Abstract
Early exposure to sevoflurane, an inhalation anesthetic, induces neurodegeneration in the developing brain and subsequent long-term neurobehavioral abnormalities. Here, we investigated whether an enriched environment could mitigate neonatal sevoflurane exposure-induced long-term cognitive and synaptic plasticity impairments. Male C57BL/6 mice were exposed to 3 % sevoflurane 2 h daily for 3 days from postnatal day 6 (P6) to P8. The exposed mice were randomly allocated to an enriched environment for 2 h daily between P8 and P42 or to a standard environment. Their behavior and cognition were assessed using open field (P35) and fear conditioning tests (P41–P42). Hematoxylin–eosin staining was used to study morphological changes in pyramidal neurons of hippocampal CA1 and CA3 regions. Synaptic plasticity alternations were assessed using western blotting, Golgi staining, and electrophysiological recording. We found that sevoflurane-exposed mice housed in a standard environment exhibited a reduced freezing response in the contextual test, decreased number of dendritic spines on pyramidal neurons and synaptic plasticity-related proteins in the hippocampus, and impaired long-term potentiation. However, in an enriched environment, some of these abnormities induced by repeated sevoflurane exposure. In conclusion, neonatal sevoflurane exposure-induced cognitive and synaptic plasticity impairments are ameliorated by an enriched environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accumulating evidence demonstrates that exposure to general anesthetics at an early stage of postnatal brain development can cause neuroapotosis and neurocognitive impairments later in life (Satomoto et al. 2009; Shen et al. 2013; Edwards et al. 2010). Although human retrospective epidemiological studies do not provide a definitive answer to the question of whether anesthesia affects brain development, they do show that the duration of anesthesia and the number of exposures to anesthetics may be among the aggravating factors (Wilder et al. 2009). One recent retrospective study investigated more than 5,000 children and demonstrated that those who received early anesthesia exposure were associated with learning disabilities as they matured (Sprung et al. 2012), which raises a significant safety concern regarding the use of general anesthetics in clinical practice (Rappaport et al. 2015; Servick 2014). Unfortunately, the mechanisms underlying general anesthetic exposure-induced cognitive impairment remain to be elucidated and effective therapy is currently limited.
The gamma-aminobutyric acid (GABA) receptor agonist pentobarbital induces impaired spatial learning and long-term suppression of hippocampal synaptic plasticity when administered at postnatal day 7 (P7) (Tachibana et al. 2011). Similarly, neonatal exposure to ketamine, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, causes impairment of long-term synaptic plasticity in the anterior cingulate cortex of rats (Wang et al. 2014). Sevoflurane, one of the most frequently used inhalation anesthetics for neonates in the clinical setting, potentiates GABA receptors and inhibits NMDA receptor function (Satomoto et al. 2009; Shen et al. 2013; Edwards et al. 2010). Sevoflurane exposure transiently reduces long-term potentiation (LTP) during development in hippocampal slice preparations (Ishizeki et al. 2008), but the persistence of this effect is not well studied. Because of the important role of hippocampal LTP in spatial learning (Li et al. 2013; Gao et al. 2014; Kato et al. 2013) and the vulnerability of hippocampal synaptic development to perinatal insults (Rood et al. 2014), one plausible mechanism underlying the neonatal sevoflurane exposure-induced cognitive impairment may be related to impaired synaptic morphology and function.
Therefore, the present study investigated the long-term consequences of repeated neonatal sevoflurane exposure on contextual fear learning and synaptic plasticity. We also determined whether these abnormalities could be reversed by environmental enrichment.
Materials and Methods
Animals and Housing
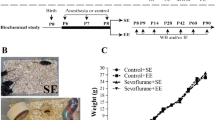
Experimental protocols were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals from the National Institutes of Health (Bethesda, MD, USA), and were approved by the Ethics Committee of Jinling Hospital, Nanjing Medical University. Six-day-old male C57BL/6 mice were purchased from Jinling Hospital, Nanjing Medical University, China. The mice were randomly assigned to one of the following four groups: control + standard environment (n = 25), control + enriched environment (n = 24), sevoflurane + standard environment (n = 25), and sevoflurane + enriched environment (n = 25). The flow chart of the study protocol is presented in Fig. 1a.
