Abstract
Purpose
We aimed to emphasize the prognostic impact of differences included in the 8th versus the previous 7th edition of AJCC (American Joint Committee on Cancer) Cancer Staging manual for hepatocellular carcinoma (HCC).
Methods
A number of 87 consecutive HCCs were retrospectively evaluated and staged, using the 7th and 8th edition of AJCC staging systems. The clinicopathological parameters were correlated with the overall survival rate. No preoperative chemotherapy was received by any of the patients.
Results
According to the 7th edition of AJCC manual, 52 of the 87 cases were staged as pT2 and 35 as pT1. After restaging, according to the 8th edition, 23 of the 52 pT2 cases were understaged as pT1b, and the rest of the 29 remained as pT2. Regarding the 35 HCCs classified as pT1, using 7th edition, all of them were restaged as pT1a. Compared to the 7th staging system, using the 8th edition of AJCC manual, the percentage of pT2 tumors significantly decreased, from 59.77 to 33.33%. The patient’s gender, age, tumor focality, and grade of differentiation did not prove to have any prognostic value. Regarding pT stage, it does not influence the overall survival rate, independently from the used staging system.
Conclusion
The staging criteria, in the most recent edition of AJCC, are simplified and allowed tumor understaging. These changes do not have independent prognostic value. The prognostic impact of pT understaging should be evaluated in larger cohorts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) represents a global health problem, mainly because of its aggressiveness, recurrence, and metastatic capacity and because it evolves a long period of time without significant, specific symptoms. For this reason, most of the patients are diagnosed in late stages, being often above today’s therapeutically resources [1, 2]. HCC ranks the 6th place in global cancer incidence and represents the 4th cause of cancer-related death worldwide, after lung, breast, and colorectal cancers [2,3,4].
Major risk factors for HCC are hepatitis B and C viruses, excessive alcohol intake, obesity, nonalcoholic steatohepatitis (NASH), exposure to aristolochic acid, diabetes, and other metabolic diseases [2, 5,6,7]. More than half of HCCs (and up to 93%) reported cases worldwide are developed on an underlying chronic liver disease, such as cirrhosis [2, 5,6,7]. Even though there have been many advancements in understanding the biology and development of this type of cancer, including molecular signatures that can be targeted by specific treatment, HCC still kills more than 750,000 people annually [2, 4, 8].
The aim of this study was to validate the impact of changes included in the 8th versus the previous 7th edition of AJCC (American Joint Committee on Cancer) Cancer Staging manual for HCC [9, 10], the latest one being considered using first time the quantitative criterion of evidence grade. This is the first study regarding prognostic validation of the 8th edition of AJCC staging system in two Eastern European countries, Romania and Hungary.
Materials and Methods
We retrospectively searched for consecutive cases reported as HCC, in the Pathology Departments of two university hospitals from two East European countries: Clinical County Emergency Hospital of Tirgu Mures, Romania, and Semmelweis University, Budapest, Hungary. Follow-up was performed between 2 and 97 months after diagnosis.
Ethics Approval and Consent to Participate
For the evaluation of the cases, the approval of Ethical Committee of University of Medicine and Pharmacy of Tirgu Mures, Romania, and Semmelweis University, Budapest, Hungary, was obtained. As retrospective evaluation was done, signed informed consent of patients was not necessary.
The following inclusion criteria were used: consecutive cases that underwent open surgery, without preoperative locoregional therapy, with patients’ survival over 2 months. In all of the cases, besides clinicopathological parameters such age, gender, tumor size, and histological type of tumor, pT restaging was done using both 7th and 8th editions of AJCC Cancer Staging manual [9, 10].
For statistical assessment, we used the GraphPad InStat and Prism softwares. Survival rate was appreciated using Kaplan-Meier curves. Categorical data analysis was conducted with t-student test. The level of significance was set at p < 0.05. We evaluated the correlation of survival rates with gender and age by categorizing the patients into two groups, under and over 65 years. The other correlative analyses were made between overall survival rate (OS) and histological grade of differentiation, tumor focality, the aspect of peritumoral liver parenchyma and OS and pT staging, according to both 7th and 8th edition of AJCC staging system.
Results
Clinicopathological Features
A number of 87 consecutive patients diagnosed with HCC were included, 35 from Romania and 52 from Hungary. Analysis of the study group demonstrated that the majority of the cases reported were males, with a male/female ratio of 2.2/1 (Table 1).
