Abstract
Schizophrenia is a severe chronic neuropsychiatric disorder, and its exact pathogenesis remains unclear. This study investigated the effect of ketamine on the expression of ErbB4 (considered a schizophrenia candidate gene) in the hippocampus and prefrontal cortex of rats. Rats were randomly divided into four groups: control, low-dose, medium-dose and high-dose groups. The low-dose, medium-dose and high-dose groups were intraperitoneally injected with 15 mg/kg, 30 mg/kg and 60 mg/kg ketamine, respectively, twice a day (9:00 a.m. and 9:00 p.m.); the control group was administered normal saline. The treatment lasted 7 days. After treatment, rats were euthanized, and their brain tissues were collected and then analyzed by immunohistochemistry. The results of immunohistochemistry staining demonstrated that the ErbB4 protein was expressed exclusively in the CA3 region of the hippocampus and the Cg1 region of the prefrontal cortex. Ketamine administration significantly decreased the expression of ErbB4 in a dose-dependent manner. The high-dose ketamine treatment was found to be optimal for establishing a rat model for schizophrenia. Ketamine induced symptoms similar to schizophrenia in humans. The ketamine-induced rat model for schizophrenia constructed in this study provides novel insights to better understand the pathogenic mechanisms of schizophrenia and aid in drug discovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ketamine, a noncompetitive and nonselective N-methyl-d-aspartate (NMDA) receptor antagonist, has been widely used clinically as an anesthetic (Huang et al. 2016). In addition, a small number of individuals use ketamine as a recreational drug. Ketamine typically induces hallucinogenic effects that alter the sense of sight, sound and touch, which was proven by research in rodents (Nikiforuk et al. 2016; Zhao et al. 2016; Kara et al. 2017). Additionally, at higher doses (>80 mg/kg) in rodent models, the drug can induce DNA damage within 12 h, as confirmed by comet assay (Leffa et al. 2016). Ketamine can also inhibit human sperm function and lead to schizophrenia (He et al. 2016; Favretto et al. 2016).
Schizophrenia is a severe chronic neuropsychiatric disorder characterized by positive symptoms (hallucinations and delusions), negative symptoms (social withdrawal) and cognitive impairments (impaired executive functions and visual memory) (Potasiewicz et al. 2017; Zugno et al. 2016; Bennett 2011). According to a study by Simeone et al. (2015), the 12-month prevalence of schizophrenia is 0.33% (with a range of 0.26–0.51%), and the median estimated lifetime prevalence is 0.48% (with a range of 0.34–0.85%) (Simeone et al. 2015). In 2013, the American Psychiatric Association estimated a lifetime prevalence of schizophrenia of approximately 0.3–0.7%. Due to the lack of biomarkers and mechanisms, while antipsychotic drugs exert beneficial effects on the positive symptoms, they have little effect on negative symptoms or cognitive deficits (Okazaki et al. 2016; Noto et al. 2016; Chen et al. 2016). Since these two types of symptoms negatively affect social functioning throughout the course of the disease, there is an urgent need to develop new effective therapeutic methods to manage them. At this point, a new biomarker for the two types of symptoms would help scientists in monitoring and studying the process.
ErbB4 plays an important role as a cell surface receptor for neuregulins and EGF family members. ErbB4 can regulate the development of the heart (Wadugu and Kuhn 2012), the central nervous system (Huang et al. 2000) and the mammary glands (Muraoka-Cook et al. 2008). ErbB4 and its ligand neuregulin1 (NRG1) have been studied extensively as candidate pathways for schizophrenia (Hahn et al. 2006; Li et al. 2007; Banerjee et al. 2010; Nicodemus et al. 2010). The dysfunction of the NRG1/ErbB4 signaling pathway has been closely associated with the pathogenesis of schizophrenia; cases of schizophrenia and childhood onset of schizophrenia have been shown to be related to mutation of the NRG1/ErbB4 signaling pathway (Dang et al. 2016; Deng et al. 2015; du Bois et al. 2012; Taylor et al. 2011; Yang et al. 2012).
In this study, we aimed to determine the effects of ketamine on ErbB4 in a ketamine-induced rat model for schizophrenia. We found that ErbB4 was expressed only in the CA3 region of the hippocampus and the Cg1 region of the prefrontal cortex under treatment with high-dose ketamine (60 mg/kg), as determined by immunohistochemistry. This work will help scientists better understand the ErbB4-related signaling pathway in schizophrenia.