Timeline of the experimental procedure in mice. Six-day-old mice were exposed to 3 % sevoflurane 2 h daily for three consecutive days and were randomly allocated to either a standard environment (SE) or enriched environment (EE) from postnatal days (P) 8–42. The behavioral tests, open field (OF) and fear conditioning (FC), were assessed at the indicated times (a). The mice used for the biochemical study were not subjected to behavioral tests (b)
Anesthesia Protocol
On P6, mice in the sevoflurane groups received 3 % sevoflurane (30 % oxygen/air) 2 h daily for three consecutive days in the anesthetizing chamber, where the animals were kept on a warm plate to maintain a rectal temperature of 37 ± 0.5 °C, as described in our previous study (Ji et al. 2015). Mice in the control group received 30 % oxygen/air at the same flow rate as that for sevoflurane in a similar chamber. The mice breathed spontaneously, and the sevoflurane and oxygen concentrations were measured continuously (GE Datex-ohmeda, Tewksbury, MA, USA). The pups rejoined their mothers after their righting reflex returned following termination of the sevoflurane exposure. The mice in the enriched environment groups were placed into an enriched environment (70 × 45 × 40 cm) every day for 2 h from P8 to P42. Animals in the standard environment groups remained in the standard environment (30 × 25 × 15 cm) throughout the experiment (Fig. 1b, c). To maintain novelty and provide challenging stimulation, the objects in the enrichment cage were changed two to three times per week. Mice in all groups remained with mothers from P8 to P21 and were weaned from P22 to P42.
Arterial Blood Gas Analysis
Arterial blood gas analysis was performed immediately after the last sevoflurane exposure or 30 % O2 exposure (P8) with an arterial blood gas analyzer (GEM Premier 3000, Instrumentation Laboratory, Guangzhou, China) as previously described (Ji et al. 2015).
Open Field Test
The open field test was used to determine locomotor activity at P35 as we previously described, with some modifications (Ji et al. 2015). The open field apparatus was a plastic chamber with dimensions of 40 × 40 × 45 cm. Each mouse was released in the center of the chamber, and its movements during the first 5 min of habituation were recorded. The total distance traveled and the time spent in the center of the open field were analyzed.
Fear Conditioning Test
This test was performed in a black plastic chamber with a stainless steel grid floor. The mice were allowed to explore for 3 min for habituation. A 30-s, 75-dB, 1-kHz tone was co-terminated with a 2-s, 0.75-mA foot shock, which was delivered through stainless steel bars by a constant current generator. A contextual test was performed in the conditioning chamber for 5 min without any stimulation 24 h after the conditioning test. Two hours after the contextual test, a cued test was performed by presentation of the cue (75 dB noise, 3 min duration) in a novel context with distinct visual and tactile cues.
Histopathological Examination
Six hours after the last sevoflurane (30 % oxygen/air) exposure, mice were killed. The hippocampus was removed and postfixed in the same fixative as that used for perfusion at 4 °C, dehydrated, and then embedded in paraffin blocks. Coronal sections of 5 mm were cut using a microtome (Leica CM30 50S, Solms, Germany) and stained with hematoxylin–eosin to study the morphological changes of pyramidal neurons in the CA1 and CA3 regions of the hippocampus. Cell counts in hippocampal CA1 and CA3 regions were conducted at ×40 magnification using a light microscope (Tokyo, Japan). Cells with a round or oval nucleus exhibiting no evidence of shrinkage or edema were scored as undamaged. Cell numbers from CA1 and CA3 were counted in five different fields of each section by an observer blinded to the treatments and conditions.
Western Blotting Analysis
The hippocampus was subjected to western blotting analysis as described in our previous studies (Ji et al. 2015; Li et al. 2013; Yang et al. 2015). Postsynaptic density protein 95 (PSD95, 1:500; Abcam, Cambridge, UK), BDNF (1:1,000; Santa Cruz Biotechnology, Dallas, TX, USA), and β-actin (1:1,000; Cell Signaling Technology, Boston, MA, USA) were used to detect PSD95, BDNF, and β-actin expression, respectively. The protein bands were detected using enhanced chemiluminescence and quantitated using ImageJ software (NIH Image, Bethesda, USA).
Golgi Staining
On P42, mice were killed by an intraperitoneal injection of 2 % sodium pentobarbitone (50 mg/kg), and their brains were collected. Sample preparation and Golgi silver staining were performed with the FD Rapid Golgi Stain Kit (FD Neurotechnologies, Columbia, MD, USA) as we previously described (Yang et al. 2015). Spine density (spine number per 10 μm) for each neuron was analyzed using MATLAB software (MathWorks, Nedik, MA, USA). The spines were counted on two or three segments of secondary dendrites.