Depending on the histological grade, the mean age varied insignificantly between G1, G2, and G3, respectively (61.33 for G1, 60.46 years for G2, and 62.52 for G3, respectively). For G4, only one case was enrolled in this study.
From the total number of 87 patients, 39 (44.82%) were 65 years or more. Out of the total number of 39 patients over 65 years, 11 (28.20%) were females, and the rest of the 28 (71.80%) were males. It was a statistically significant correlation between gender and histological grade, females tending to be affected by more undifferentiated tumors than males (p = 0.003) (Fig. 1). A proportion of 67.41% of the cases reported under the age of 65 were graded as moderately differentiated (G2), while the same G2 grade was represented by 48.08% from the patients with age 65 or more.
8th Versus 7th Edition of AJCC TNM Staging System
Compared with the 7th edition of AJCC [9], the latest 8th edition [10] brings the following modifications, which were synthesized in Table 2:
-
Stage pT1 was sub-devised in pT1a and pT1b, respectively; the previous edition pT1 stage included only tumors smaller than 2 cm, while subdivision 1b of 8th edition comprises also tumors greater than 2 cm in diameter, but without vascular invasion.
-
The newest edition brings a clarification upon the interval of dimensions for stage pT2 (tumors from 2 to 5 cm in diameter).
-
The previous pT3a becomes pT3, so stage IIIA remains unmodified.
-
The new pT4 stage integrates the characteristics of pT3b from 7th ed., so stages IIIB (T3BN0M0) and IIIC (T4N0M0) becomes stage IIIB (T4N0M0) of the 8th edition.
-
Stages IVA (any T, N1, M0) and IVB (any T, any N, M1) remain the same.
According to the 7th edition of AJCC staging system, 52 tumors were classified as T2, and the rest of the 35 were T1. After restaging all of them by using the criteria of the 8th edition of AJCC staging system, almost half of T2 were understaged as T1b tumors, the percentage of pT2 tumors decreasing significantly from 59.77 to 33.33%, and all of the cases staged as T1 were classified as T1a (Table 2).
Correlations Regarding the Survival Rates
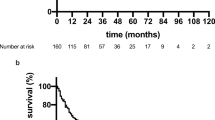
The average 5 years survival was 55.55%. The statistical assessment showed no statistically correlations of OS with the gender or age of patients, either with the number of tumor foci (Fig. 2). As expected, the survival rates were greater for tumors graded as G1 and G2, compared to G3 and G4 tumors. With a p value of 0.22, these results are without statistical significance (Fig. 2).
The OS for patients with tumors staged as pT1 decreases linearly after 3 years from diagnosis. For the first 5 years, the OS was greater for pT1 tumors, but after this point, pT2 tumor survival curve overcomes pT1 curve. The results are without statistical significance, using the 7th edition of AJCC staging system (Fig. 3a).
The survival curves realized after restaging the tumors by using the criteria from 8th edition of AJCC are almost supposable for the first 2 years after diagnosis for all pT1a, pT1b, and pT2 stages. After this point, they diverge paradoxically, with pT2 tumors showing a greater survival rate, due to the fact that the number of cases included is relatively small, with a large proportion of them being staged pT2 (Fig. 3b).
By analyzing the survival rates depending on whether the tumors are developed on a background of cirrhosis or not, tumors with this preexisting condition have a worse prognosis, cirrhosis-free tumor curve being situated above cirrhosis-associated tumor curve on the entire period of the follow-up (Fig. 4). In most of the cases, it was about alcohol-induced cirrhosis. It is necessary mentioning that a significant number of cases from the non-cirrhotic group (11/28) showed associated hepatitis C infection (Table 1).
Discussion
Our study demonstrated a predominance of male gender for HCC (68.96%), with a gender ratio M/F of 2.2/1. The results correlates with those reported by other authors, the M:F ratio ranging between 2:1 and 4:1, probably because of the region-related variation of the risk factors [4, 6, 11, 12].
In the present study, G2-HCC was the most frequently reported histological grade, representing more than 60% of cases. Bibliographical data return results between 37 and 79% for G1/G2 [2, 4, 13]. A systematic review of literature regarding the histological grade of HCC demonstrated a heterogeneity on the microscopic assessment, with divergences in the histological grade, showing the need for a more detailed standardization [2, 14].
The TNM staging system for HCC, proposed by AJCC, along with Barcelona Clinic Liver Cancer Staging System, represents the most commonly used systems, to predict the prognosis [15,16,17]. They use the size of the tumor, the number of tumors, the vascular invasion if present, the number of regional lymph nodes affected by the tumoral process, and distant metastasis, if present. Liver Cancer Study Group of Japan and Chinese staging systems are also proposed and are in use in Asia [15,16,17].
