Abstract
Ketogenic diet (KD) is a high-fat, low-protein and low-carbohydrate diet. It is reported that KD can provide the neuroprotection for the neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease (PD) and amyotrophic lateral sclerosis. The main clinical symptom of PD is motor dysfunction derived from the loss of dopaminergic neurons in the substantia nigra (SN) and dopamine content in the striatum subsequently. It is well known that treatments with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in mice produce motor dysfunction, biochemical, and neurochemical changes remarkably similar to idiopathic PD patients. In this study, we investigated the neuroprotective and anti-inflammatory effects of KD in MPTP-treated mice. The data showed that pretreatment with KD alleviated the motor dysfunction induced by MPTP. The decrease of Nissl-staining and tyrosine hydroxylase (TH)-positive neurons induced by MPTP was inhibited in the SN. The change of dopamine was very similar to dopaminergic neurons in the SN. KD inhibited the activation of microglia induced by MPTP in the SN. The levels of proinflammatory cytokines (interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha) in the SN were also decreased and induced by MPTP. So, we concluded that KD was neuroprotective and anti-inflammatory against MPTP-neurotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second most common and chronic age-related neurodegenerative disease after Alzheimer’s disease (AD). It is characterized by the selective progressive loss of dopaminergic neurons in the substantia nigra (SN) and their projection fibers in the striatum subsequently (Hirsch et al. 1988; Hornykiewicz and Kish 1987). The clinical progressive motor dysfunction in PD including resting tremor, bradykinesia, rigidity, slowness of movement, and postural instability results from degeneration of dopaminergic neurons in the SN (Ghosh et al. 2007). According to epidemiology, the average age of PD onset is 55 years old and the disease affects over 1% of the population over the age of 60 (Dauer and Przedborski 2003; de Lau and Breteler 2006; Godoy et al. 2008). The replacement therapy of l-DOPA and its derivatives only alleviates the clinical symptoms of PD and do not halt the progressive neurodegeneration of dopaminergic neurons (Yokoyama et al. 2008).
Although the etiology and mechanism of neurodegeneration in the SN with PD is not still clear, considerable studies indicate that activated microglia mediating the neuroinflammation in the SN of PD plays an important role to the pathogenesis and progression of PD in recent years (Hirsch et al. 1998). Microglia are the main immune cells in the brain and are rich in the SN of human midbrain (Gehrmann et al. 1995). They provide immune protection against infection under normal physiologic conditions. In many neurodegenerative diseases, microglial activation is observed in PD, AD, multiple sclerosis, and the AIDS dementia complex (Kim et al. 2000). Postmortem shows that activated microglia are found in the SN of PD, and positron emission tomography images of the human brain also show the existence of microglia activation in the brain of the PD patients (McGeer et al. 1988; Gerhard et al. 2006). It is reported that activated microglia are closely related to dying or damaged dopaminergic neurons. Activated microglia overproduce large amounts of proinflammatory cytokines including interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) (Liu and Hong 2003). Those are primary mediators of the inflammatory response leading to dopaminergic neurodegeneration. The therapies of anti-inflammation are demonstrated to slow down the neurodegeneration of dopaminergic neurons and alleviate the motor dysfunction in many PD models of rodents (Kim et al. 2004).