Materials and Methods
Drugs, Reagents and Apparatus
The reagents/materials were purchased as follows: ketamine hydrochloride (cat. #H35020148, Fujian Gutian Pharmaceutical Co., Ltd.); rabbit monoclonal anti-ErbB4 antibody (cat. #05–1133, MilliporeSigma, Billerica, MA, USA); DAB detection kit (cat. #PV-9000, Beijing Zhong Shan Jin Qiao Biotechnology Co., Ltd.); microscope (Olympus BX53, Japan).
Animals
Three-week-old male Sprague–Dawley (SD) rats, weighing 110–125 g, were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. All the animals were housed in an environment with temperature of 22 ± 1 °C, relative humidity of 50 ± 1% and a light/dark cycle of 12/12 h, and were fed a standard diet (Beijing KeaoXieli Feed Co., Ltd., SPF rat feed); proper water was given. All animal studies (including the rat euthanasia procedure) were performed in compliance with the regulations and guidelines of Kunming Medicine University institutional animal care and conducted according to Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and institutional animal care and use committee (IACUC) guidelines.
Drug Administration
A total of 24 SD rats were acclimatized to the environment for 1 week. Subsequently, rats were randomly divided into four experimental groups (n = 6/group): control, low-dose, medium-dose and high-dose groups. The low-dose, medium-dose and high-dose groups were intraperitoneally administered 15 mg/kg, 30 mg/kg and 60 mg/kg ketamine, respectively, twice each day (9:00 a.m. and 9:00 p.m); the control group was administered normal saline. The ketamine was diluted with normal saline (Bian et al. 2009). The treatment lasted for 7 days.
Tissue Preparation
Rats were euthanized 13 h after the last injection and perfused with normal saline until the liver became white and the effluent was clarified. A blade was used to remove the unwanted parts of the brain, rostral and caudal to the region of interest. In the case of the hippocampus, the brain was placed ventral side down, the superior colliculus was located and a transverse cut was made, and the caudal part was discarded (see details in supplemental figure). Brain tissues from the hippocampus and prefrontal cortex regions were collected, fixed in 4% paraformaldehyde (PFA) and subsequently embedded in paraffin.
Immunohistochemistry
The paraffin-embedded tissues from the two regions were cut coronally into 5-μm sections. Slices containing coronal sections were incubated at 70 °C for 3 h, deparaffinized and subsequently rehydrated using a graded series of ethanol to PBS. Slices were then treated with 1% (w/w) citrate buffer (pH 6.0) and endogenous peroxidase inhibitors. The measured areas were delineated with an immunohistochemical pen. The samples were sequentially incubated with rabbit monoclonal anti-ErbB4 antibody (1:400) and goat anti-mouse/rabbit IgG polymer-marked horseradish peroxidase. Lastly, the sections were stained with DAB stain agent for 5 min, and then rendered transparent with dimethylbenzene (Liu et al. 2013; Ho et al. 2014; Li et al. 2014). Stained sections were visualized under a microscope (Olympus BX53, Japan).
Quantitative Methods
All images and data were acquired and analyzed using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA). The gray value of ErbB4 protein was measured by the Image-Pro Plus 6.0 analysis system, and then converted into an optical density (OD) value. The ratio of optical density to the whole image, referred to as OD/area, was calculated, and the expression of ErbB4 protein was quantified by the value of the OD/area ratio (Liu et al. 2014; Koh et al. 2016; Onaka et al. 2016; Farahmandfar et al. 2016; Zhang et al. 1631).
Statistical Methods
All data ae presented as mean ± standard error of the mean (SEM). Statistical analysis and generation of graphs were carried out using GraphPad Prism 5 software (San Diego, CA, USA). Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by the Newman–Keuls post hoc test (*p < 0.05; **p < 0.01; ***p < 0.001).
Results
Expression and Localization of ErbB4 in the Hippocampus Region
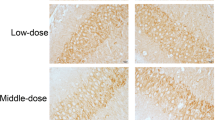
To investigate the effect of ketamine on the expression and localization of ErbB4 in the hippocampus, we performed an immunohistochemistry (IHC) staining experiment for ErbB4. As shown in Fig. 1a, ErbB4-positive cells were localized in the CA3 region of the hippocampus. Our data indicate that treatment with ketamine decreased the expression of ErbB4 in a dose-dependent manner (Fig. 1a and b). The expression of ErbB4 was significantly decreased in the medium-dose and high-dose groups (p < 0.01), while the low-dose group showed no significant reduction (Newman–Keuls post hoc test) (Fig. 1a and b). Together, the results showed that ketamine decreased the ErbB4 protein level in the CA3 region of the hippocampus.
