao YJ, et al. Curcumin induces down-regulation of EZH2 expression through the MAPK pathway in MDA-MB-435 human breast cancer cells. Eur J Pharmacol. 2010;637(1–3):16–21.
Gonzalez ME, Li X, Toy K, et al. Downregulation of EZH2 decreases growth of estrogen receptor–negative invasive breast carcinoma and requires BRCA1. Oncogene. 2009;28:843–53.
Rennstam K, McMichael N, Berglund P, et al. Numb protein expression correlates with a basal-like phenotype and cancer stem cell markers in primary breast cancer. Breast Cancer Res Treat. 2010;122:315–24.
Simmons MJ, Serra R, Hermance N, et al. NOTCH1 inhibition in vivo results in mammary tumor regression and reduced mammary tumorsphere-forming activity in vitro. Breast Cancer Res. 2012;14(5):R126.
Wang Z, Li Y, Sarkar FH. Notch signaling proteins: legitimate targets for cancer therapy. Curr Protein Pept Sci. 2010;11(6):398–408.
Li XJ, Ji MH, Zhong SL, et al. MicroRNA-34a modulates chemosensitivity of breast cancer cells to Adriamycin by targeting Notch1. Arch Med Res. 2012;43(7):514–21.
Liu S, Ma X, Ai Q, et al. NOTCH1 functions as an oncogene by regulating the PTEN/PI3K/AKT pathway in clear cell renal cell carcinoma. Urol Oncol. 2011 October 10.
Wang Z, Li Y, Sarkar FH. Notch signaling proteins: legitimate targets for cancer therapy. Curr Protein Pept Sci. 2010;11:398–408.
Wang M, Wu L, Lin W, et al. Down-regulation of Notch1 by gamma-secretase inhibition contributes to cell growth inhibition and apoptosis in ovarian cancer cells A2780. Biochem Biophys Res Commun. 2010;393(1):144–9.
Qiu M, Peng Q, Jiang I, et al. Specific inhibition of Notch1 signaling enhances the antitumor efficacy of chemotherapy in triple negative breast cancer through reduction of cancer stem cells. Cancer Lett. 2013;328(2):261–70.
Chen N, Szalay AA. Oncolytic vaccinia virus: a theranostic agent for cancer. Futur Virol. 2010;5:763–84.
Sivendran S, Pan M, Kaufman HL, et al. Herpes simplex virus oncolytic vaccine therapy in melanoma. Expert Opin Biol Ther. 2010;10(7):1145–53.
Merrick AE, Ilett EJ, Melcher AA. JX-594, a targeted oncolytic poxvirus for the treatment of cancer. Curr Opin Investig Drugs. 2009;10(12):1372–82.
Alam S, Bowser BS, Conway MJ, et al. Adeno-associated virus type 2 infection activates caspase dependent and independent apoptosis in multiple breast cancer lines but not in normal mammary epithelial cells. Mol Cancer. 2011;10(1):97.
Muharram G, Le Rhun E, Loison I, et al. Parvovirus H-1 induces cytopathic effects in breast carcinoma-derived cultures. Breast Cancer Res Treat. 2009;30(121):23–33.
Wang Huiqiang, Chen NG, Minev BR, et al. Oncolytic vaccinia virus GLV-1h68 strain shows enhanced replication in human breast cancer stem-like cells in comparison to breast cancer cells. J transl med. 2012;10:167.
Li J, Zeng W, Huang Y, Zhang Q, et al. Treatment of breast cancer stem cells with oncolytic herpes simplex virus. Cancer Gene Ther. 2012;19:707–14.
Gholami S, Chen CH, Lou E, et al. Vaccinia virus GLV-1h153 is effective in treating and preventing metastatic triple-negative breast cancer. Ann Surg. 2012;256(3):437–45.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahamodhossen, Y.A., Liu, W. & Rong-Rong, Z. Triple-negative breast cancer: new perspectives for novel therapies. Med Oncol 30, 653 (2013). https://doi.org/10.1007/s12032-013-0653-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0653-1