Abstract
Background and Aims
Pivotal phase III trials indicated that the anti–PD-1 inhibitor nivolumab prolongs overall survival in patients with advanced gastric cancer. Nivolumab is currently used in the first- or later-line treatment of patients with advanced gastric cancer in Japan. The efficacy of immune check inhibitor rechallenge after progression has been reported in other cancers. Therefore, this study investigated the clinical outcome of nivolumab rechallenge in patients with advanced gastric cancer who received nivolumab in a previous systemic line.
Methods
We retrospectively reviewed the medical records of six patients with advanced or recurrent gastric cancer who received nivolumab rechallenge.
Results
During initial nivolumab therapy, three patients experienced partial responses, and one patient achieved stable disease. The reasons for discontinuing initial nivolumab therapy were progressive disease in five patients and immune-related adverse events in one patient. The median interval duration of treatment for patients receiving both nivolumab regimens was 13.7 (range: 5.1–17.8) months. During nivolumab rechallenge, no patients achieved partial responses, whereas two patients had stable disease. Median progression-free survival was 2.5 (95% confidence interval [CI] = 1.6–not available [NA]) months, and median overall survival was 7.4 (95% CI = 2.3–NA) months. Although one patient had discontinued prior nivolumab therapy because of immune-related adverse events, there were no immune-related adverse events associated with nivolumab rechallenge.
Conclusions
The benefit of nivolumab rechallenge in patients with advanced gastric cancer was limited. Rechallenge with the same immune check inhibitor might be ineffective in patients with advanced gastric cancer.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Immune check inhibitors are widely used to treat a variety of cancer types, such as gastric, lung, breast, and head and neck cancers. Nivolumab is an immune check inhibitor and monoclonal antibody that targets the PD-1 receptor. Major clinical trials were conducted to analyze the clinical outcomes of nivolumab in patients with advanced gastric cancer. The first positive Phase III trial in advanced gastric cancer was the ATTRACTION-2 study, which was conducted in patients receiving third-line or later treatment. The results indicated that nivolumab monotherapy significantly prolonged overall survival (OS) compared with placebo [1]. Another Phase III study, the CHECKMATE 649 trial, was conducted in patients with previously untreated advanced gastric cancer, and the study demonstrated that nivolumab in combination with chemotherapy achieved superior OS versus chemotherapy alone [2]. Based on these promising results, nivolumab was recently proposed as an important component of treatment in patients with advanced gastric cancer.
Nivolumab is currently used in the first-line treatment of patients with advanced gastric cancer. However, progression is inevitable, and the treatment of advanced gastric patients after anti–PD-1 therapy is challenging. A previous study reported that rechallenge with immunotherapy beyond progression might be effective in other cancer types [3,4,5]. These studies described the efficacy of immune check inhibitor rechallenge in some patients who became refractory to the previous treatment. However, little research has been conducted in patients with advanced gastric cancer.
To evaluate the efficacy of nivolumab rechallenge in patients with advanced gastric cancer who had been treated on previous nivolumab treatment, we reviewed the clinical data of six patients.
Patients and Methods
This case series was approved by the Institutional Review Board of our institution and conducted in accordance with the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. We retrospectively reviewed the medical records of patients at Ishikawa Prefectural Central Hospital between 2019 and 2021. The inclusion criteria were as follows: (1) histologically confirmed adenocarcinoma, (2) prior treatment with nivolumab alone or in combination with chemotherapy regardless of the duration of administration, and (3) treated the rechallenge of nivolumab monotherapy. Six patients meeting the inclusion criteria were enrolled.
Assessment
Tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors. Patients without measurable lesions were excluded from the response rate analysis. Toxicity was assessed using the Common Toxicity Criteria for Adverse Events, version 5.0.
Progression-free survival (PFS) was measured from the first day of nivolumab rechallenge until the date of confirmed disease progression or the last day of follow-up without disease progression. OS was measured from the first day of nivolumab rechallenge until the date of death from any cause and was censored at the date of the last follow-up visit for surviving patients on treatment. Survival was calculated using the Kaplan–Meier method. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical fusions frequently used in biostatistics [6].
Results
Patients
Six patients were included in this analysis. The detailed characteristics of the patients are summarized in Table 1. The median patient age was 67.5 years; five patients were male, and three patients had an Eastern Cooperative Oncology Group performance status of 0. Two patients received a combined nivolumab and platinum doublet regimen as first-line chemotherapy, and the other patients were treated with nivolumab monotherapy.