The ketogenic diet (KD) is a high-fat, low-protein, low-carbohydrate diet. It has long been widely and successfully used to medically treat intractable refractory epilepsy of children in clinical use for over 80 years since the 1920s (Freeman et al. 2007). The blood levels of the ketone bodies (KB: d-β-hydroxybutyrate, acetoacetate, and acetone) are overproduced significantly by the liver and treated with KD, and the uptake of KB by brain is enhanced on KD or during fasting measured by positron emission tomography (Veech et al. 2001; Bentourkia et al. 2009). KB can cross through the blood–brain barrier (Pierre and Pellerin 2005). In the absence of glucose, the main fuel for the human brain, KB act as an alternative fuel for brain (Owen et al. 1967). In recent studies, KD not only treat epilepsy but also show neuroprotection for brain injuries and neurodegenerative disease (Prins et al. 2005). KD can inhibit the neurodegeneration induced by cardiac arrest-induced cerebral ischemic injury in the CA1 region of the hippocampus, the cerebellum, and the thalamic reticular nucleus and protect from neuronal loss in the striatum, neocortex, and the hippocampus induced by transient cerebral ischemia (Marie et al. 1990; Tai et al. 2008). d-β-hydroxybutyrate prevents the death of hippocampal neurons exposed to Aβ1–42, protects cultured mesencephalic dopaminergic neurons from the toxic effects of 1-methyl-4-phenylpyridinium (MPP+, an inhibitor of nicotinamide adenine dinucleotide (NADH) dehydrogenase that increases oxygen radical formation), and reduces brain injury in rodents subjected to glycolysis inhibition and focal or generalized ischemia (Kashiwaya et al. 2000). In a rotenone model of PD, in vitro d-β-hydroxybutyrate protects dopaminergic SH-SY5Y cells from rotenone-neurotoxicity (Imamura et al. 2006). Infusion with d-β-hydroxybutyrate prevents the loss of dopaminergic neurons in the SN of mice (Tieu et al. 2003). Clinically, KD alleviates the symptoms of PD patients (Vanitallie et al. 2005). Yet the neuroprotective mechanism of KD is not entirely clear.
In this study, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice of PD model were used to investigate the neuroprotection of KD. We found that KD alleviated the motor dysfunction, inhibited the loss of Nissl-staining and TH-positve neurons in the SN, decreased the activation of microglia, and down-regulated the levels of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) compared to MPTP-treated mice fed by standard diet (SD).
Methods
Outline

Animals
Male C57BL/6 J mice, 8 weeks of age and weighing 24–30 g, were used in our present work. All the mice were housed in a constant temperature-controlled room (23 ± 1°C) under a 12:12 light:dark cycle and fed by SD and water ad libitum for 1 week after shipment in order to acclimate to the housing facility. The experiments were carried out strictly in accordance to the Guidelines for Animal Experiments.
Diets Regime and MPTP Lesion
KD or SD was used in our experiments according to Xu et al. (2006; Ziegler et al. 2002). After shipment for 1 week, all the mice were divided randomly into four groups: KD + MPTP, SD + MPTP, KD + saline, and SD + saline. Following 1 week, SD + saline and SD + MPTP groups continued with SD, and KD and KD + MPTP groups were treated by KD. One week after KD, SD + MPTP and KD + MPTP groups were given four injections of MPTP-HCl (i.p., 20 mg·kg-1/injection, the total dose per mouse being 80 mg/kg, Sigma) at 2-h intervals, and KD + saline and SD + saline groups were given four injections of the same volume saline at 2-h intervals. After MPTP injection, four groups received their diets, respectively, for 2 days or 1 week continually for the following researches.
Rota-rod
Quantitative measurements of motor coordination in mice were measured by an accelerating rota-rod. When the mice were treated with KD, all the groups were pretrained at the acceleration of 4–40 rpm on rod for the following 1 week. The length of time was recorded before the MPTP injections. After MPTP injection for 1 week, the measurement of rota-rod was measured again, and the length of time was recorded.
Tissue Preparation
After the second measurement of rota-rod and MPTP injection for 2 days, the mice were sacrificed and transcardially perfused with 4% paraformaldehyde in phosphate buffered saline (PBS) (pH 7.4). Brains were dissected and kept in 4% paraformaldehyde. After 2 days, they were taken into 30% sucrose in PBS at 4°C. The tissue was sunk and cut at 30 μm thickness through the midbrain.
Nissl-staining of SN
One section was selected from every sixth sections in the SN using 1 week after MPTP injection, and five sections were selected from each sample and stained with cresyl violet. Then, all the sections were mounted on the slides, dried out, and dehydrated to cover slip.
TH Immunohistochemistry of SN
One section was selected from every sixth sections in the SN using 1 week after MPTP injection and five sections were selected from each sample. Then all the sections were washed with PBS. Following treatment with 3% hydrogen peroxide for 5 min, the sections were washed with PBS and blocked by blocking buffer (0.4% Triton X-100 in PBS, 20 ml; 0.04% normal goat serum, 800 μL; bovine serum albumin, 0.2 g). The sections were incubated by polyclonal anti-TH antibody (1:2000, Santa Cruz, USA) at 4°C overnight. The sections were rinsed and incubated with secondary antibody at 37°C for 1 h. After washing with PBS, the sections were incubated by avidin-biotin complex (ABC) (Vector Laboratories, USA) for 1 h and developed in diaminobenzidine. Finally, all the sections were mounted on the slides and were covered with cover slips.