Electrophysiological Analysis
Electrophysiological recording was performed as we previously described (Yang et al. 2015). Briefly, transverse hippocampal slices (350 μm thick) were prepared from P39 to P42 male mice. After decapitation, the brain was removed and placed in oxygenated (95 % O2/5 % CO2) artificial cerebrospinal fluid (ACSF; sucrose 75 mM, NaCl 87 mM, KCl 2.5 mM, NaH2PO4 1.25 mM, NaHCO3 21.4 mM, CaCl2 0.5 mM, MgCl2 7 mM, and D-glucose 20 mM) at 4 °C. Slices were cut using a Leica VT1000S vibratome (Leica Instruments Ltd., Wetzlar, Germany) and maintained at 32 °C for 1 h in a holding chamber filled with oxygenated ACSF. After an equilibration period of at least 1 h at room temperature, a single slice was transferred to the recording chamber and continuously perfused with the same oxygenated ACSF (23–25 °C) at a flow rate of 2.5–3 mL/min.
Statistical Analysis
For all statistical evaluations, SPSS Statistics version 16 software (SPSS Inc., Chicago, IL, USA) was used. Averaged values are given as mean ± SEM. The normality assumption test was performed using the Shapiro–Wilk test, with the P value set at 0.05. Comparisons were performed using Student’s t test or one-way analysis of variance followed by a Tukey test as appropriate. A P value <0.05 was regarded as a statistically significant difference.
Results
Effects of Repeated Sevoflurane Exposure on Blood Gas Values in Mice
Repeated exposure of neonatal mice to sevoflurane did not significantly change the pH, arterial oxygen (PO2), or carbon dioxide tension (PCO2) values compared with the control group (pH, 7.35 ± 0.07 and 7.39 ± 0.07; PCO2, 46.2 ± 5.4 and 43.2 ± 6.1 mmHg, and PO2, 132.6 ± 9.2 and 138.5 ± 7.8 mmHg; all P > 0.05), suggesting repeated sevoflurane exposure did not affect arterial blood gas.
Repeated Sevoflurane Exposure-Induced Cognitive Impairment is Ameliorated by an Enriched Environment
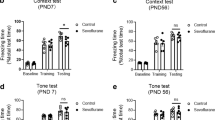
No significant difference was observed in the total locomotor activity (P > 0.05; Fig. 2a) and the time spent in the center (P > 0.05; Fig. 2b) during the open field test among the four groups. The freezing time of mice exposed to sevoflurane was significantly reduced in the contextual test when compared with those of controls at P42 (P < 0.05; Fig. 2c). However, there was no significant difference in the freezing time to tone between the four groups at P42 (P > 0.05; Fig. 2d). These results suggested that repeated sevoflurane exposure impaired hippocampal-dependent memory without affecting locomotor activity. An enriched environment significantly reversed the decreased freezing time observed during the contextual test in the sevoflurane-treated mice.
Sevoflurane exposure-induced cognitive impairment is reversed by an enriched environment. No difference is observed in the total locomotor activity (a) and time spent in the center (b) during the open field test among the four groups. The freezing response of mice exposed to sevoflurane is significantly reduced in the contextual test, (c) but not the freezing time to tone (d) compared with those of control + standard environment (SE) group at P42. EE enriched environment. Each value represents the mean ± SEM (n = 12 per group), *P < 0.05
Repeated Sevoflurane Exposure Induces Morphological Changes in Pyramidal Neurons in Hippocampal CA1 and CA3 Regions
The cell outline in the control groups was clear and the structure was compact. Although the number of pyramidal neurons did not change, repeated sevoflurane exposure resulted in neuronal cell edema and nuclear shrinkage in the CA1 and CA3 regions of the hippocampus (Fig. 3), indicating sevoflurane exposure caused abnormal morphology changes in the hippocampus.
Repeated sevoflurane exposure-induced morphological changes of pyramidal neurons in hippocampal CA1 and CA3 regions. Hematoxylin–eosin staining and cell counts per visual field (×400). Con control, Sev sevoflurane, SE standard environment, EE enriched environment. Each value represents the mean ± SEM (n = 5 per group). Arrows indicate neuronal cell edema and nuclear shrinkage
Repeated Sevoflurane Exposure-Induced Dendritic Spine Abnormalities in Hippocampal CA1 and CA3 are Ameliorated by an Enriched Environment
The number of dendritic spines in hippocampal CA1 and CA3 regions decreased significantly in the sevoflurane + standard environment group compared with the control + standard environment group, suggesting that neonatal sevoflurane exposure-induced cognitive impairment was associated with decreased dendritic spine number. However, an enriched environment ameliorated the sevoflurane-induced decrease in dendritic spine number compared with the sevoflurane + standard environment group (P < 0.05; Fig. 4).