Efficacy
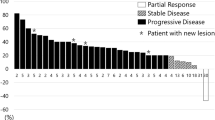
Upon initial nivolumab treatment, three patients achieved partial responses, and one patient had stable disease (Table 2). Median PFS after the initial nivolumab regimen was 9.0 (range: 1.5–31.0) months. The reasons for discontinuing initial nivolumab therapy were progressive disease in five patients and immune-related adverse events in one patient.
During the rechallenge with nivolumab, no patients achieved partial responses, and two patients exhibited stable disease (Table 2). Median PFS after nivolumab rechallenge was 2.5 (95% confidence interval [CI] = 1.6–not available [NA]) months (Fig. 1), and median OS was 7.4 (95% CI = 2.3–NA) months (Fig. 2).
Safety
Although one patient had discontinued prior nivolumab therapy and treated prednisolone because of immune-related arthritis. However, no immune-related adverse events were observed in any patients, and no treatment-related deaths were observed in nivolumab rechallenge.
Discussion
The present retrospective study evaluated the efficacy and safety of nivolumab rechallenge in patients with advanced gastric cancer who had received nivolumab in a prior treatment line. Rechallenge with immune check inhibitors has been reported [3,4,5]. However, these studies involved small numbers of patients and investigated other cancer types. Thus, a definitive conclusion could not be drawn regarding the immune check inhibitor rechallenge in patients with advanced gastric cancer. To the best of our knowledge, this is the first case series to examine nivolumab rechallenge in advanced gastric cancer. Although our study included a limited number of patients in clinical practice, several possibilities can be derived from our study.
First, the efficacy of nivolumab rechallenge in patients with advanced gastric cancer was limited in this study. The overall response rate (ORR) and disease control rate were 0% and 40%, respectively. Although most patients in this study responded to nivolumab in the prior line, and there was a sufficient interval between prior nivolumab therapy and nivolumab rechallenge, these data were not expected. Previous reports illustrated that the ORR was 20–30% for nivolumab and pembrolizumab in patients with ipilimumab-refractory melanoma [7, 8]. However, the response rate was lower for anti–PD-1 rechallenge after prior anti–PD-1 therapy [7, 8]. Another report recorded no responses after switching to another anti–PD1-1/anti–PD-L1 antibody [9]. It is well known that durable responses occur over a long period even after the discontinuation of immune check inhibitor therapy [10]. Nivolumab interferes with the suppressive signaling between cytotoxic T cells and tumors. Although a single dose of nivolumab has a half-life of only 13 ± 7 days [11], it was previously reported that the level of PD-1 occupancy on circulating T cells persists for much longer [11, 12]. The duration for which the effects of nivolumab on the immune system persist and affect subsequent therapy is unclear. Our study suggests that the effects of prior nivolumab therapy can persist through the period of nivolumab rechallenge, thereby limiting the efficacy of rechallenge with the same immune checkpoint inhibitor.
Second, there were no immune-related adverse events associated with nivolumab rechallenge. Nevertheless, our study included one patient who experienced an immune-related adverse event during previous nivolumab therapy. Although it is difficult to draw a firm conclusion because of the small number of patients in this study, previous research described the safety profile of immune check inhibitor rechallenge [13, 14]. One of these studies reported that retreatment with immune check inhibitors was feasible after severe immune-related hepatitis, even with the same immune check inhibitor [14]. Another systematic review mentioned that rechallenge with single-agent anti–PD-1 or anti–PD-L1 therapy had an acceptable safety profile that was similar to that observed in treatment-naïve patients [15]. Our study suggests that rechallenge with nivolumab might be well tolerated in the vast majority of patients as observed during the initial treatment.
Third, OS was relatively better with nivolumab rechallenge despite the low response rate and short PFS. The recommended drugs of third or later-line treatment for advanced gastric cancer include trifluridine/tipiracil (FTD/TPI), irinotecan, and so on. The median OS was reported to be 5.7 months in the FTD/TPI in the pivotal phase III study, the TAGS trial [16]. And the median OS was 6.61 months in the irinotecan in the retrospective study [17]. The OS in this study showed a longer trend compared to these studies. The treatment periods of nivolumab rechallenge are short, and thus, either the prior chemotherapy or the subsequent therapy might have positively influenced OS. From the perspective that our study included heavily pretreated patients and subsequent therapy options are limited, prior nivolumab treatment might provide a durable survival benefit.
This study had several limitations. First, this was a retrospective study in a single institution. Second, the sample size was insufficient for drawing definitive conclusions. Further studies considering these limitations should be conducted to verify the conclusions.