The expression of ErbB4 in tissues of the CA3 region of the hippocampus. a Data shown are representative images of staining for ErbB4 protein in tissues of the CA3 region of the hippocampus. Scale bar, 20 μm. Four-week-old male Sprague–Dawley (SD) rats were randomly assigned to one of four groups (n = 6 per group): the control group (treated with equal volume of normal saline), low-dose group (treated with ketamine at a dose of 15 mg /kg body weight), medium-dose group (treated with ketamine at a dose of 30 mg/kg body weight), and high-dose group (treated with ketamine at a dose of 60 mg/kg body weight). Rats were intraperitoneally administered ketamine or normal saline twice daily (9 a.m. and 9 p.m.) for 7 consecutive days. Rats were euthanized 13 h after the last injection of ketamine or saline, and the hippocampal tissues were subjected to immunohistochemistry staining. b Quantitation of the ErbB4 protein expression levels in the CA3 region of the hippocampus tissues from rats treated with normal saline or different doses of ketamine. The relative ErbB4 expression levels in tissues from the CA3 region of the hippocampus from the four groups were quantitated by OD/area. n = 6 for each group; **p < 0.01, compared with the control group.
Expression and Localization of ErbB4 in the Prefrontal Cortex
The same approach as that described above was used to examine the expression and localization of ErbB4 in the prefrontal cortex of rats. As shown in Fig. 2a, ErbB4-positive cells were localized in the Cg1 region of the prefrontal cortex. Moreover, the quantitative results indicated that ketamine reduced the protein level of ErbB4 in a dose-depended manner. The expression of ErbB4 was significantly lower in medium-dose and high-dose groups than in the control group (p < 0.01), and the protein level in the low-dose group did not show a significant reduction (Newman–Keuls post hoc test) (Fig. 2a and b). Together, the results showed that ketamine could decrease the ErbB4 protein level in the Cg1 region of the prefrontal cortex.
The expression of ErbB4 in the Cg1 region of the prefrontal cortex. a. Data shown are representative images of staining for ErbB4 protein in the Cg1 region of the prefrontal cortex. Scale bar, 20 μm. Experimental procedures were as described in Fig. 1a, except that the ErbB4 protein expression was examined in the postmortem tissue from rat prefrontal cortex. b. Quantitation of the ErbB4 protein expression levels in the Cg1 region of the prefrontal cortex from rats treated with normal saline or different doses of ketamine. The relative expression levels of ErbB4 protein in the Cg1 region of the prefrontal cortex from the four groups were quantitated by OD/area. n = 6 for each group; **p < 0.01, compared with the control group.
Discussion
Similar to methylamphetamine-induced animal models, ketamine can induce both positive and negative symptoms of schizophrenia (Frohlich and Van Horn 2014). In this study, a ketamine-induced model was used to investigate the effect of the drug on the expression of ErbB4 in the hippocampus and prefrontal cortex of rats.
The experimental results showed that ketamine treatment indeed influenced the expression of ErbB4 specifically in both the CA3 region of the hippocampus and the Cg1 region of the prefrontal cortex. In addition, the effects of ketamine acted in a strictly dose-dependent manner. The study also demonstrated that a dose of 60 mg/kg (ketamine) was the best dosage for establishing a rat model of schizophrenia. Although ketamine doses of 30 mg/kg and 60 mg/kg caused similar effects, the higher-dose ketamine (60 mg/kg) led to more obvious symptoms of schizophrenia. Therefore, based on these results, the higher dose (60 mg/kg) is recommended in order to ensure a reliable, stable and secure model.
The high-dose group (60 mg/kg) showed significant positive symptoms of schizophrenia, including apparent aggressive behavior starting from the third day of ketamine treatment. On the other hand, the medium-dose and low-dose groups (15 mg/kg and 30 mg/kg) exhibited less significant symptoms of schizophrenia than the high-dose group, even after all scheduled treatments. A dose higher than 60 mg/kg led to the death of the rats in our study. This suggests that high-dose ketamine is a requirement in a chronic ketamine exposure-induced rat model of schizophrenia.
Importantly, there are no relevant articles arguing that changes in ErbB4 expression in different encephalon regions are associated with ketamine. Our work thus reveals the relationship between ketamine and protein reduction (ErbB4) in different brain tissues, which could improve our knowledge regarding schizophrenia, and all the results here also provide a potential biomarker to aid in drug discovery. However, schizophrenia is a complex disorder involving multiple disease pathways, and therefore a comprehensive therapeutic methodology is needed. This work may provide a potential target and open a new path to the final objective.