Cell Counting of SN
The number of the neurons was counted by Zeiss Axioplan 2 microscope using the optical fractionator method in Nissl-staining, and the dopaminergic neurons were counted using the same way in TH staining of SN (West et al. 1991).
Measurement of Dopamine in the Striatum
After the measurement of rota-rod, the mice were killed and the striatum was taken out and kept in -80°C refrigerator. The levels of dopamine in the striatum were measured using high-performance liquid chromatography (HPLC). Data were expressed as nanogram per gram wet weight of tissue.
Ionized Calcium-Binding Adaptor Molecule 1 (IBA-1) Immunohistochemistry of SN
One section was selected from every sixth sections in the SN using 2 days after MPTP injection, and five sections were selected from each group. IBA-1 (1:2000, Wako, Japan) immunohistochemistry was carried in the SN using the same method as TH immunohistochemistry. The activated microglial cells were analyzed like the TH immunohistochemistry as above.
Enzyme-Linked Immunosorbent Assay for the Levels of Proinflammatory Cytokines
The levels of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) was detected in the SN by enzyme-linked immunosorbent assay (ELISA) kits according to the protocols (R & D Systems). After MPTP injection for 2 days, all the mice were taken out to get the SN and were kept at -80°C. When the measurements were carried, all the tissues were homogenized on ice and centrifuged. The supernatant was collected and the total protein concentration was measured by Bradford protein assays in all samples. Then, the mice IL-1β, mice TNF-α, and mice IL-6 were measured by the corresponding kits. The results were presented as percent of control.
Statistical Analysis
All the data were expressed as mean ± SEM and statistical significance was analyzed by one-way analysis of variance. P < 0.05 was considered significant. Each group consisted of 5–6 mice.
Results
KD Improved the Movements against the Motor Deficits Induced by MPTP in C57BL/6J Mice
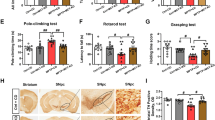
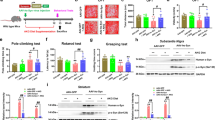
To explore the effect of KD on the motor function in the mice model of PD, the rota-rod test was used, and the results were shown as Fig. 1. When KD was carried out, all the groups were pretrained on the rota-rod every 2 days for 1 week, and the time on the rod before the MPTP injection was measured. After MPTP injection, four groups received their diets, respectively, for 1 week continually. Then all the mice were tested on the rod and the time were recorded. The length of time on rod was no difference between SD + saline and KD + saline groups without MPTP injections (P > 0.05). However, the length of time on rod in SD + MPTP group was decreased more significantly than SD + saline group (P < 0.05). The length of time on rod in KD + MPTP group was longer than SD + MPTP group significantly (P < 0.05). The data showed that KD improved the movements against the motor deficits induced by MPTP in C57BL/6J mice.
The effects of KD on dysfunction in MPTP-treated mice by rota-rod. MPTP decreased the length of the time on the rod in mice treated with SD. The length of time on rod in KD + MPTP group was longer than SD + MPTP group significantly. A: SD + saline; B: KD + MPTP; C: SD + MPTP; D: KD + MPTP. *P < 0.05
KD Protected Dopaminergic Neurons against MPTP-induced Degeneration
As shown in Figs. 2 and 3, we used the cell count numbers of Nissl-staining and TH immunohistochemistry to explore the neuroprotective effects of KD. After 1 week of MPTP injection, the number of the dopaminergic neurons of SN in SD + MPTP group was decreased markedly compared to SD + saline group (P < 0.05). There was no difference between SD + saline and KD + saline groups without MPTP injections (P > 0.05). Pretreatment with KD significantly decreased the loss of dopaminergic neurons induced by MPTP (P < 0.05). The data showed that KD protected the dopaminergic neurons against MPTP-neurotoxicity.