Recent studies bring evidence that expression of some molecular markers should be integrated with TNM staging system, to create a more complex classification for the improvement of prognostic accuracy [18,19,20]. Other authors proposed, for patients with resectable HCC, preoperative quantification of liver surface nodularity, as an indicator of posthepatectomy liver failure [6].
A study published in 2011, which analyses the prognostic value of the 7th edition of AJCC, concludes that even though its prognostic power is greater than the previous 6th edition, it should be used with caution for patients with advanced stages. Furthermore, this study showed that BCLC staging system seems to have a better prognostic power [21].
Recently released 8th edition staging system for HCC incorporates several changes, compared to the previous 7th edition. In literature, it is a relatively accepted idea that even though the newest edition brings a clarification upon the interval of dimensions for tumors classified as T2, it may require a further stratification for this stage, specifically by differentiating solitary tumors greater than 2 cm in diameter from multiple tumors smaller than 5 cm. The prognostic impact of whether a multifocal tumor with a diameter smaller than 5 cm invades or not one or more of the major portal or hepatic vein branches should be further analyzed [22].
The most recent studies in this field showed that, according to the 7th edition of AJCC, 41% of patients were diagnosed in stage I, 38.8% in stage II, 6.4% in stage IIIA, 5.7% in stage IIIB, and 7.3% in stage IIIC. After restaging by using the 8th edition’s criteria, over 65% of patients were diagnosed in stages I and II [4]. Stage I was represented by 13.8%, stage IB 29.7%, stage II 36.4%, stage IIIA 6.4%, and stage IIIB 13% [23].
In line to our data, comparison of the 5-year overall survival rate between 7th and 8th edition of AJCC did not show no major differences: Using 7th edition, for stage I, the 5-year overall survival rate was 86.8%, for stage II 63.5%, for stage IIIA 38.2%, for stage IIIB 28.9%, and for stage IIIC 28.6%. Using 8th edition, the 5-year overall survival rate was 90.4% for stage IA, 85% for stage IB, 62% for stage II, 38.2% for stage IIIA, and 29.1% for stage IIIB [4, 22,23,24,25].
The present study revealed that the latest AJCC edition of HCC staging system includes an understaging of the T2 tumors, which induce stratification of the previous T1 (7th ed.) into T1a and T1b (8th ed.), respectively, with T1b receiving some of the criteria from the old T2. As no prognostic impact was proved in our material, further studies are necessary to analyze the clinical value of these modifications on the staging system, as a prognostic tool.
Conclusions
Although the pT2 tumors are understaged, according to the 8th AJCC system, it does not add new prognostic parameters. Large cohorts need to be analyzed, to emphasize the possible impact of the newest staging system that is more easily to be used, compared with the older one.
Availability of Data and Materials
The used data were stored in a database but are confidential data, based on our institutional rules.
References
Rastogi A. Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma. World J Gastroenterol. 2018;24:4000–13.
Turdean S, Gurzu S, Turcu M, Voidazan S, Sin A. Current data in clinicopathological characteristics of primary hepatic tumors. Romanian J Morphol Embryol. 2012;53:719–24.
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. Accessed on 22/03/2019
Wan S, Nie Y, Zhu X. Development of a prognostic scoring model for predicting the survival of elderly patients with hepatocellular carcinoma. Peer J. 2020. https://doi.org/10.7717/peerj.8497.
Pittman M, Brunt E. Anatomic pathology of hepatocellular carcinoma: histopathology using classic and new diagnostic tools. Clin Liver Dis. 2015;19:239–59.
Hobeika C, Cauchy F, Sartoris R, Beaufrere A, Yoh T, Vilgrain V, et al. Relevance of liver surface nodularity for preoperative risk assessment in patients with resectable hepatocellular carcinoma. Br J Surg. 2020. https://doi.org/10.1002/bjs.11511.
Bara T Jr, Jung I, Sugimura H, Bara T, Beleaua MA, Gurzu S. A systematic review of the possible carcinogenic role of the aristolochic acid. Romanian J Morphol Embryol. 2017;58:41–4.
Saleh D, Muhammad M. Current and future treatment of hepatocellular carcinoma: an updated comprehensive review. J Clin Transl Hepatol. 2018;6:69–78.
Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A. AJCC Cancer staging manual, vol. 18. 7th ed. Chicago: Springer International Publishing: American Joint Commission on Cancer; 2009. p. 191–200.
Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer staging manual, vol. 22. 8th ed. Chicago: Springer International Publishing: American Joint Commission on Cancer; 2017. p. 287–93.
Liu P, Xie S, Hu S, Cheng X, Gao T, Zhang C, et al. Age specific sex difference in the incidence of hepatocellular carcinoma in the United States. Oncotarget. 2017;8:68131–7.
Omata M, Cheng A, Kokudo N, Kudo M, Lee J. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–70.
Guo W, Zhao S, Yang Y, Shao G. Histological grade of hepatocellular carcinoma predicted by quantitative diffusion-weighted imaging. Int J Clin Exp Med. 2015;8:4164–9.
Martins-Filho SN, Paiva C, Azevedo RS, Alves VAF. Histological grading of hepatocellular carcinoma-a systematic review of literature. Front Med. 2017;4:193.
Kee K, Wang J, Lin C, Wang C, Cheng Y, Lu S. Validation of the 7th edition TNM staging system for hepatocellular carcinoma: an analysis of 8,828 patients in a single medical center. Dig Dis Sci. 2013;58:2721–8.
Santambrogio R, Salceda J, Costa M. External validation of a simplified BCLC staging system for early hepatocellular carcinoma. Eur J Surg Oncol. 2013;39:850–7.
Huang XT, Chen LH, Huang CS, Li JH, Cai JP, Chen W, et al. Establishment of a nomogram by integrating molecular markers and tumor-node-metastasis staging system for predicting the prognosis of hepatocellular carcinoma. Dig Surg. 2019;36:426–32.
Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245:909–22.
Fodor D, Jung I, Turdean S, Satala C, Gurzu S. Angiogenesis of hepatocellular carcinoma: an immunohistochemistry study. World J Hepatol. 2019;11:294–304.
Gurzu S, Kobori L, Fodor D, Jung I. Epithelial mesenchymal and endothelial mesenchymal transitions in hepatocellular carcinoma: a review. Biomed Res Int. 2019;2962580. https://doi.org/10.1155/2019/2962580.
Chun YH, Kim SU, Park JY, Kim DY, Han KH, Chon CY, et al. Prognostic value of the 7th edition of the AJCC staging system as a clinical staging system in patients with hepatocellular carcinoma. Eur J Cancer. 2011;47:2568–75.
Kamarajah SK, Frankel TL, Sonnenday C, Cho CS, Nathan H. Critical evaluation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with hepatocellular carcinoma (HCC): a surveillance, epidemiology, end results (SEER) analysis. J Surg Oncol. 2018;117:644–50.
Kim IG, Hu XG, Wang HJ, Kim BW, Hong SY, Shen XY. The 7th/8th American Joint Committee on Cancer and the modified Union for International Cancer Control staging system for hepatocellular carcinoma. Yonsei Med J. 2019;60:140–7.
Sun YY, Soon HU, Chung GS, Yoo JL, Tae HK, Yeon SS, et al. Validation of American Joint Committee on Cancer 8th staging system in patients with resected hepatocellular carcinoma. Gut Liver. 2019;13:103.
Ueno M, Morizane C, Ikeda M, Okusaka T, Ishii H, Furuse J. A review of changes to and clinical implications of the eight TNM classification of hepatobiliary and pancreatic cancers. Jpn J Clin Oncol. 2019;49:1073–82.
Funding
This study was funded by Romanian National Authority for Scientific Research, CNCS – UEFISCDI, project number 20 PCCF/2018, code: PN-III-P4-ID-PCCF-2016-0006. The financial resources were used for data interpretation only.
Author information
Authors and Affiliations
Contributions
SC wrote the manuscript and collected data about Romanian patients, JI performed interpretation of histopathological data, KL supervised collection of data regarding Hungarian patients and performed liver transplantation; KZ performed statistical assessment; SR contributed to data collection and literature review; FD collected clinical data about Hungarian patients; SG supervised the study design and allowed final variant of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
For the evaluation of the cases, the approval of Ethical Committee of University of Medicine and Pharmacy of Targu-Mures, Romania, and Semmelweis University, Budapest, Hungary, was obtained. As retrospective evaluation was done, signed informed consent of patients was not necessary.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Satala, C.B., Jung, I., Kobori, L. et al. Benefits of the 8th American Joint Committee on Cancer System for Hepatocellular Carcinoma Staging. J Gastrointest Canc 52, 243–248 (2021). https://doi.org/10.1007/s12029-020-00394-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-020-00394-z