Conclusions
In this study, we showed that in vivo administration of ketamine in rats could reduce the expression of the ErbB4 protein in specific regions of the hippocampus (CA3 region) and the prefrontal cortex (Cg1 region), and that the reduction occurred in a dose-dependent manner. Moreover, ketamine at 60 mg/kg was found to be effective for establishing an optimal rat model of schizophrenia. In summary, this study investigated the protein distribution and expression of ErbB4 in the encephalon regions of a schizophrenia-induced rat model chronically treated with high-dose ketamine, and provides novel insights that will deepen our understanding of the pathogenic mechanisms of schizophrenia and aid in drug discovery. Further studies are still needed to elucidate the exact molecular mechanisms involved in the pathogenesis of schizophrenia.
References
Banerjee A, Macdonald ML, Borgmann-Winter KE et al (2010) Neuregulin 1-erbB4 pathway in schizophrenia: from genes to an interactome. Brain Res Bull 83(3–4):132–139
Bennett MR (2011) Schizophrenia: susceptibility genes, dendritic-spine pathology and gray matter loss. Prog Neurobiol 95(3):275–300
Bian SZ, Liu WL, Zhang ZX et al (2009) The correlation between ketamine-induced schizophrenia-like signs in mice and the expressions of NRG1, ErbB4 mRNA. Fa Yi Xue Za Zhi 25(5):348–351 358
Chen SD, Sun XY, Niu W et al (2016) A preliminary analysis of microRNA-21 expression alteration after antipsychotic treatment in patients with schizophrenia. Psychiatry Res 244:324–332
Dang R, Cai H, Zhang L et al (2016) Dysregulation of Neuregulin-1/ErbB signaling in the prefrontal cortex and hippocampus of rats exposed to chronic unpredictable mild stress. Physiol Behav 154:145–150
Deng C, Pan B, Hu CH et al (2015) Differential effects of short- and long-term antipsychotic treatment on the expression of neuregulin-1 and ErbB4 receptors in the rat brain. Psychiatry Res 225(3):347–354
du Bois TM, Newell KA, Huang XF (2012) Perinatal phencyclidine treatment alters neuregulin 1/erbB4 expression and activation in later life. Eur Neuropsychopharmacol 22(5):356–363
Farahmandfar M, Bakhtazad A, Akbarabadi A et al (2016) The influence of dopaminergic system in medial prefrontal cortex on ketamine-induced amnesia in passive avoidance task in mice. Eur J Pharmacol 781:45–52
Favretto D, Vogliardi S, Tucci M et al (2016) Occupational exposure to ketamine detected by hair analysis: a retrospective and prospective toxicological study. Forensic Sci Int 265:193–199
Frohlich J, Van Horn JD (2014) Reviewing the ketamine model for schizophrenia. J Psychopharmacol 28(4):287–302
Hahn CG, Wang HY, Cho DS et al (2006) Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med 12(7):824–828
He Y, Zou Q, Li B et al (2016) Ketamine inhibits human sperm function by ca(2+)-related mechanism. Biochem Biophys Res Commun 478(1):501–506
Ho TY, Tang NY, Hsiang CY et al (2014) Uncaria rhynchophylla and rhynchophylline improved kainic acid-induced epileptic seizures via IL-1beta and brain-derived neurotrophic factor. Phytomedicine. 21(6):893–900
Huang YZ, Won S, Ali DW et al (2000) Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 26(2):443–455
Huang H, Liu CM, Sun J et al (2016) Ketamine affects the neurogenesis of the hippocampal dentate gyrus in 7-day-old rats. Neurotox Res 30(2):185–198
Kara NZ, Agam G, Anderson GW et al (2017) Lack of effect of chronic ketamine administration on depression-like behavior and frontal cortex autophagy in female and male ICR mice. Behav Brain Res 317:576–580
Koh MT, Shao Y, Sherwood A et al (2016) Impaired hippocampal-dependent memory and reduced parvalbumin-positive interneurons in a ketamine mouse model of schizophrenia. Schizophr Res 171(1–3):187–194
Leffa DD, Bristot BN, Damiani AP et al (2016) Anesthetic ketamine-induced DNA damage in different cell types in vivo. Mol Neurobiol 53(8):5575–5581
Li B, Woo RS, Mei L et al (2007) The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 54(4):583–597
Li J, Liu W, Peng Q et al (2014) Effect of rhynchophylline on conditioned place preference on expression of NR2B in methamphetamine-dependent mice. Biochem Biophys Res Commun 452(3):695–700
Liu Y, Yu Y, Schachner M et al (2013) Neuregulin 1-beta regulates cell adhesion molecule L1 expression in the cortex and hippocampus of mice. Biochem Biophys Res Commun 441(1):7–12