The effects of KD on the neurons of SN in MPTP-treated mice by Nissl-staining. The number of the neurons of SN in SD + MPTP group was decreased markedly compared to SD + saline group. Pretreatment with KD significantly decreased the loss of neurons induced by MPTP. A: SD + saline; B: KD + MPTP; C: SD + MPTP; D: KD + MPTP. *P < 0.05
The effects of KD on dopaminergic neurons of SN in MPTP-treated mice by TH immunohistochemistry. The number of the dopaminergic neurons of SN in SD + MPTP group was decreased markedly compared to SD + saline group. Pretreatment with KD significantly decreased the loss of the dopaminergic neurons induced by MPTP. A: SD + saline; B: KD + MPTP; C: SD + MPTP; D: KD + MPTP. *P < 0.05
KD Inhibited the Decrease of Dopamine in the Striatum Induced by MPTP Treatment
There was no difference of the level of dopamine in the striatum between SD + saline and KD + saline groups without MPTP injections (P > 0.05), as shown Fig. 4. After MPTP injection for 1 week, the level of dopamine in the striatum was decreased significantly in SD + MPTP group compared to the SD + saline group (P < 0.05). KD prevented a significant decrease of dopamine content in the striatum after MPTP treatment. The level of dopamine in the striatum was preserved significantly in the KD + MPTP group compared to SD + MPTP group (P < 0.05). The results revealed that KD inhibited the decrease of dopamine in the striatum induced by MPTP treatment.
The effects of KD on the level of the dopamine in the striatum in MPTP-treated mice by HPLC. MPTP decreased the level of dopamine in the striatum significantly in SD + MPTP group compared to the SD + saline group. The level of dopamine in the striatum was preserved significantly in the KD + MPTP group compared to SD + MPTP group. A: SD + saline; B: KD + MPTP; C: SD + MPTP; D: KD + MPTP. *P < 0.05
KD Decreased the Activation of the Microglial Cells Induced by MPTP in the SN of C57BL/6J Mice
Figure 5 shows the morphological changes of activated microglia in the SN showed by IBA-1 immunohistochemistry. Previous studies reported that MPTP induced the activation of microglia. In the present study, microglial cells in the SN were observed to activate significantly in SD + MPTP group compared to the SD + saline group after 2 days of MPTP injection. No difference was observed between SD + saline and KD + saline groups. However, the activated microglial cells were decreased more significantly in KD + MPTP group than SD + MPTP group (data were not shown). Therefore, KD decreased the activation of the microglial cells induced by MPTP in the SN of C57BL/6J mice.
The effects of KD on activated microglia in MPTP-treated mice by IBA-1 immunohistochemistry (only observing and no data analyzing). The microglial cells in the SN were observed to activate significantly in SD + MPTP group compared to the SD + saline group after 2 days of MPTP injection. However, the activated microglial cells were decreased more significantly in KD + MPTP group than SD + MPTP group (data were not shown). A: SD + saline; B: KD + MPTP; C: SD + MPTP; D: KD + MPTP
KD Inhibited the Increase of Proinflammatory Cytokines (IL-1β, IL-6, and TNF-α) Induced by MPTP in the SN of C57BL/6J Mice
As shown in Fig. 6, MPTP induced the increase of the proinflammatory cytokines (IL-1β, IL-6, and TNF-α) more significantly in SD + MPTP group than in KD + MPTP group (P < 0.05). There was no difference between KD + saline and SD + saline groups (P > 0.05). The levels of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) in the SN induced by MPTP decreased more significantly in KD + MPTP group than SD + MPTP group. The data showed that KD inhibited the increase of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) in the SN induced by MPTP in the SN of C57BL/6J mice.
The effects of KD on the proinflammatory cytokines releasing in the SN in MPTP-treated mice by ELISA. MPTP induced the increase of the proinflammatory cytokines (IL-1β, IL-6, and TNF-α) more significantly in SD + MPTP group than in KD + MPTP group. The levels of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) releasing in the SN induced by MPTP decreased more significantly in KD + MPTP group than SD + MPTP group. A: SD + saline; B: KD + MPTP; C: SD + MPTP; D: KD + MPTP. *P < 0.05
Discussion
In the present study, MPTP induced the deficits of motor function, degeneration of dopaminergic neurons in the SN, depletion of dopamine in the striatum, activation of microglial cells in the SN, and increase of the proinflammatory cytokines (IL-1β, IL-6, and TNF-α) in the SN. However, pretreatment with KD on MPTP-treated mice alleviated motor dysfunction, increased the number of dopaminergic neurons, restored the level of dopamine in the striatum, inhibited the activation of microglial cells in the SN, and decreased the levels of the proinflammatory cytokines (IL-1β, IL-6, and TNF-α) in the SN. The data showed that KD had the neuroprotection on MPTP-treated mice through the inhibition of neuroinflammation.