Sevoflurane exposure-induced loss of dendritic spines in hippocampal CA1 and CA3 regions is reversed by an enriched environment. Representative micrographs of dendritic segments of pyramidal neurons in hippocampal CA1 and CA3 regions in the four groups (a, c). Histograms represent the number of dendritic spines per 10 μm length of primary dendrites after sevoflurane exposure (b, d). Con control, Sev sevoflurane, SE standard environment, EE enriched environment. Each value represents the mean ± SEM (n = 8 per group), *P < 0.05
Repeated Sevoflurane Exposure-Induced Loss of Synaptic Plasticity-related Protein in the Hippocampus is Ameliorated by an Enriched Environment
The levels of BDNF and PSD95 were decreased at P42 after repeated sevoflurane exposure compared with those in the control group. These results suggested that sevoflurane exposure-induced cognitive impairment was associated with the loss of synaptic plasticity-related proteins in the hippocampus. An enriched environment rescued these abnormalities, as shown by comparisons with the sevoflurane + standard environment group (P < 0.05; Fig. 5).
Sevoflurane exposure-induced loss of synaptic plasticity-related protein in the hippocampus is reversed by an enriched environment. Representative micrographs of BDNF and PSD95 were assessed by western blotting at P42 (a). Histograms represent the levels of BDNF and PSD95 among the four groups (b). Con control, Sev sevoflurane, SE standard environment, EE enriched environment. Each value represents the mean ± SEM (n = 4 per group), *P < 0.05
Repeated Sevoflurane Exposure-Induced Impaired LTP is Ameliorated by an Enriched Environment
As mentioned above, compared with the control + standard environment group, an enriched environment did not further increase the freezing behavior or enhance synaptic function of mice. Therefore, we only measured LTP in the control + standard environment, sevoflurane + standard environment group, and sevoflurane + enriched environment group to minimize the number of animals used. There was no difference in the input–output curves among the three groups (P > 0.05; Fig. 6a). The paired-pulse facilitation was similar among all groups (P > 0.05; Fig. 6b). The Schaffer CA3-CA1 hippocampal collateral pathway displayed synaptic plasticity deficits in the sevoflurane + standard environment group as compared with the other two groups (P < 0.05; Fig. 6c). The slopes of the last 10 min in the LTP were analyzed. The value of the slope in the sevoflurane + standard environment group (111 ± 0.8 %) was lower than those in the control + standard environment (164 ± 2 %) and sevoflurane + enriched environment groups (158 ± 0.54 %; P < 0.05; Fig. 6d). These results suggested that an enriched environment prevented neonatal sevoflurane exposure-induced synaptic plasticity impairments.
Sevoflurane-impaired hippocampal synaptic plasticity is rescued by an enriched environment. The amplitude of the evoked field excitatory post-synaptic potentials (fEPSP) is similar among the groups (a). No difference is observed in paired-pulse facilitation among the groups (b). Long-term potentiation (LTP) is impaired in the sevoflurane + standard environment group compared with that in the control + standard environment group. This impairment is reversed by an enriched environment (c). Insert in Fig. 4c shows superimposed sample sweeps from the last 10 min of the recording in the three groups. The slopes of last 10 min in the LTP were analyzed (d). Con control, Sev sevoflurane, EE enriched environment. Data are shown as mean ± SEM (n = 4–5 per group), *P < 0.05
Discussion
Our study demonstrated that repeated exposure of neonatal mice to sevoflurane induced significant cognitive impairment that was accompanied by impaired synaptic plasticity. However, environmental enrichment ameliorated these abnormalities.