Liu W, Peng QX, Lin XL et al (2014) Effect of rhynchophylline on the expression of p-CREB and sc-Fos in triatum and hippocampal CA1 area of methamphetamine-induced conditioned place preference rats. Fitoterapia. 92:16–22
Muraoka-Cook RS, Feng SM, Strunk KE et al (2008) ErbB4/HER4: role in mammary gland development, differentiation and growth inhibition. J Mammary Gland Biol Neoplasia 13(2):235–246
Nicodemus KK, Law AJ, Radulescu E et al (2010) Biological validation of increased schizophrenia risk with NRG1, ERBB4, and AKT1 epistasis via functional neuroimaging in healthy controls. Arch Gen Psychiatry 67(10):991–1001
Nikiforuk A, Holuj M, Kos T et al (2016) The effects of a 5-HT5A receptor antagonist in a ketamine-based rat model of cognitive dysfunction and the negative symptoms of schizophrenia. Neuropharmacology. 105:351–360
Noto C, Ota VK, Santoro ML et al (2016) Depression, cytokine, and cytokine by treatment interactions modulate gene expression in antipsychotic naive first episode psychosis. Mol Neurobiol 53(8):5701–5709
Okazaki S, Boku S, Otsuka I et al (2016) The cell cycle-related genes as biomarkers for schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 70:85–91
Onaka Y, Shintani N, Nakazawa T et al (2016) Prostaglandin D2 signaling mediated by the CRTH2 receptor is involved in MK-801-induced cognitive dysfunction. Behav Brain Res 314:77–86
Potasiewicz A, Holuj M, Kos T et al (2017) 3-Furan-2-yl-N-p-tolyl-acrylamide, a positive allosteric modulator of the alpha7 nicotinic receptor, reverses schizophrenia-like cognitive and social deficits in rats. Neuropharmacology 113(Pt A):188–197
Simeone JC, Ward AJ, Rotella P et al (2015) An evaluation of variation in published estimates of schizophrenia prevalence from 1990 horizontal line 2013: a systematic literature review. BMC Psychiatry 15:193
Taylor SB, Taylor AR, Markham JA et al (2011) Disruption of the neuregulin 1 gene in the rat alters HPA axis activity and behavioral responses to environmental stimuli. Physiol Behav 104(2):205–214
Wadugu B, Kuhn B (2012) The role of neuregulin/ErbB2/ErbB4 signaling in the heart with special focus on effects on cardiomyocyte proliferation. Am J Physiol Heart Circ Physiol 302(11):H2139–H2147
Yang CP, Wang HA, Tsai TH et al (2012) Characterization of the neuropsychological phenotype of glycine N-methyltransferase−/− mice and evaluation of its responses to clozapine and sarcosine treatments. Eur Neuropsychopharmacol 22(8):596–606
Zhang Y, Sun J, Zhu S et al (1631) The role of rhynchophylline in alleviating early brain injury following subarachnoid hemorrhage in rats. Brain Res 2016:92–100
Zhao T, Li C, Wei W et al (2016) Prenatal ketamine exposure causes abnormal development of prefrontal cortex in rat. Sci Rep 6:26865
Zugno AI, Canever L, Heylmann AS et al (2016) Effect of folic acid on oxidative stress and behavioral changes in the animal model of schizophrenia induced by ketamine. J Psychiatr Res 81:23–35
Funding
This study was supported by the National Natural Science Foundation of China [Grant No. 81373239], the Yunnan Applied Basic Research/Kunming Medical University Union Project [Grant Nos. 2017FE467-(166) and 2018FE001-(011)], and the Kunming Medical University Innovation Group Project [Grant No. CXTD201604].
Author information
Authors and Affiliations
Contributions
R-F Xie and L-C Liao: study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding. R-F Xie and S-J Hong contributed equally to this work. R-F Xie, S-J Hong, Y Ye, X-Y Wang: the acquisition of samples or data, statistical analysis, review of the manuscript, obtained funding. F Chen, L Yang, Y-Y Yan: statistical analysis, review of the manuscript, material support.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that the submitted manuscript does not contain previously published material and is not under consideration for publication elsewhere. The manuscript is a truthful original work without fabrication, fraud or plagiarism. Each author has made an important scientific contribution to the study and is thoroughly familiar with the primary data. All the listed authors have read the complete manuscript and have approved the publication of the paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PPTX 142 kb)
Rights and permissions
About this article
Cite this article
Xie, R., Hong, S., Ye, Y. et al. Ketamine Affects the Expression of ErbB4 in the Hippocampus and Prefrontal Cortex of Rats. J Mol Neurosci 70, 962–967 (2020). https://doi.org/10.1007/s12031-020-01502-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01502-1