KD first described by Wilder has been successfully used to treat intractable childhood epilepsy since the early 1920s, and more and more studies reported that KD has a neuroprotective function on the neurodegeneration induced by hypoxia, epilepsy, ischemia, glutamate excitotoxicity, traumatic brain injury (TBI), PD, amyotrophic lateral sclerosis, and AD (Tai et al. 2008; Hartman et al. 2007; Puchowicz et al. 2008; Appelberg et al. 2009a; Hu et al. 2009; Maalouf et al. 2007; Zhao et al. 2006; Van der Auwera et al. 2005).
As described by Vanitallie et al. (2005), PD patients were treated by KD for 28 days and Unified Parkinson’s Disease Rating Scale scores improved significantly. In vitro, the major component (d-β-hydroxybutyrate) of KB had the neuroprotective effect against rotenone toxicity on SH-SY5Y dopaminergic neuroblastoma and MN9D cells (Imamura et al. 2006; Kweon et al. 2004). Tieu et al. (2003) found that d-β-hydroxybutyrate had therapeutical function on MPTP-treated mice of PD model. KD alleviated the motor dysfunction in the models of the neurological diseases. Exposure to KD in rats’ locomotor activity was increased significantly, and KD had improved the behavioral function after TBI (Appelberg et al. 2009b). MPTP is a neurotoxin that produces an irreversible and severe Parkinsonian syndrome such as tremor, rigidity, slowness of movement, postural instability, and freezing in both humans and experimental animals (monkeys and mice) (Watanabe et al. 2008; Tillerson et al. 2002). Therefore, it is widely used as models of PD (Tanji et al. 1999). MPTP induced the decrease of the time on rota-rod in previous (Tieu et al. 2003) and our present studies. The important component of KB, d-β-hydroxybutyrate continual infusion, inhibited the decrease of the time on rod in MPTP-treated mice (Tieu et al. 2003). KD improved the motor function against MPTP-treated mice.
Evidences showed that KD and its components d-β-hydroxybutyrate or acetoacetate were neuroprotective for neurodegeneration. Acetoacetate protects hippocampal neurons against glycolysis inhibition in vivo and in vitro (Massieu et al. 2003). Treatment with d-β-hydroxybutyrate alleviated brain damage and improve neuronal function in models of brain hypoxia, anoxia, and ischemia (Suzuki et al. 2001). d-β-hydroxybutyrate was neuroprotective against the neurodegeneration induced by ischemia of brain during some surgeries such as cardiopulmonary bypass (Smith et al. 2005). Our previous study shows that KD protects dopaminergic neurons against 6-hydroxydopamine (6-OHDA) neurotoxicity in a rat model of PD (Cheng et al. 2009). d-β-hydroxybutyrate reduced glutamate-induced neuronal damage, protected mesencephalic neuronal cultures from MPP + toxicity, hippocampal neurons in culture from Aβ1–42, and decreased the neurodegeneration of SN induced by MPTP (Tieu et al. 2003; Maalouf et al. 2007). Our work showed that KD protected the dopaminergic neurons from MPTP-neurotoxicity. The decrease of dopamine content was restored by KD (Tieu et al. 2003), and we also found that KD inhibition of the depletion of dopamine content in the striatum were induced by MPTP.
It was indicated that the neuroinflammation that participated in the mechanisms of PD and microglial cells of SN were activated (Hirsch et al. 2005). Proinflammatory cytokines (IL-1β, IL-6, and TNF-α) were partially released from activated microglia (Nagatsu et al. 2000). In MPTP-treated mouse, model of PD microglia in the SN were activated and proinflammatory cytokines led to damage dopaminergic neurons (Whitton 2007). Therefore, the inhibition of microglia could rescue the dopaminergic neurons from MPTP-neurotoxicity (Kurkowska-Jastrzebska et al. 1999). For example, anti-inflammatory drugs such as ibuprofen, aspirin, and exogenous corticosterone have potential therapeutic effects on PD model (Aubin et al. 1998; Casper et al. 2000; Barnum et al. 2008). In our present study, KD inhibited the activation of microglial cells partly induced by MPTP and decreased the levels of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) in the SN.