Sevoflurane is an inhalation anesthetic commonly used in obstetric or pediatric surgical procedures and is an NMDA receptor antagonist (Satomoto et al. 2009; Shen et al. 2013; Edwards et al. 2010). It has been proposed that P6 mice exposed to 3 % sevoflurane for 6 h show long-lasting neurocognitive dysfunction (Satomoto et al. 2009). However, in another study comparing isoflurane to sevoflurane exposure in P7 rats, neither isoflurane nor sevoflurane was associated with cognitive impairment when the rats were tested from 31 to 40 days after the exposure (Liang et al. 2010). One recent study suggested that sevoflurane anesthesia even improved cognitive impairment in both young adult and aged rats when tested 1 week after sevoflurane exposure (Callaway et al. 2012). These results suggest that the cognitive effects of anesthetics in rodents critically depend on the test anesthetic concentrations, anesthetic duration, rodent age, and time between anesthesia and cognitive testing. In the present study, we demonstrated that repeated sevoflurane exposure induced significant hippocampal-dependent cognitive impairment, as reflected by the decreased freezing response when assessed at P42. Our results are supported by previous studies demonstrating that anesthesia with 3 % sevoflurane 2 h daily for 3 days in young (P6) mice induced cognitive impairment detected at a later time (P30–P36; Shen et al. 2013).
Although acute widespread cell apoptosis has been observed, several studies have shown increased neurogenesis and apparent anatomical recovery after acute histopathological damage induced by neonatal anesthesia (Stratmann et al. 2009). In those studies, rat pups exposed to anesthesia showed minimal to no histopathological damage as they matured, indicating the developing brain may have a high capacity for self-repair of morphological changes. These results suggest that additional mechanisms may mediate anesthetic-induced long-term cognitive dysfunction. During the developmental period, intense synaptic formation and refinement occur in the hippocampus (Williams et al. 2015). Studies performed in mature rodents have shown that hippocampal LTP could be altered by various experimental physiological challenges (Lynch 2004). Commonly used general anesthetics with GABAergic or glutamatergic properties, such as pentobarbital, propofol, isoflurane, and ketamine, administered at P7 suppress LTP later in life (Tachibana et al. 2011; Wang et al. 2014; Ishizeki et al. 2008; Gao et al. 2014; Kato et al. 2013; Simon et al. 2001). Thus, hippocampal LTP may be associated with the pathophysiology of neonatal anesthetic-related learning deficits later in adulthood. Based on the findings that synapses are particularly vulnerable in neurodegenerative conditions, we studied the effects of repeated sevoflurane exposure on the regulation of synaptic proteins (Leal et al. 2014), which have important functions in learning and memory. In the present study, repeated sevoflurane exposure induced distinct alterations at the synaptic protein level, and more specifically, a significant reduction in PSD95 and BDNF might have contributed to the observed LTP and cognition impairments. These findings led us to propose that intervention at an early period may alter hippocampal neural function, including synaptic plasticity, and consequently, change neurocognitive function later in life. In contrast to previous studies demonstrating that environmental enrichment has beneficial effects on wild-type mice (Novkovic et al. 2015), our data suggested that environmental enrichment did not further increase the freezing behavior or enhance synaptic function compared with mice exposed to a standard environment. The inconsistency between the results of the current study and the aforementioned reports may be due to environmental enrichment during different postnatal periods, which is thought to have a critical impact on brain development.
Environmental enrichment, a complex, multifactorial, non-invasive intervention that can incorporate physical, social, somatosensory, and cognitive components, has been found to improve memory and its neuroanatomical and biochemical substrates in a number of cognitive disorders, including sevoflurane exposure-induced cognitive impairment (Nithianantharajah and Hannan 2006; Shih et al. 2012). Consistent with these findings, we observed that 1 month in an enriched environment was sufficient to reverse sevoflurane exposure-induced cognitive impairment and prevent the disrupted LTP. Given that the formation and retention of contextual memory is critically dependent on synaptic plasticity in the hippocampus (Kratzer et al. 2012), establishing hippocampal LTP using periodic placement in an enriched environment may contribute to improving the fear conditioning learning impaired by repeated sevoflurane exposure.
In conclusion, the results of the present study suggested that repeated sevoflurane exposure in neonatal mice caused long-term cognitive and hippocampal synaptic plasticity impairments. However, environmental enrichment following sevoflurane exposure ameliorated the abnormalities in hippocampal synaptic plasticity and cognitive function observed later in life for those mice remaining in a standard environment. Our study suggests that environmental enrichment may serve as a novel therapy for treating cognitive impairments induced by sevoflurane exposure.