In conclusion, the efficacy of nivolumab rechallenge in patients with advanced gastric cancer was limited even in cases in which prior nivolumab therapy was effective. The effects of prior nivolumab therapy might persist through the period of nivolumab rechallenge, thereby limiting the efficacy of rechallenge with the same immune check inhibitor. Further prospective studies are needed to explore more promising methods of immune check inhibitor rechallenge for patients with advanced gastric cancer.
References
Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–71. https://doi.org/10.1016/S0140-6736(17)31827-5. (PMID: 28993052).
Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. https://doi.org/10.1016/S0140-6736(21)00797-2. PMID: 34102137; PMCID: PMC8436782.
Ravi P, Mantia C, Su C, et al. Evaluation of the safety and efficacy of immunotherapy rechallenge in patients with renal cell carcinoma. JAMA Oncol. 2020;6:1606–10. https://doi.org/10.1001/jamaoncol.2020.2169. PMID: 32469396; PMCID: PMC7260689.
Gul A, Shah NJ, Mantia C, et al. Ipilimumab plus nivolumab (Ipi/Nivo) as salvage therapy in patients with immunotherapy (IO)-refractory metastatic renal cell carcinoma (mRCC). J Clin Oncol. 2019;37(suppl):669. https://doi.org/10.1200/JCO.2019.37.7_suppl.669.
Zhang T, Xie J, Arai S, et al. The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory cancers: a meta-analysis. Oncotarget. 2016;7:73068–79. https://doi.org/10.18632/oncotarget.12230. PMID: 27683031; PMCID: PMC5341964.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8. https://doi.org/10.1038/bmt.2012.244. PMID: 23208313; PMCID: PMC3590441.
Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–18. https://doi.org/10.1016/S1470-2045(15)00083-2. PMID: 26115796; PMCID: PMC9004487.
Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–84. https://doi.org/10.1016/S1470-2045(15)70076-8. PMID: 25795410.
Fujita K, Uchida N, Yamamoto Y, et al. Retreatment with anti-PD-L1 antibody in advanced non-small cell lung cancer previously treated with anti-PD-1 antibodies. Anticancer Res. 2019;39:3917–21. https://doi.org/10.21873/anticanres.13543. (PMID: 31262921).
Swami U, Monga V, Bossler AD, et al. Durable clinical benefit in patients with advanced cutaneous melanoma after discontinuation of anti-PD-1 therapies due to immune-related adverse events. J Oncol. 2019;2019:1856594. https://doi.org/10.1155/2019/1856594. PMID: 31428149; PMCID: PMC6683789.
Yamamoto N, Nokihara H, Yamada Y, et al. Phase I study of nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Invest New Drugs. 2017;35:207–16. https://doi.org/10.1007/s10637-016-0411-2. PMID: 27928714; PMCID: PMC5352798.
Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. https://doi.org/10.1200/JCO.2009.26.7609. PMID: 20516446; PMCID: PMC4834717.
Scheiner B, Roessler D, Phen S, et al. Efficacy and safety of immune checkpoint inhibitor rechallenge in individuals with hepatocellular carcinoma. JHEP Rep. 2022;5:100620. https://doi.org/10.1016/j.jhepr.2022.100620. PMID: 36578451; PMCID: PMC9791167.
Riveiro-Barciela M, Barreira-Díaz A, Callejo-Pérez A, et al. Retreatment with immune checkpoint inhibitors after a severe immune-related hepatitis: results from a prospective multicenter study. Clin Gastroenterol Hepatol. 2023;21:732–40. https://doi.org/10.1016/j.cgh.2022.03.050. (PMID: 35487453).
Yang K, Li J, Sun Z, et al. Retreatment with immune checkpoint inhibitors in solid tumors: a systematic review. Ther Adv Med Oncol. 2020;12:1758835920975353. https://doi.org/10.1177/1758835920975353. PMID: 33294036; PMCID: PMC7705192.
Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437–1448. https://doi.org/10.1016/S1470-2045(18)30739-3. PMID: 30355453.
Makiyama A, Arimizu K, Hirano G, et al. Irinotecan monotherapy as third-line or later treatment in advanced gastric cancer. Gastric Cancer. 2018;21(3):464–72. https://doi.org/10.1007/s10120-017-0759-9. (PMID: 28799048).
Acknowledgements
We thank Joe Barber Jr., PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
KT wrote the main manuscript text and prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsuji, K., Miyajima, S. & Kito, Y. Nivolumab Rechallenge After Prior Nivolumab Therapy in Advanced Gastric Cancer: A Single-Center Case Series and Literature Review. J Gastrointest Canc 55, 956–960 (2024). https://doi.org/10.1007/s12029-023-01011-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-023-01011-5