Taken together, KD had the therapeutical function on experimental model of PD. It could alleviate the motor dysfunction, protect the dopaminergic neurons, increase the level of dopamine (DA), and inhibit the activation of microglial cells and production of proinflammatory cytokines. Therefore, KD was an alternative method to slow down the progressive neurodegeneration of dopaminergic neurons in the SN for the treatment of PD in the future.
References
Appelberg KS, Hovda DA, Prins ML (2009a) The effects of a ketogenic diet on behavioral outcome after controlled cortical impact injury in the juvenile and adult rat. J Neurotrauma 26:497–506
Appelberg KS, Hovda DA, Prince ML (2009b) The effects of a ketogenic diet on behavioral outcome after controlled cortical impact injury in the juvenile and adult rat. J Neurotrauma 26(4):497–506
Aubin N, Curet O, Deffois A, Carter C (1998) Aspirin and salicylate protect against MPTP-induced dopamine depletion in mice. J Neurochem 71:1635–1642
Barnum CJ, Eskow KL, Dupre K, Blandino P Jr, Deak T, Bishop C (2008) Exogenous corticosterone reduces l-DOPA-induced dyskinesia in the hemi-Parkinsonian rat: role for interleukin-1beta. Neuroscience 156:30–41
Bentourkia M, Tremblay S, Pifferi F, Rousseau J, Lecomte R, Cunnane S (2009) PET study of 11C-acetoacetate kinetics in rat brain during dietary treatments affecting ketosis. Am J Physiol Endocrinol Metab 296:E796–E801
Casper D, Yaparpalvi U, Rempel N, Werner P (2000) Ibuprofen protects dopaminergic neurons against glutamate toxicity in vitro. Neurosci Lett 289:201–204
Cheng B, Yang X, An L, Gao B, Liu X, Liu S (2009) Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson’s disease. Brain Res 1286:25–31
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
de Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet neurology 5:525–535
Freeman JM, Kossoff EH, Hartman AL (2007) The ketogenic diet: one decade later. Pediatrics 119:535–543
Gehrmann J, Matsumoto Y, Kreutzberg GW (1995) Microglia: intrinsic immune effector cell of the brain. Brain Res Brain Res Rev 20:269–287
Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A et al (2006) In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis 21:404–412
Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM et al (2007) Selective inhibition of NF-kappa B activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA 104:18754–18759
Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ (2008) Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain 131:1880–1894
Hartman AL, Gasior M, Vining EP, Rogawski MA (2007) The neuropharmacology of the ketogenic diet. Pediatr Neurol 36:281–292
Hirsch E, Graybiel AM, Agid YA (1988) Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature 334:345–348
Hirsch EC, Hunot S, Damier P, Faucheux B (1998) Glial cells and inflammation in Parkinson’s disease: a role in neurodegeneration? Ann Neurol 44:S115–120
Hirsch EC, Hunot S, Hartmann A (2005) Neuroinflammatory processes in Parkinson’s disease. Parkinsonism Relat Disord 11(Suppl 1):S9–S15
Hornykiewicz O, Kish SJ (1987) Biochemical pathophysiology of Parkinson’s disease. Adv Neurol 45:19–34
Hu ZG, Wang HD, Qiao L, Yan W, Tan QF, Yin HX (2009) The protective effect of the ketogenic diet on traumatic brain injury-induced cell death in juvenile rats. Brain Inj 23:459–465
Imamura K, Takeshima T, Kashiwaya Y, Nakaso K, Nakashima K (2006) d-beta-hydroxybutyrate protects dopaminergic SH-SY5Y cells in a rotenone model of Parkinson’s disease. J Neurosc Res 84:1376–1384
Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL (2000) D-beta-hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc Natl Acad Sci USA 97:5440–5444
Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS (2000) Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci 20:6309–6316
Kim HP, Son KH, Chang HW, Kang SS (2004) Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci 96:229–245
Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A (1999) The inflammatory reaction following 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine intoxication in mouse. Exp Neurol 156:50–61
Kweon GR, Marks JD, Krencik R, Leung EH, Schumacker PT, Hyland K et al (2004) Distinct mechanisms of neurodegeneration induced by chronic complex I inhibition in dopaminergic and non-dopaminergic cells. J Biol Chem 279:51783–51792