References
Callaway JK, Jones NC, Royse AG, Royse CF (2012) Sevoflurane anesthesia does not impair acquisition learning or memory in the Morris water maze in young adult and aged rats. Anesthesiology 117:1091–1101
Edwards DA, Shah HP, Cao W, Gravenstein N, Seubert CN, Martynyuk AE (2010) Bumetanide alleviates epileptogenic and neurotoxic effects of sevoflurane in neonatal rat brain. Anesthesiology 112:567–575
Gao J, Peng S, Xiang S, Huang J, Chen P (2014) Repeated exposure to propofol impairs spatial learning, inhibits LTP and reduces CaMKIIα in young rats. Neurosci Lett 560:62–66
Ishizeki J, Nishikawa K, Kubo K, Saito S, Goto F (2008) Amnestic concentrations of sevoflurane inhibit synaptic plasticity of hippocampal CA1 neurons through gamma-aminobutyric acid-mediated mechanisms. Anesthesiology 108:447–456
Ji MH et al (2015) Pre-administration of curcumin prevents neonatal sevoflurane exposure-induced neurobehavioral abnormalities in mice. Neurotoxicology 46:155–164
Kato R et al (2013) Neonatal exposure to sevoflurane causes significant suppression of hippocampal long-term potentiation in postgrowth rats. Anesth Analg 117:1429–1435
Kratzer S, Mattusch C, Kochs E, Eder M, Haseneder R, Rammes G (2012) Xenon attenuates hippocampal long-term potentiation by diminishing synaptic and extrasynaptic N-methyl-D-aspartate receptor currents. Anesthesiology 116:673–682
Leal G, Comprido D, Duarte CB (2014) BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 76 Pt C:639–656
Li S et al (2013) Environmental novelty activates β2-adrenergic signaling to prevent the impairment of hippocampal LTP by Aβ oligomers. Neuron 77:929–941
Liang G, Ward C, Peng J, Zhao Y, Huang B, Wei H (2010) Isoflurane causes greater neurodegeneration than an equivalent exposure of sevoflurane in the developing brain of neonatal mice. Anesthesiology 112:1325–1334
Lynch MA (2004) Long-term potentiation and memory. Physiol Rev 84:87–136
Nithianantharajah J, Hannan AJ (2006) Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 7:697–709
Novkovic T, Mittmann T, Manahan-Vaughan D (2015) BDNF contributes to the facilitation of hippocampal synaptic plasticity and learning enabled by environmental enrichment. Hippocampus 25:1–15
Rappaport BA, Suresh S, Hertz S, Evers AS, Orser BA (2015) Anesthetic neurotoxicity—clinical implications of animal models. N Engl J Med 372:796–797
Rood BD, Calizo LH, Piel D, Spangler ZP, Campbell K, Beck SG (2014) Dorsal raphe serotonin neurons in mice: immature hyperexcitability transitions to adult state during first three postnatal weeks suggesting sensitive period for environmental perturbation. J Neurosci 34:4809–4821
Satomoto M et al (2009) Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology 10:628–637
Servick K (2014) Biomedical research. Researchers struggle to gauge risks of childhood anesthesia. Science 346:1161–1162
Shen X et al (2013) Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology 118:502–515
Shih J et al (2012) Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology 116:586–602
Simon W, Hapfelmeier G, Kochs E, Zieglgänsberger W, Rammes G (2001) Isoflurane blocks synaptic plasticity in the mouse hippocampus. Anesthesiology 94:1058–1065
Sprung J et al (2012) Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc 87:120–129
Stratmann G et al (2009) Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology 110:834–848
Tachibana K, Hashimoto T, Kato R, Tsuruga K, Ito R, Morimoto Y (2011) Long-lasting effects of neonatal pentobarbital administration on spatial learning and hippocampal synaptic plasticity. Brain Res 1388:69–76
Wang RR et al (2014) Neonatal ketamine exposure causes impairment of long-term synaptic plasticity in the anterior cingulate cortex of rats. Neuroscience 268:309–317
Wilder RT et al (2009) Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110:796–804
Williams MR, DeSpenza T Jr, Li M, Gulledge AT, Luikart BW (2015) Hyperactivity of newborn Pten knock-out neurons results from increased excitatory synaptic drive. J Neurosci 35:943–959
Yang J et al (2015) Maged1 co-interacting with CREB through a hexapeptide repeat domain regulates learning and memory in mice. Mol Neurobiol 51:8–18
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was supported by the grants from the National Science Foundation of China (Nos. 81271216, 81300946, and 81471105).
Author information
Authors and Affiliations
Corresponding author
Additional information
Mu-huo Ji and Xing-ming Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ji, Mh., Wang, Xm., Sun, Xr. et al. Environmental Enrichment Ameliorates Neonatal Sevoflurane Exposure-Induced Cognitive and Synaptic Plasticity Impairments. J Mol Neurosci 57, 358–365 (2015). https://doi.org/10.1007/s12031-015-0627-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0627-1