Liu B, Hong JS (2003) Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther 304:1–7
Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM (2007) Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience 145:256–264
Marie C, Bralet AM, Gueldry S, Bralet J (1990) Fasting prior to transient cerebral ischemia reduces delayed neuronal necrosis. Metab Brain Dis 5:65–75
Massieu L, Haces ML, Montiel T, Hernandez-Fonseca K (2003) Acetoacetate protects hippocampal neurons against glutamate-mediated neuronal damage during glycolysis inhibition. Neuroscience 120:365–378
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38:1285–1291
Nagatsu T, Mogi M, Ichinose H, Togari A (2000) Cytokines in Parkinson’s disease. J Neural Transm 143–151
Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF Jr (1967) Brain metabolism during fasting. J Clin Invest 46:1589–1595
Pierre K, Pellerin L (2005) Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem 94:1–14
Prins ML, Fujima LS, Hovda DA (2005) Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J Neurosci Res 82:413–420
Puchowicz MA, Zechel JL, Valerio J, Emancipator DS, Xu K, Pundik S et al (2008) Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J Cereb Blood Flow Metab 28:1907–1916
Smith SL, Heal DJ, Martin KF (2005) KTX 0101: a potential metabolic approach to cytoprotection in major surgery and neurological disorders. CNS Drug Rev 11:113–140
Suzuki M, Suzuki M, Sato K, Dohi S, Sato T, Matsuura A et al (2001) Effect of beta-hydroxybutyrate, a cerebral function improving agent, on cerebral hypoxia, anoxia and ischemia in mice and rats. Jpn J Pharmacol 87:143–150
Tai KK, Nguyen N, Pham L, Truong DD (2008) Ketogenic diet prevents cardiac arrest-induced cerebral ischemic neurodegeneration. J Neural Transm 115:1011–1017
Tanji H, Araki T, Nagasawa H, Itoyama Y (1999) Differential vulnerability of dopamine receptors in the mouse brain treated with MPTP. Brain Res 824:224–231
Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD et al (2003) d-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest 112:892–901
Tillerson JL, Caudle WM, Reveron ME, Miller GW (2002) Detection of behavioral impairments correlated to neurochemical deficits in mice treated with moderate doses of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. Exp Neurol 178:80–90
Van der Auwera I, Wera S, Van Leuven F, Henderson ST (2005) A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutrition & Metabolism 2:28
Vanitallie TB, Nonas C, Di Rocco A, Boyar K, Hyams K, Heymsfield SB (2005) Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology 64:728–730
Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GF Jr (2001) Ketone bodies, potential therapeutic uses. IUBMB life 51:241–247
Watanabe Y, Kato H, Araki T (2008) Protective action of neuronal nitric oxide synthase inhibitor in the MPTP mouse model of Parkinson’s disease. Metab Brain Dis 23:51–69
West MJ, Slomianka L, Gundersen HJ (1991) Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231:482–497
Whitton PS (2007) Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol 150:963–976
Xu XP, Sun RP, Jin RF (2006) Effect of ketogenic diet on hippocampus mossy fiber sprouting and GluR5 expression in kainic acid induced rat model. Chin Med J 119:1925–1929
Yokoyama H, Takagi S, Watanabe Y, Kato H, Araki T (2008) Role of reactive nitrogen and reactive oxygen species against MPTP neurotoxicity in mice. J Neural Transm 115:831–842
Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J et al (2006) A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neuroscience 7:29
Ziegler DR, Araujo E, Rotta LN, Perry ML, Goncalves CA (2002) A ketogenic diet increases protein phosphorylation in brain slices of rats. J Nutr 132:483–487
Author information
Authors and Affiliations
Corresponding author
Additional information
Baohua Cheng and Xinxin Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, X., Cheng, B. Neuroprotective and Anti-inflammatory Activities of Ketogenic Diet on MPTP-induced Neurotoxicity. J Mol Neurosci 42, 145–153 (2010). https://doi.org/10.1007/s12031-010-9336-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-010-9336-y